Abstract
Corticotropin releasing factor (CRF) is the primary mediator of stress responses, and nociceptin/orphanin FQ (N/OFQ) plays an important role in the modulation of these stress responses. Thus, in this multidisciplinary study, we explored the relationship between the N/OFQ and the CRF systems in response to stress. Using in situ hybridization (ISH), we assessed the effect of body restraint stress on the gene expression of CRF and N/OFQ-related genes in various subdivisions of the amygdala, a critical brain structure involved in the modulation of stress response and anxiety-like behaviors. We found a selective upregulation of the NOP and downregulation of the CRF1 receptor transcripts in the CeA and in the BLA after body restraint. Thus, we performed intracellular electrophysiological recordings of GABAA-mediated IPSPs in the central nucleus of the amygdala (CeA) to explore functional interactions between CRF and N/OFQ systems in this brain region. Acute application of CRF significantly increased IPSPs in the CeA, and this enhancement was blocked by N/OFQ. Importantly, in stress-restraint rats, baseline CeA GABAergic responses were elevated and N/OFQ exerted a larger inhibition of IPSPs compared with unrestraint rats. The NOP antagonist [Nphe1]-nociceptin(1–13)NH2 increased the IPSP amplitudes in restraint rats but not in unrestraint rats, suggesting a functional recruitment of the N/OFQ system after acute stress. Finally, we evaluated the anxiety-like response in rats subjected to restraint stress and nonrestraint rats after N/OFQ microinjection into the CeA. Intra-CeA injections of N/OFQ significantly and selectively reduced anxiety-like behavior in restraint rats in the elevated plus maze. These combined results demonstrate that acute stress increases N/OFQ systems in the CeA and that N/OFQ has antistress properties.
Introduction
From a biological perspective, stress may be viewed as external demands placed on a living organism that in turn reacts to them with a highly dynamic combination of physiological, emotional, cognitive, and behavioral responses that have evolved to be adaptive, although they may be more or less successful in a given instance. These reactions are initially aimed at regaining a preexisting equilibrium; however, this may occur by establishing a new setpoint. This allostatic change may be viewed as only a partially successful adaptive response, which occurs in the presence of prolonged stress exposure, at the cost of altering normal physiological balance (McEwen and Gianaros, 2011; Schank et al., 2012). Setpoint shift involves neuroadaptations within neurocircuitry that mediate stress responses, and are influenced by several peptidergic neuromodulators. Corticotropin releasing factor (CRF) via its actions on hypothalamic and extrahypothalamic brain sites is recognized as a primary mediator of stress responses in mammals (McEwen and Gianaros, 2011; Schank et al., 2012). Other peptidergic systems, including nociceptin/orphanin FQ (N/OFQ), have been identified as important players in contrasting stress responses mediated by CRF. We previously found that N/OFQ, an opioid-like peptide that binds with high affinity to its NOP, exerts potent functional antistress and anti-CRF actions. In particular, activation of brain NOPs by N/OFQ or other selective agonists prevents anorexia elicited by intracerebroventricular administration of CRF or by exposure to stress (Ciccocioppo et al., 2001, 2002). N/OFQ also prevents foot-shock stress-induced reinstatement of alcohol seeking (Martin-Fardon et al., 2000), whereas alcohol withdrawal in dependent animals, a condition associated with high-stress vulnerability, is associated with an increased N/OFQ and NOP gene expression in the central nucleus of the amygdala (CeA) and in the bed nucleus of the stria terminalis (Aujla et al., 2013). N/OFQ also blocks the ethanol- and CRF-induced increases in GABAergic transmission in the CeA (Veinante and Freund-Mercier, 1998; Roberto and Siggins, 2006; Cruz et al., 2012).
Thus, in this study, we explored the relationship between the CRF and the N/FQ systems in response to stress. We elicited stress by physically restraining rats and then measured the expression of CRF and N/OFQ-related genes in various subdivisions of the amygdala. We found significant and selective changes in NOP and CRF1 receptor transcript expression in the CeA and BLA of restraint rats compared with unrestraint ones. Because our gene expression results also show that CeA, but not BLA, expresses both N/OFQ and CRF, we assessed whether changes occurred in CeA GABA systems after acute physical stress restraint. Thus, focusing on the CeA we performed in vitro electrophysiological recordings to explore the interactions between N/OFQ and CRF and possible neuroplastic changes in the two peptidergic systems induced by restraint stress. Finally, we investigated the effect of NOP manipulation in the CeA on the elevated plus maze (EPM) test in rats subjected to body restraint and in unrestraint controls.
Materials and Methods
Subjects.
Male Wistar rats (N = 108, Charles River) weighing 250–300 g at the beginning of the experiments were used. Animals were housed in standard cages, in a room with artificial 12:12 h light/dark cycle (lights off at 8:00 A.M.) at constant temperature (20–22°C) and humidity (45–55°), with food and water ad libitum. Rats were handled once a day for 5 min during the first week after arrival to the vivarium. All procedures were conducted during the dark cycle and met the guidelines of the European Community Council Directive for Care and Use of Laboratory Animals and The Scripps Research Institute Institutional Animal Care and Use Committee and National Institutes of Health guidelines on the care and use of laboratory animals.
Intracranial surgery and histological analysis.
For intracranial surgery, animals were anesthetized by intramuscular injection of 100–150 μl of a solution containing tiletamine chlorohydrate (58.17 mg/ml) and zolazepam chlorohydrate (57.5 mg/ml). To reach the CeA, guide cannulae were implanted bilaterally using the following coordinates with reference to Bregma: CeA, anteroposterior −1.8; lateral ±3.9, and ventral −7.0 (Paxinos and Watson, 1998). For intracranial injection, N/OFQ was dissolved in artificial CSF (ACSF) and given bilaterally as nmol doses into the CeA in a volume of 0.5 μl/site by means of a stainless-steel injector 1.5 mm longer than the guide cannula, so that its tip protruded into the area. After the experiments, to verify the cannula placement, 0.5 μl/site of black India ink was injected into the CeA immediately before the rat was euthanized, and ink diffusion into the CeA was histologically evaluated. Animals (N = 3) with wrong placed cannulae were excluded from the analysis.
Drugs.
Nociceptin/orphanin FQ (Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asp-Glu) (N/OFQ) and [Nphe1]-nociceptin(1–13)NH2 were purchased from Tocris Bioscience. CGP 55845A was a gift from Novartis Pharma. dl-AP5, DNQX, picrotoxin, and bicuculline were obtained from Sigma, whereas CRF was purchased from Chempacific.
Acute restraint stress.
