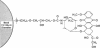Abstract
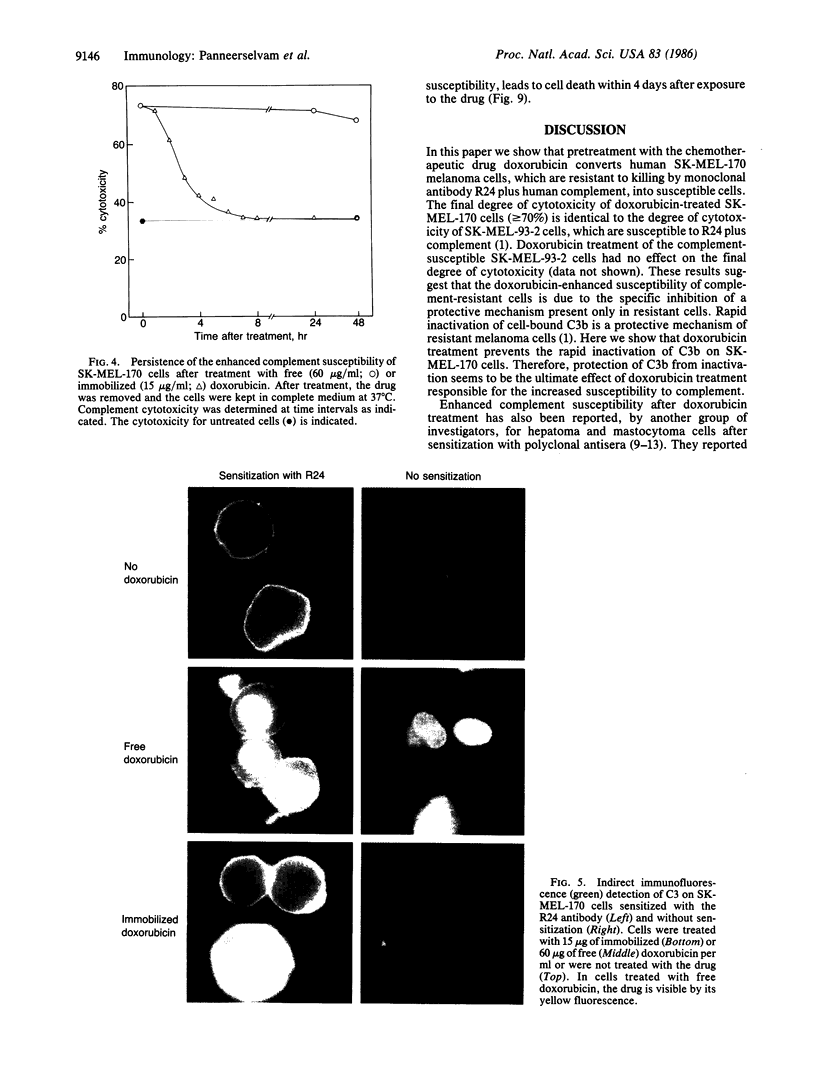
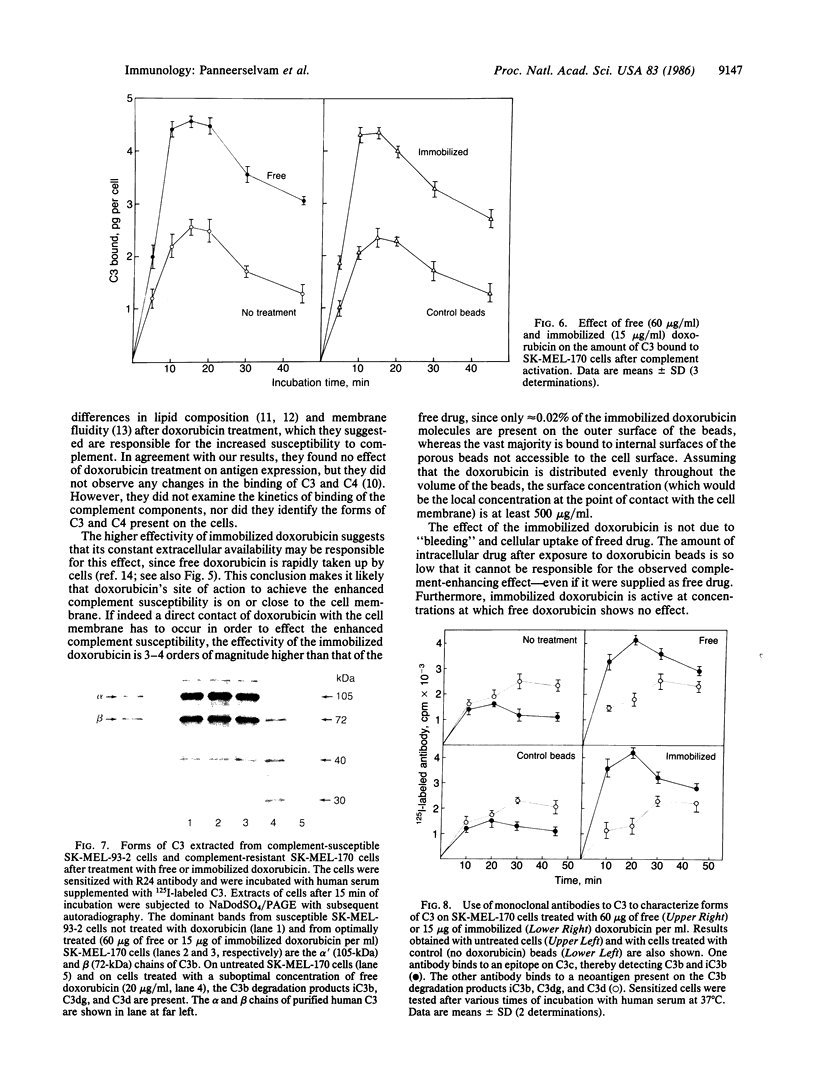
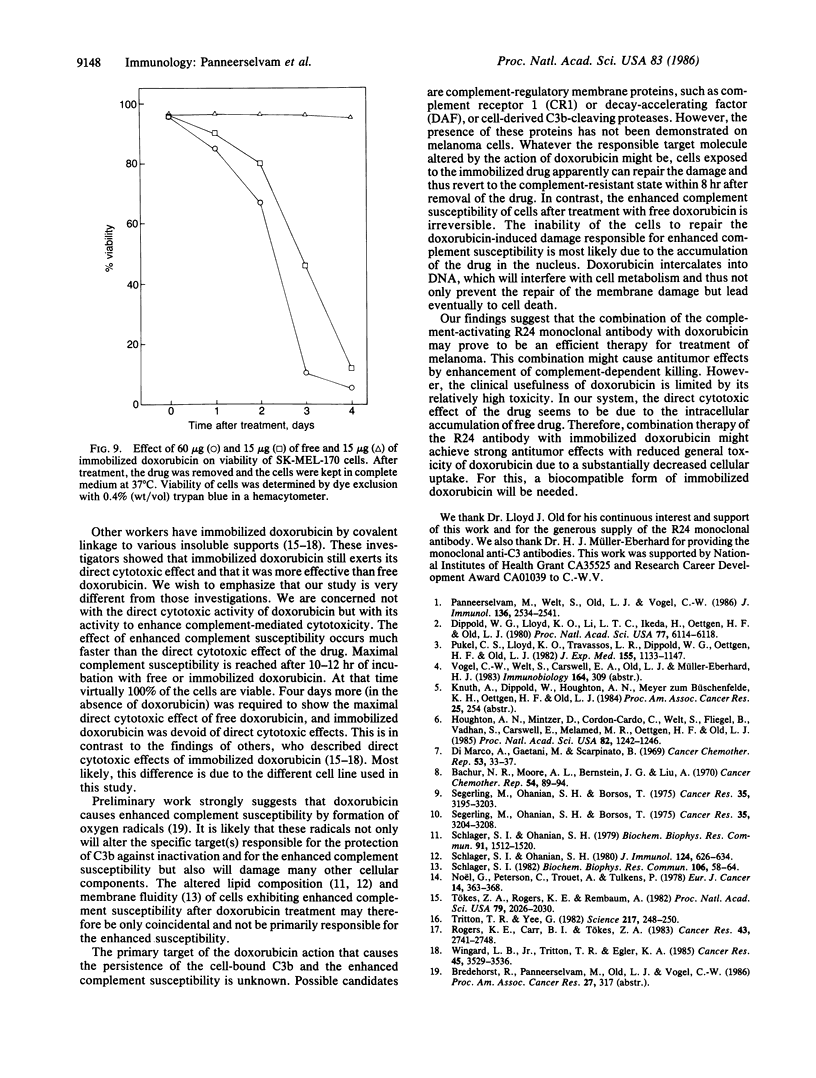
Human melanoma cells resistant to killing by monoclonal antibody R24 plus human complement became susceptible after treatment with doxorubicin (adriamycin). Treatment with doxorubicin prevented the rapid degradation of surface-bound complement component C3b that has been identified as a protective mechanism of complement-resistant melanoma cells. Doxorubicin caused the increased complement susceptibility as free drug and after immobilization onto glass beads to prevent cellular uptake. Immobilized doxorubicin was more effective than free drug, causing enhanced complement susceptibility at concentrations where the free drug was no longer active. In contrast to free doxorubicin, which exhibited a direct cytotoxic effect leading to cell death within 4 days, immobilized doxorubicin did not affect cell viability. These findings suggest that combination therapy of the complement-activating monoclonal antibody R24 with the complement-enhancing drug doxorubicin may be a promising approach for the treatment of melanoma.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachur N. R., Moore A. L., Bernstein J. G., Liu A. Tissue distribution and disposition of daunomycin (NCS-82151) in mice: fluorometric and isotopic methods. Cancer Chemother Rep. 1970 Apr;54(2):89–94. [PubMed] [Google Scholar]