For the acute restraint stress, male Wistar rats were restrained, for 1 h in a cylindrical tube made of clear Plexiglas measuring 21.5-cm-long, 6.3 cm internal diameter. Restraint tubes were round slotted Plexiglas cylinders with sliding plugs to allow adjustment of the tube length for each animal size. The end of the tube had a sliding plastic plug that was secured in place by a screw and adjusted to fit the size of the rat. A slotted opening in the plug allowed for free mobility of the tail. For behavioral studies, animals were divided in two groups. The first one was then sorted into three smaller groups (N = 7 or 8 per group) and received N/OFQ (0.5 or 1.0 nmol/rat) or vehicle (0.0 nmol/rat) 5 min before they were immobilized in the restraint tube. They were restrained for 1 h and then returned to their home cages. Six hours later, their anxiety-like behavior was measured on the EPM, for 5 min. The same identical procedure was performed for the control/nonrestraint rats (N = 7 or 8 per group).
For the electrophysiological (N = 28) and ISH (N = 16) studies, the same procedure was performed to stress the animals. The recordings from the slices were made 6 h later. The control rats (not subjected to the restraint stress) were euthanized at the same time of the day as the acutely restraint animals. For the ISH study, the animals were euthanized 6 h after the stress procedure.
EPM.
The EPM is a widely used test of anxiety-like behavior, which is sensitive to anxiogenic and anxiolytic drugs (Pellow et al., 1985). We used this test to study the effect of N/OFQ on anxiety-like responses in restraint versus unrestraint animals (controls). All behavioral testing took place in a dimly lit room. The apparatus consisted of four black wooden arms (50 cm long × 10 cm wide), arranged such that the respective closed and open arms were opposite to each other. The maze, elevated 50 cm above the floor, was cleaned with water and dried after each trial. The 5 min test procedure began when the animals were individually placed in the center of the maze, facing a closed arm. A rat was considered to be on the central platform when at least two of its paws were on it. An entry was defined as the presence of all four paws in the arms. Percentage of time spent in open arms [% OAT = (time in open arm/time in “open arm” + time in “closed” arm) × 100] and percentage of open arm entries [% OAE = (number of open arm entries/number of “open + closed” arm entries) × 100] were considered as an index of anxiety, and the number of total arm entries was used as a measure of spontaneous locomotor activity.
ISH.
Six hours after restraint stress, rats were rapidly decapitated. Brains were quickly removed, snap frozen in −40°C isopentane, and stored at −70°C; 10 μm coronal brain sections were taken at Bregma levels according to the atlas of Paxinos and Watson (1998) and stored at −70°C until use. Specific rat riboprobes for N/OFQ (gene reference sequence in PubMed database: NM_013007, position 275–535 bp), NOP (gene reference sequence in PubMed database: NM_031569, position 879–1142 bp), CRF (gene reference sequence in PubMed database: NM_031019, position 590–748 bp), and CRF1R (gene reference sequence in PubMed database: NM_030999, position 808–1318 bp) were used as previously reported (Hansson et al., 2006). Procedures for S35 labeled probes RNA probe synthesis in both antisense and sense direction and all the hybridization steps have been described in detail in (Hansson et al., 2003, 2006). For data visualization, phosphor-imaging plates (Fujifilm for BAS-5000, Fujifilm) were exposed for 48 h to hybridized sections. Phosphor imager (Fujifilm Bio-Imaging Analyzer Systems, BAS-5000, Fujifilm) generated digital images were analyzed using MCID Image Analysis Software (Imaging Research). Regions of interest were defined by anatomical landmarks as described in the atlas of Paxinos and Watson (1998). Signal density was measured as photostimulable luminescence per millimeter squared and converted into integrated optical density values, expressed in calibration standards units (nCi/g) using a C14 standard curve (Microscale C14, GE Healthcare). For detailed visualization, films (Kodak BioMax MR, Eastman Kodak Company) were subsequently exposed for 1 month to hybridized sections.
Electrophysiology.
The brain slices were prepared 3 h after the termination of the restraint period. Electrophysiological recordings were done between 5 and 7 h from restraint.
Slice preparation.
CeA slices were prepared as previously described (Ciccocioppo et al., 2004) from 41 male Wistar rats (264.2 ± 17 g) that were anesthetized with isoflurane (3%) and decapitated. The brains were rapidly removed and placed into ice-cold ACSF equilibrated with 95% O2 and 5% CO2.
Transverse, 400-μm-thick slices were cut on a Vibratome Series 3000 (Technical Products International), incubated in an interface configuration for ∼20 min, and subsequently completely submerged and continuously superfused (flow rate of 2–4 ml/min) with warm (31°C), gassed ACSF of the following composition in mm: NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4-7H2O, 1.5; CaCl2, 2.0; NaHCO3, 24; glucose, 10. Drugs were added to the ACSF from stock solutions to obtain known molar concentrations in the superfusate.
Electrophysiology.
Neurons were recorded principally in the medial subdivision of the CeA with sharp micropipettes filled with 3M KCl using current-clamp mode. Most neurons were held near their resting membrane potential. Pharmacologically isolated GABAA receptor-mediated IPSPs were evoked by local stimulation within the CeA through a bipolar stimulating electrode while superfusing the slices with the glutamate receptor blockers DNQX (20 μm) and DL-2-amino-5-phosphonovalerate (dl-AP5; 30 μm), and the GABAB receptor antagonist (CGP 55845A; 1 μm). Bicuculline (30 μm) or picrotoxin (50 μm) was often superfused at the end of the experiment to confirm the GABAA nature of the IPSP. Data were acquired with an Axoclamp-2A preamplifier (Molecular Devices) and stored for later analysis using pClamp software (Molecular Devices). To determine the experimental response parameters for each cell, we performed an input–output protocol consisting of a range of current stimulations (typically between 50 and 250 mA; 0.125 Hz), starting at the threshold current required to elicit an IPSP up to the strength required to elicit the maximum amplitude. The stimulus strength was maintained throughout the entire duration of the experiment. We normalized three stimulus intensities of equal steps (threshold, half-maximal, and maximal) as 1–3×. Stability of IPSPs was established by local stimulation for at least 15 min before beginning experiments. The synaptic responses were quantified by averaging two consecutive responses (30 s apart, i.e., 1 data point/min) and calculating the IPSP amplitude with Clampfit software (Molecular Devices). We also applied hyperpolarizing and depolarizing current steps (200 pA increments, 750 ms duration) to generate voltage-current curves. We examined paired-pulse facilitation (PPF) in each neuron using paired stimuli at 50 ms interstimulus intervals. The stimulus strength was adjusted such that the amplitude of the first IPSP was 50% of the maximal amplitude as determined from the input–output relationship. We calculated the PPF ratio as the second IPSP amplitude over that of the first IPSP amplitude (Roberto et al., 2003; Cruz et al., 2012). All measures were taken before CRF or N/OFQ superfusion (control), during drug superfusion (5–15 min), and after washout (20–30 min). All values are mean ± SEM.
Statistical analysis.
Behavioral data were analyzed by means of two-way ANOVA with two between-subjects factors (restraint and N/OFQ treatment). When appropriate, post hoc comparisons were performed with the Newman–Keuls tests.
For gene expression analyses, region-wise comparisons by mean of Students's t test were adopted to evaluate statistical significance.
Electrophysiological data were expressed as mean ± SEM. Statistical analysis was performed with GraphPad Prism 5.0 software (GraphPad Software). We analyzed the data using a one-way repeated measure ANOVA or two-way repeated measure ANOVA followed by a Newman–Keuls post hoc test; p < 0.05 was considered statistically significant. In some cases, the Student's paired or unpaired t test for individual means comparisons was used. Statistical significance was set at p < 0.05.
Results
Restraint stress-induced changes in N/OFQ and CRF-related genes
To assess the effect of stress on the regional distribution of N/OFQ, NOP, CRF, and CRF1 mRNA, rat brains were analyzed by ISH. The expression of N/OFQ in all areas analyzed is reported in Table 1. In general, N/OFQ mRNA distribution was similar to that previously reported in other studies with hybridization signals found at comparable levels of expression in the medial amygdala (MeA) and the CeA. In these regions, restraint stress did not induce significant variations in N/OFQ mRNA expression. The distribution density of NOP mRNA is reported in Table 2. The highest expression level was found in the MeA followed by the CeA and the BLA. When expression levels were compared between restraint and unrestraint rats, t test revealed statistically significant higher expression levels in restraint rats versus controls in the CeA (t(14) = 3.02; p < 0.01) and in the BLA (t(13) = 4.02; p < 0.01). No significant differences were observed in the MeA.
Table 1.
N/OFQ mRNA levels in different forebrain regions of restraint and control Wistar ratsa
| Brain region | Treatment | nCi/g (mean ± SEM) | N |
|---|---|---|---|
| CeA | Control | 17.81 ± 0.94 | 8 |
| Restraint | 18.60 ± 0.69 | 6 | |
| MeA | Control | 16.94 ± 2.17 | 8 |
| Restraint | 15.24 ± 2.77 | 6 | |
| BLA | Control | ND | 8 |
| Restraint | ND | 6 |
aData are expressed as nCi/g (mean ± SEM); n = 6–8/group. Statistical analysis has been performed by Student's t test. ND, Signal not detected.
Table 2.
NOP mRNA levels in different forebrain regions of restraint and control Wistar ratsa
| Brain region | Treatment | nCi/g (mean ± SEM) | N |
|---|---|---|---|
| CeA | Control | 3.25 ± 0.51 | 8 |
| Restraint | 5.22 ± 0.41* | 8 | |
| MeA | Control | 8.63 ± 0.60 | 8 |
| Restraint | 8.28 ± 0.51 | 8 | |
| BLA | Control | 2.27 ± 0.19 | 8 |
| Restraint | 3.66 ± 0.27* | 7 |
aData are expressed as nCi/g (mean ± SEM); n = 7 or 8/group. Statistical analysis has been performed by Student's t test.
*p < 0.01, restraint versus control.
As shown in Table 3, detectable CRF mRNA levels were found in the CeA. However, we did not find a significant difference between restraint and unrestraint rats in the CeA. The distribution density of CRF1 mRNA is reported in Table 4. The highest expression density was observed in the BLA followed by the CeA, and the MeA where lower but comparable levels of expression were detected. When CRF1 mRNA expression densities were compared between restraint and unrestraint rats, t test revealed a significantly lower mRNA levels in the CeA (t(14) = 3.36; p < 0.01) and BLA (t(13) = 3.42; p < 0.01) of stressed animals.
Table 3.
CRF mRNA levels in different forebrain regions of restraint and control Wistar ratsa
| Brain region | Treatment | nCi/g (mean ± SEM) | N |
|---|---|---|---|
| CeA | Control | 1.70 ± 0.12 | 6 |
| Restraint | 2.42 ± 0.30 | 6 | |
| MeA | Control | ND | 6 |
| Restraint | ND | 6 | |
| BLA | Control | ND | 6 |
| Restraint | ND | 6 |
aData are expressed as nCi/g (mean ± SEM); n = 6–8/group. Statistical analysis has been performed by Student's t test. ND, Signal not detected.
Table 4.
CRF1 mRNA levels in different forebrain regions of restraint and control Wistar ratsa
| Brain region | Treatment | nCi/g (mean ± SEM) | N |
|---|---|---|---|
| CeA | Control | 4.72 ± 0.29 | 8 |
| Restraint | 3.31 ± 0.30* | 8 | |
| MeA | Control | 4.61 ± 0.26 | 8 |
| Restraint | 3.99 ± 0.38 | 7 | |
| BLA | Control | 8.56 ± 0.23 | 7 |
| Restraint | 6.31 ± 0.58* | 8 |
aData are expressed as nCi/g (mean ± SEM); n = 7 or 8/group. Statistical analysis has been performed by Student's t test.
*p < 0.01, restraint versus control.
Basal GABAergic transmission is increased in CeA neurons from stress-restraint rats compared with unrestraint rats
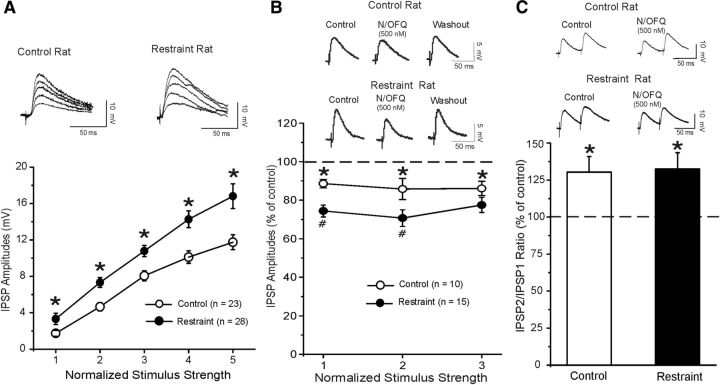
Because the CeA is a brain region well known to be involved in the physiological response to stressors and our gene expression results show that CeA is the only amygdala nucleus expressing both N/OFQ and CRF and their respective receptors, we assessed whether changes occurred in CeA GABA systems after acute physical stress restraint. We performed all the in vitro electrophysiological experiments in the CeA slices from either restraint or unrestraint control rats. We recorded from 105 CeA neurons with mean resting membrane potential of −76.7 ± 1.9 mV and a mean input resistance of 114 ± 5.6 mΩ. The restraint stress procedure did not significantly affect the basic membrane properties of the CeA neurons. We evoked pharmacologically isolated GABAA receptor-mediated IPSPs by local stimulation within the CeA. Interestingly, baseline IPSP input–output curves generated by equivalent stimulus intensities were significantly (F(1,253) = 5,749; p < 0.001) higher in slices from restraint (n = 28) compared with those from unrestraint rats (n = 23; Fig. 1A), suggesting an increased GABAergic tone in neurons from animals exposed to the acute stress. In CeA neurons of restraint rats, the baseline PPF ratio of IPSPs was significantly (t(47) = 2.700; p < 0.001, by unpaired t test) lower (0.85 ± 0.05, n = 28) than in CeA neurons of unrestraint (1.12 ± 0.09; n = 21) rats, suggesting increased baseline GABA release.