- Di Marco A., Gaetani M., Scarpinato B. Adriamycin (NSC-123,127): a new antibiotic with antitumor activity. Cancer Chemother Rep. 1969 Feb;53(1):33–37. [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A. N., Mintzer D., Cordon-Cardo C., Welt S., Fliegel B., Vadhan S., Carswell E., Melamed M. R., Oettgen H. F., Old L. J. Mouse monoclonal IgG3 antibody detecting GD3 ganglioside: a phase I trial in patients with malignant melanoma. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1242–1246. doi: 10.1073/pnas.82.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel G., Peterson C., Trouet A., Tulkens P. Uptake and subcellular localization of daunorubicin and adriamycin in cultured fibroblasts. Eur J Cancer. 1978 Apr;14(4):363–368. doi: 10.1016/0014-2964(78)90206-2. [DOI] [PubMed] [Google Scholar]
- Panneerselvam M., Welt S., Old L. J., Vogel C. W. A molecular mechanism of complement resistance of human melanoma cells. J Immunol. 1986 Apr 1;136(7):2534–2541. [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers K. E., Carr B. I., Tökés Z. A. Cell surface-mediated cytotoxicity of polymer-bound Adriamycin against drug-resistant hepatocytes. Cancer Res. 1983 Jun;43(6):2741–2748. [PubMed] [Google Scholar]
- Schlager S. I. Ability of tumor cells to resist humoral vs. cell-mediated immune attack is controlled by different membrane physical properties. Biochem Biophys Res Commun. 1982 May 14;106(1):58–64. doi: 10.1016/0006-291x(82)92057-5. [DOI] [PubMed] [Google Scholar]