Figure 1.
A, Central amygdala basal GABAergic transmission is enhanced in restraint rats compared with control unrestraint rats. Top, Representative evoked CeA GABAA IPSPs from the input–output curves generated in control and restraint rats. Bottom, Input–output curves of mean GABAA IPSP amplitudes. The IPSPs are larger in restraint rats using five equivalent stimulus intensities. The mean baseline GABAergic transmission is significantly increased in slices from restraint rats (n = 21) compared with unrestraint rats (n = 17): *p < 0.05 (unpaired t test). B, Top, Representative evoked CeA IPSPs from control and restraint rats during the control, 500 nm N/OFQ application and washout. Bottom, A total of 500 nm N/OFQ significantly (*p < 0.05) decreases the mean IPSP amplitudes of evoked IPSP over the middle three stimulus strength intensities tested. The N/OFQ-induced inhibition of IPSP amplitudes is larger (#p < 0.05) in CeA of restraint compared with unrestraint rats. C, Top, Representative PPF of evoked CeA IPSPs from control and restraint rats during control and 500 nm N/OFQ application. Bottom, Histograms representing percentage increase in mean ± SEM PPF ratios of IPSPs using 50 ms interstimulus interval in CeA of control and restraint rats. N/OFQ significantly increased the PPF ratio in both groups: *p < 0.05 (paired t test).
N/OFQ inhibition of GABAergic transmission is greater in restraint rats compared with unrestraint rats
We previously reported that N/OFQ decreased the amplitudes of evoked IPSPs in CeA GABAergic synapses from naive Sprague Dawley rats via a decrease in GABA release (Roberto and Siggins, 2006). Consistent with this earlier report, here we found that 500 nm N/OFQ significantly (p < 0.05) reduced the amplitudes of evoked IPSPs to 87.0 ± 0.9% of control (Fig. 1B; n = 10) in CeA from naive Wistar rats over all stimulus strengths. To assess whether the site of action was presynaptic, we examined PPF of IPSPs. Generally, changes in PPF are inversely related to transmitter release (Jensen et al., 1994), such as an increase in PPF ratio suggests decreased GABA release and vice versa. N/OFQ significantly (t(9) = 2.290; p < 0.05) increased the PPF ratio of IPSPs to 130.5% of control (control 1.16 ± 0.14, N/OFQ 1.44 ± 0.16, n = 10; Fig. 1C), suggesting that N/OFQ decreases GABA release. Then we assessed whether acute restraint stress induced relevant alterations in the N/OFQ effects on GABAergic transmission in the CeA. Interestingly, in 15 CeA neurons from restraint rats, the N/OFQ-induced inhibition of evoked IPSP amplitudes was significantly [two-way repeated-measures ANOVA (stress restraint × drug)], F(1,23) = 9.56; p < 0.05] larger (∼25%) compared with the inhibition (∼13%) observed in control unrestraint animals (n = 10; Fig. 1B). In CeA of restraint rats, the baseline PPF ratio of IPSPs was 0.94 ± 0.07, lower than in naive (1.16 ± 0.1) rats, suggesting increased baseline GABA release. N/OFQ also significantly (t(11) = 2.352; p < 0.05; n = 12) increased the PPF ratio of IPSPs to 132.5% of control (restraint: baseline 0.94 ± 0.07; N/OFQ 1.25 ± 0.18, n = 12; Fig. 1C).
N/OFQ inhibits the CRF-induced increase in GABAergic transmission
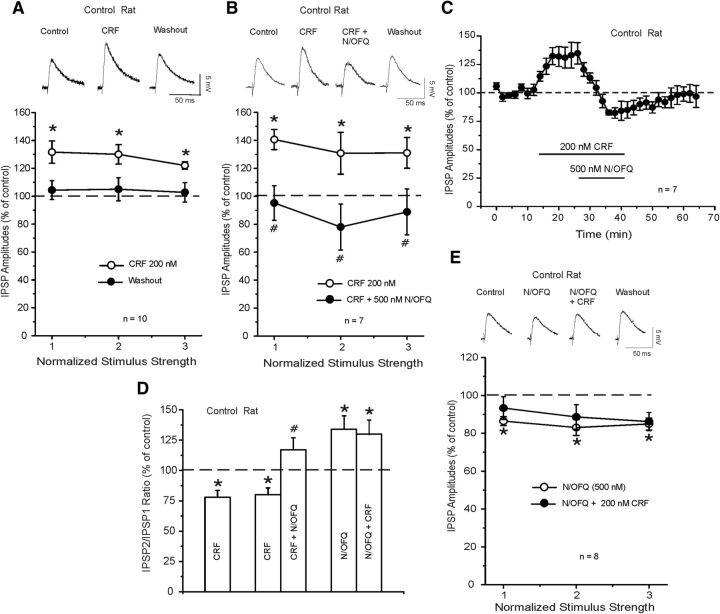
Next, we examined the effect of acute CRF (200 nm, a maximally effective concentration) (Roberto et al., 2010) application in CeA of naive rats. In agreement with previous studies, we found that CRF significantly (p < 0.05) and reversibly increased to 128.2 ± 2.9% (Fig. 2A; n = 10) of the mean amplitude of evoked IPSPs measured over all stimulus strengths. This CRF-induced increase in IPSP amplitudes was associated with a significant (t(7) = 3.916; p < 0.05) decrease in PPF ratios (naive: baseline 1.21 ± 0.2; CRF 0.6 ± 0.10, n = 8; Fig. 2D).
Figure 2.
A, Top, Representative evoked CeA IPSPs from a control unrestraint rat during control, CRF, and washout. Bottom, CRF application reversibly and significantly increased the IPSP amplitude in CeA neurons of control rats: *p < 0.05. B, Top, Representative recordings of evoked IPSPs during CRF, CRF + N/OFQ, and washout of the two peptides. Bottom, CRF significantly (*p < 0.05; Newman–Keuls post hoc) increases mean IPSP amplitudes and subsequent application of N/OFQ significantly (#p < 0.05; Newman–Keuls post hoc) diminishes the CRF-induced enhancement. C, Time course of changes in evoked IPSP amplitude (at half-maximal intensity) induced by CRF, concurrent application of N/OFQ, and washout of the two peptides. D, Histograms summarized the percentage change in mean (± SEM) PPF ratio of IPSPs in the experimental conditions of A, B, and E. CRF significantly decreases the PPF ratio of IPSPs: *p < 0.05 (Newman–Keuls post hoc). #p < 0.05, significance between CRF alone and concurrent application of CRF and N/OFQ (Newman–Keuls post hoc). N/OFQ significantly increases PPF ratios compared with control (*p < 0.05; Newman–Keuls post hoc), and CRF coapplied with N/OFQ did not alter these PPF ratios. E, In the CeA of control rats, N/OFQ 500 nm significantly (*p < 0.05; Newman–Keuls post hoc) decreases the mean IPSP amplitudes, whereas subsequent CRF 200 nm has no effect.
In another set of experiments, we tested the CRF-N/OFQ interaction at the CeA GABAergic synapses from control rats, applying first CRF alone and then with N/OFQ. Superfusion of 200 nm CRF significantly (p < 0.05) increased to 138.2 ± 3.7% (Fig. 2B; n = 7) the mean amplitude of evoked IPSPs measured over all stimulus strengths. N/OFQ (500 nm) added to the ACSF containing CRF reversed the CRF-induced augmentation and reduced the mean evoked IPSP amplitude to 86.5 ± 2.8% of control with recover upon washout (Fig. 2B,C). The ability of N/OFQ to reverse the CRF-induced increase in IPSPs correlated with a significant increase in the PPF ratio (Fig. 2D). Specifically, CRF decreased the mean PPF ratio of IPSPs to 75.3 ± 6% (baseline 1.24 ± 0.2; CRF 0.89 ± 0.12) and N/OFQ reversed the PPF ratio to pre-CRF values (1.31 ± 0.17; Fig. 2D).
Because N/OFQ counteracted the effects of CRF, we then inverted the order of peptide application and tested whether N/OFQ occluded CRF. In 8 CeA neurons of control unrestraint rats, we first applied 500 nm N/OFQ for 15 min and then added 200 nm CRF. Overall, ANOVA revealed a significant effect (F(2,23) = 19.51; p < 0.001), and Newman–Keuls post hoc analysis revealed that nociceptin significantly (p < 0.05) decreased the mean amplitude of evoked IPSPs over all the stimulus intensities, and subsequent coapplication of CRF did not alter these amplitudes (Fig. 2E). Specifically, N/OFQ reduced the amplitude of evoked IPSPs to ∼85.4 ± 1.5% of control over all stimulus strengths and completely prevented the CRF-induced enhancement of IPSPs (84.6 ± 2.6%, n = 8; Fig. 2E). Overall, ANOVA revealed a significant effect (F(2,6) = 6.07951; p < 0.001), with nociceptin significantly (p < 0.05 by Newman–Keuls post hoc test) increasing the PPF ratios of IPSPs. Subsequent application of CRF in the presence of nociceptin did not (p > 0.05 by Newman–Keuls post hoc test) alter these ratios (Fig. 2D).
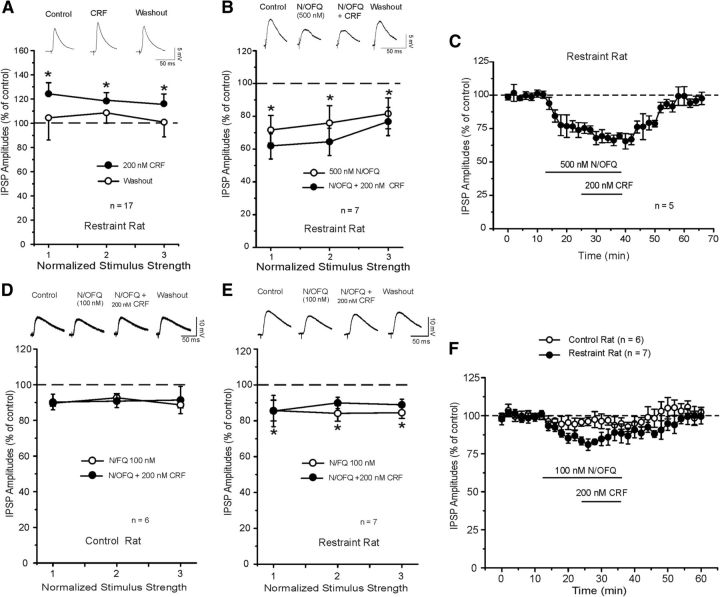
Then we assessed whether nociceptin would block the CRF effects in CeA of restraint rats. In 17 CeA neurons from restraint rats, we first tested the effect of acute CRF application alone. We found that CRF significantly (p < 0.05) and reversibly increased the evoked IPSP amplitude by 20%, over all the stimulus intensities (Fig. 3A). Although this CRF-induced increase in GABAergic responses was slighter smaller, it was not significantly different from the one observed in unrestraint rats (Figs. 2A and 3E). CRF also reversibly and significantly (t(9) = 2.752; p < 0.05) decreased PPF ratio of IPSPs from 0.96 ± 0.06 to 0.80 ± 0.06 (data not shown), suggesting a presynaptic effect on GABA release.
Figure 3.
A, In the CeA from restraint rats, superfusion of CRF alone significantly increased the mean IPSP amplitudes to 120% of control (similar to control rats): *p < 0.05 (paired t test). B, Top, Representative evoked CeA IPSPs from a restraint rat during the N/OFQ, coapplication with CRF, and washout. Bottom, Superfusion of N/OFQ alone significantly decreased the mean IPSP amplitudes to 74% of control and prevented the enhancement of IPSPs induced by subsequent CRF (as unrestraint rats, E): *p < 0.05. C, Time course of the changes in evoked IPSP amplitude induced by N/OFQ, concurrent application of CRF, and washout of the two peptides in CeA of restraint rats. D, Top, Representative IPSPs during application of evoked CeA IPSPs from unrestraint rat during the 100 nm N/OFQ, coapplication with CRF, and washout. Bottom, Superfusion of 100 nm N/OFQ alone did not affect the mean IPSP amplitudes, but it prevented the enhancement of IPSPs induced by CRF. E, Top, Representative IPSPs during application of evoked CeA IPSPs from a restraint rat during the 100 nm N/OFQ, coapplication with CRF, and washout. Bottom, Superfusion of 100 nm N/OFQ alone significantly decreased the mean IPSP amplitudes and blocked the CRF-induced increase of IPSPs: *p < 0.05 (Newman–Keuls post hoc). F, Time course of changes in evoked IPSP amplitude (at half-maximal intensity) induced by N/OFQ, concurrent application of CRF, and washout of the two peptides in the control (n = 6) and restraint (n = 7) rats.
In another set of 7 CeA neurons from restraint rats, we examined the interaction of the two peptides applying nociceptin and then CRF in the presence of nociceptin. Overall, ANOVA indicated a significant effect (F(2,20) = 29.64; p < 0.001). Post hoc analysis revealed that nociceptin significantly (p < 0.001) decreased (to 74.3 ± 2.0% of control) the evoked IPSPs over all three stimulus intensities. Subsequent coapplication of CRF with nociceptin did not alter (p > 0.05) IPSP amplitudes (67.5 ± 4.2% of control, n = 7; Fig. 3B,C), suggesting blockade of the CRF-induced enhancement of IPSPs. This effect was reversible upon washout. N/OFQ also increased the mean PPF ratio of IPSPs to 135.4 ± 13% (baseline 1.06 ± 0.2; N/OFQ 1.45 ± 0.16; n = 5) and blocked the CRF-induced increase of PPF ratio (1.33 ± 0.25) (data not shown). In 4 separate CeA neurons of restraint rats, we found that longer (25 min) application of nociceptin alone produced a persistent decrease (73.8 ± 4.6% at 15 min and 71.2 ± 5.3% at 25 min; n = 4) in the IPSP amplitudes that returned to baseline levels 25 min into washout (data not shown).