- Schlager S. I., Ohanian S. H. Physico-chemical properties of tumor cells that influence their susceptibility to humoral immune killing. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1512–1520. doi: 10.1016/0006-291x(79)91236-1. [DOI] [PubMed] [Google Scholar]
- Schlager S. I., Ohanian S. H. Tumor cell lipid composition and sensitivity to humoral immune killing. I. Modification of cellular lipid and fatty acid content by metabolic inhibitors and hormones. J Immunol. 1980 Feb;124(2):626–634. [PubMed] [Google Scholar]
- Segerling M., Ohanian S. H., Borsos T. Effect of metabolic inhibitors on the ability of tumor cells to express antigen and bind complement components C4 and C3. Cancer Res. 1975 Nov;35(11 Pt 1):3204–3208. [PubMed] [Google Scholar]
- Segerling M., Ohanian S. H., Borsos T. Enhancing effect by metabolic inhibitors on the killing of tumor cells by antibody and complement. Cancer Res. 1975 Nov;35(11 Pt 1):3195–3203. [PubMed] [Google Scholar]
- Tokes Z. A., Rogers K. E., Rembaum A. Synthesis of adriamycin-coupled polyglutaraldehyde microspheres and evaluation of their cytostatic activity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2026–2030. doi: 10.1073/pnas.79.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Wingard L. B., Jr, Tritton T. R., Egler K. A. Cell surface effects of adriamycin and carminomycin immobilized on cross-linked polyvinyl alcohol. Cancer Res. 1985 Aug;45(8):3529–3536. [PubMed] [Google Scholar]