Because nociceptin strongly opposes CRF effects at GABAergic CeA synapses, we tested whether a low (100 nm) concentration of nociceptin could antagonize an efficacious dose of CRF in both unrestraint and restraint rats. Nociceptin did not significantly affect (p > 0.05) baseline-evoked IPSP amplitudes in control unrestraint naive rats (Fig. 3D,F), but it still prevented the CRF-induced increase of evoked IPSPs. Interestingly, in CeA neurons from restraint rats, 100 nm nociceptin significantly (F(2,20) = 24.44; p < 0.001) decreased baseline-evoked IPSPs to 84.7 ± 2.5% % of control over all three stimulus intensities (Fig. 3E,F). Coapplication of CRF (200 nm) with N/OFQ did not affect (p > 0.05) the IPSP amplitudes compared with the application of N/OFQ alone. Nociceptin did not alter the 50 ms PPF ratio of IPSPs (unrestraint rats: baseline 1.02 ± 0.09; N/OFQ: 1.1 ± 0.08; N/OFQ + CRF: 1.13 ± 0.1; n = 6 and restraint rats: baseline 0.73 ± 0.08; N/OFQ: 0.88 ± 0.07; N/OFQ + CRF: 0.83 ± 0.1; n = 7; data not shown).
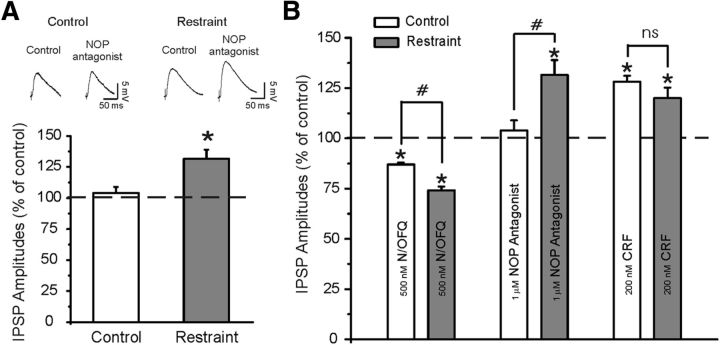
Because in CeA of restraint rats, the N/OFQ inhibitory effect on GABAergic synapses is larger and there is a significant increment of NOP expression compared with control rats, we determined whether NOPs regulate baseline evoked GABAergic transmission using a putative selective NOP antagonist, [Nphe1]-nociceptin(1–13)NH2 (Ciccocioppo et al., 2004; Roberto and Siggins, 2006; Cruz et al., 2012). Similarly to our previous studies, 1 μm [Nphe1]-nociceptin(1–13)NH2 applied alone for 15 min had no effect (104 ± 5% of control; p > 0.05; n = 6; Fig. 4A) on basal evoked IPSPs, suggesting lack of a tonic activity of the endogenous N/OFQ transmission in CeA. Interestingly, in CeA neurons from restraint rats, the NOP antagonist significantly (p < 0.05; n = 6) increased the evoked IPSP amplitudes to 131.5 ± 7.4% of control (Fig. 4A).
Figure 4.
A, Top, Representative IPSPs during application of an NOP antagonist ([Nphe1]nociceptin(1–13)NH2) at 1 μm concentration. Bottom, The NOP antagonist does not alter the basal-evoked IPSP amplitudes (stimulus intensity equal to half-maximal IPSP amplitude) in control rats, but significantly increases their amplitudes in CeA neurons of restraint rats: *p < 0.05 (paired t test). B, Histograms summarized the percentage change in mean (± SEM) evoked IPSP amplitudes measured during N/OFQ, NOP antagonist, and CRF application in CeA of control and restraint rats. #p < 0.05, significance between drug effects in the two animal groups. *p < 0.05, significance between drug effects and baseline control within the same group. ns, Not significant.
In our recent studies (Cruz et al., 2012), we reported that application of the CRF1 antagonist R121919 (1 μm) slightly decreases evoked CeA IPSP amplitudes of naive rats. Coapplication of 500 nm nociceptin significantly decreases evoked IPSP amplitudes, indicating that blockade of CRF1 with R121919 does not prevent nociceptin-induced decreases in GABAergic response. Here, we tested whether NOP blockade would alter the CRF-induced facilitation of GABAergic transmission in CeA of unrestraint rats. In CeA neurons pretreated with 1 μm [Nphe1]-nociceptin(1–13)NH2 for 15 min, CRF (200 nm) significantly increased the amplitude of IPSPs to 133.2 ± 4.4% of control (n = 4; data not shown), ruling out noncompetitive allosteric pharmacological antagonism of NOPs. In addition, in the presence of the [Nphe1]-nociceptin(1–13)NH2, the CRF-induced facilitation (133.2 ± 4.4%, n = 4) of IPSPs was not significantly (t(12) = 0.2559; p = 0.8; by unpaired t test) different from the CRF-induced facilitation (128.2 ± 2.9%, n = 10) of IPSPs observed in absence of the NOP antagonist.
Figure 4B summarizes the main effects of 500 nm N/OFQ, 1 μm [Nphe1]-nociceptin(1–13)NH2, and 200 nm CRF in both control unrestraint and restraint rats. In restraint rats, the effects of both N/OFQ and a NOP antagonist are significantly different from that in control unrestraint rats, suggesting an upregulation of this system.
Effect of intra-CeA injection of N/OFQ on stress-induced increase in anxiety-like behavior
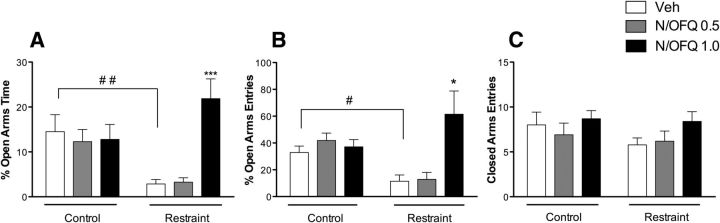
To provide additional functional correlates for the interaction between stress and NOPs in CeA, we microinjected N/OFQ directly into the CeA of restraint and unrestraint rats. Six hours after restraint stress, animals were tested on EPM for anxiety as illustrated in Figure 5. Overall, ANOVA revealed significant differences between restraint rats and unrestraint controls in the percentage of time spent in open arms (F(5,53) = 5.54; p < 0.01) (Fig. 5A).
Figure 5.
Effect of intra-CeA N/OFQ (0.0, 0.5, and 1.0 nmol/rat) injection on EPM test. A, Time spent in open arms. ##p < 0.01, Veh control versus Veh restraint (Newman–Keuls post hoc test). ***p < 0.001, N/OFQ 1.0 nmol versus Veh in the restraint group. B, Open arms entries. #p < 0.01, Veh control versus Veh restraint (Newman–Keuls post hoc test). *p < 0.05, N/OFQ 1.0 nmol versus Veh. C, Closed arm entries. The number of closed arm entries was not affected by the restraint procedure or N/OFQ treatment. In summary, our data show higher anxiety-like responses in restraint compared with unrestraint rats. N/OFQ significantly attenuated the effect of restraint stress on anxiety-like responses. Data are mean ± SEM (n = 9 or 10 rats/group).
The Newman–Keuls post hoc test showed that restraint animals spent less time in the open arm compared with unrestraint rats (p < 0.01), suggesting a higher level of anxiety. After injection of 1.0 nmol/rat of N/OFQ, the time spent in the open arms of restraint rats increased (p < 0.001), whereas no effect was observed in unrestraint controls (Fig. 5A). No significant effects were observed after 0.5 nmol/rat of N/OFQ. Overall, ANOVA also showed a significant effect in the percentage of open arm entries (F(5,53) = 3.2; p < 0.05) (Fig. 5B). Post hoc comparisons demonstrated a significantly lower level of entries after restraint stress in vehicle groups (p < 0.05). Intra-CeA N/OFQ significantly (p < 0.001) increased the number of entries in the open arms at the dose of 1 nmol/rat. No effect was observed at the lower dose of the peptide. Finally, the number of closed arm entries was not affected by the restraint procedure or N/OFQ treatment (Fig. 5C).
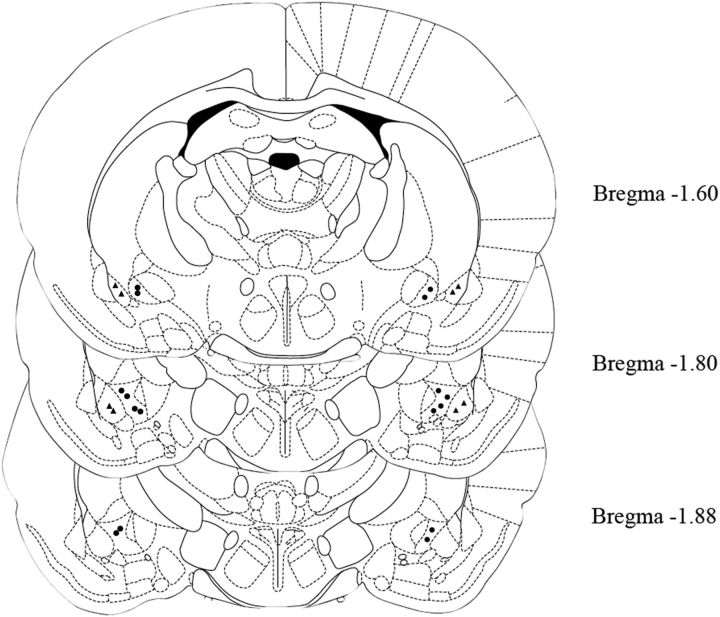
Notably, in four rats of the restraint group, histological analysis revealed incorrect cannula placement. In two of these rats, the CeA was reached only on one side of the brain, whereas in the other two animals cannulae were placed too lateral and reached the BLA. In these four animals, N/OFQ injection did not result in changes in EPM responses, thus providing some evidence that bilateral activation of CeA NOPs is needed to achieve anxiolytic-like responses after stress exposure. In unrestraint controls, wrong cannula placement was observed in two rats. Animals with incorrect cannula placement were not included in the statistical analysis. Histological reconstruction showing correct and incorrect injections into the CeA is presented in Figure 6.
Figure 6.
Histological reconstructions showing correct (filled circles) and incorrect (filled triangles) injections into the CeA. Data presented in the figures are indicative of the criteria used for identification of correct cannulae sites. Drawing is from the atlas by Paxinos and Watson (1998).
Discussion
Physical restraint in rodents is widely used to investigate neurobiological readaptations and pathological conditions associated with stress exposure (Glavin et al., 1994; McEwen and Magarinos, 1997). Overactivation of the extrahypothalamic CRF system has been identified as a hallmark of stress-related pathologies, including anxiety, depression, eating disorders, and addiction (Walker and Davis, 2008; Shalev et al., 2010; Gilpin, 2012). On the other hand, a broad set of studies have shown that activation of the N/OFQ system may represent a mechanism through which an organism attempts to attenuate the pathological consequences arising from stress exposure (Martin-Fardon et al., 2000; Ciccocioppo et al., 2003). Based on this background, here we used physical restraint stress to investigate functional interaction between these two systems. Our attention was focused on the amygdala an area known for mediating interactions between CRF and N/OFQ neurotransmission (Ciccocioppo et al., 2003; Economidou et al., 2008).
In the ISH experiment, we found that exposure to restraint stress elicited a highly significant overexpression of NOP mRNA in the CeA and in the BLA amygdala. No differences were observed in the medial amygdala. Conversely, expression of N/OFQ transcript was not affected by restraint stress in all brain regions tested. On the other hand, the CeA and the BLA showed a reduction in CRF1 receptor gene expression after restraint. This is consistent with a scenario in which stress leads to the recruitment of the CRF system in specific brain areas, including CeA and PVN (Sterrenburg et al., 2012).
This is also in line with what we observed in the N/OFQ system in which NOP transcript overexpression may also be viewed as a physiological compensatory reaction aimed at potentiating N/OFQ signaling within the amygdala nuclei. To prove our hypothesis, we focused on the CeA. In the CeA, GABAergic neurons express CRF (Veinante et al., 1997) and CRF, like ethanol, enhances GABA release (Nie et al., 1994; Roberto et al., 2010) via activation of the CRF1R, whereas N/OFQ counteracts both the CRF and ethanol effects (Roberto and Siggins, 2006; Cruz et al., 2012). Thus, here we recorded CeA GABA IPSPs in restraint and unrestraint animals and tested N/OFQ and CRF interactions. Notably, the amplitude of the baseline CeA IPSPs recorded in restraint rats was significantly elevated compared with control rats. Despite these higher baseline-evoked GABAergic responses, CRF was able to further increase them, although the CRF-induced enhancement was slightly smaller than the one observed in control unrestraint rats. In contrast to the upregulated CRF1/CRF signaling observed in CeA of alcohol-dependent rats (Roberto et al., 2010), and as seen in the dorsal raphe after stress (Waselus et al., 2009), we report reduced CRF1 expression in CeA. Because the CRF system overall maintains its facilitatory effects on CeA GABAergic transmission of restraint rats, we speculate that reduction of CRF1 expression might also have been accompanied by compensatory post-translational changes in the CRF1 system. We also found that N/OFQ reduced the amplitudes of evoked IPSPs of control animals and prevented the CRF-induced increase of IPSPs, confirming previous findings (Cruz et al., 2012). Interestingly, we found that the depressant effect of N/OFQ on evoked IPSP amplitudes was significantly stronger in CeA neurons from restraint rats compared with unrestraint control animals, indicating that stress leads to an increased sensitivity of the CeA synapses to N/OFQ exogenous application. Importantly, the NOP antagonist did not alter basal evoked IPSP amplitudes in control rats but significantly increased IPSP amplitudes in restraint rats, suggesting neuroadaptations of the N/OFQ system during acute stress (i.e., increased sensitivity and expression of NOPs). We speculate that acute stress enhances tonic activity of the endogenous nociceptin/NOP system that may account for the increased sensitivity of the CeA GABA system to exogenous nociceptin. Enhancement of nociceptin-NOP function is also suggested by gene expression data indicating an increased activity of NOP system after stress. Of note, increased function of N/OFQ was also observed in alcohol-dependent rats under withdrawal, a condition associated with dysregulation of the stress system and increased CRF tone in the CeA (Roberto et al., 2010; Economidou et al., 2011; Cruz et al., 2012). Thus, the present findings together with our previous studies (Cruz et al., 2012) in ethanol-dependent rats point to a critical role of the N/OFQ system in opposing the recruitment of the CeA CRF system associated with stress-related stimuli and to restore functional equilibrium in the CeA.
Nociceptin fibers, nociceptin-containing neurons, and NOPs are widely distributed in the central amygdala (Neal et al., 1999), suggesting both local CeA production of nociceptin and from cell bodies outside the CeA. Nociceptin and CRF had opposite effects on CeA GABAergic transmission at the presynaptic level. Importantly, this interaction between peptides is more than a mere summation of opposing effects because the nociceptin-induced inhibition of GABA responses completely abolished the large amplitude increase elicited by an efficacious concentration of CRF alone. Particularly, a low (100 nm) subthreshold concentration of nociceptin did not alter baseline GABAergic responses but blocked the CRF-induced effects at these synapses in unrestraint rats. This suggests that the anti-CRF actions of nociceptin were the result of functionally independent actions and not simply the summation of opposing effects on GABAergic transmission. Interestingly, in CeA neurons from restraint rats, 100 nm nociceptin significantly decreased baseline GABA responses, supporting our hypothesis of enhanced tonic activity of the endogenous N/OFQ after exposure to acute stress.
NOP activation is known to inhibit adenylate cyclase and decrease PKA activity (via Gi protein) (Meunier, 1997). PKA is also one of the second messengers engaged by CRF1 receptor activation (via Gs and Gq proteins) (Papadopoulou et al., 2004; Cruz et al., 2012). Our recent studies demonstrated that nociceptin presynaptically preempts the action of CRF in CeA neurons via adenylate cyclase/PKA signaling (Cruz et al., 2012). Thus, the presynaptic PKA pathway represents a common signaling pathway targeted in opposing directions by nociceptin and CRF in regulating GABA release, and the balance is tilted in favor of nociceptin, perhaps via a molecular entity upstream from CRF activation. We found specificity of the two neuropeptides at their respective receptors, such that blockade of NOP did not alter CRF-induced facilitation of GABAergic response and blockade of CRF1 did not alter N/OFQ induced inhibition of GABAergic response (Cruz et al., 2012), ruling out noncompetitive allosteric pharmacological antagonism at the level of receptors. Although the blockade of the NOP receptor by the competitive antagonist [Nphe1]-nociceptin(1–13)NH2 blocks the inhibition of the adenylate cyclase (facilitating the intracellular signaling pathway), in unrestraint rats this blockade of the NOP receptor has no significant effects on the amplitudes of basal GABAergic responses (revealing no endogenous N/OFQ tone) but is able to prevent the effect of exogenous N/OFQ by blocking receptor activation. Thus, because there is little endogenous N/OFQ tone, blockade of the NOP (which blocks the inhibition of adelylate cyclase) does not significantly affect (facilitate) the CRF-induced facilitation of GABA responses.
To further confirm the antistress properties of N/OFQ, we monitored the consequences of body restraint stress on anxiety-like behavior in the EPM test. Moreover, we studied the EPM effects of N/OFQ injected directly into the CeA, comparing peptide effects in physically restraint and unrestraint rats. Results revealed that activation of NOP in the CeA leads to a pronounced anxiolytic-like profile in restraint rats. At the same dose, no effects were detected in unrestraint controls. This finding further demonstrates that stress exposure leads to increased efficacy of N/OFQ as anxiolytic, this effect of the peptide is mediated by activation of NOPs in the CeA and is likely dependent upon the antistress action of N/OFQ. Notably, in restraint rats with incorrect cannula placement, central injection of N/OFQ did not show EPM effects, thus providing some evidence that bilateral activation on CeA NOP is needed to achieve anxiolytic-like responses after stress exposure. Another consideration is that N/OFQ did not appear to have a dose-related effect on anxiety. This is, however, not surprising because the EPM is a paradigm where dose–response relationships are not easily captured. Moreover, this finding is in line with previous studies showing that the EPM effects of N/OFQ are not dose-related (Uchiyama et al., 2008a, b; Economidou et al., 2011).
Overall, our findings are partially in agreement with a previous study in which it was shown that activation of the stress system by intracerebroventricular infusion of CRF leads to a compensatory overexpression of NOP transcript and to a heightened sensitivity to the anxiolytic-like effects of N/OFQ (Rodi et al., 2008). The electrophysiological and microinjection results clearly point to the specificity of the role of the CeA in N/OFQ function in response to restraint stress exposure. In agreement with our findings, other studies have shown that site-specific microinjection of N/OFQ into the CeA resulted in significant anxiolytic-like actions in the EPM test, supporting the anxiolytic-like effects of this peptide (Uchiyama et al., 2008b). In contrast, N/OFQ effects were observed in rodents that were not subjected to stress procedures (Uchiyama et al., 2008a). It is well known that environmental factors, such as housing conditions, have an important impact in the regulation of stress mechanisms. Hence, it is possible that, if in these latter studies animals were maintained or tested under a more stressful environment, the N/OFQ system might have been recruited as well. In support of this view, there are data showing that NOP or N/OFQ gene expression and function are upregulated after exposure to environmental stress, such as social crowding or maternal separation (Ploj et al., 2002; Reiss et al., 2007). Additional factors may have also contributed to this discrepancy in the anxiolytic effect of intra-CeA injection of N/OFQ in normal nonstressed rats. For example, here the study was conducted in Wistar rats during the dark period of the light dark cycle using a four arm elevated maze, whereas in the study by Uchiyama et al. (2008a), Sprague Dawley rats were tested during the light phase of the light dark cycle using a three arm elevated maze.
In conclusion, through a multidisciplinary approach, the present study provides converging evidence demonstrating that stress leads to an increased efficacy of NOP-mediated neuronal activity in the CeA. This change in N/OFQ neurotransmission may be viewed as an adaptive response to overactivation of the extrahypothalamic CRF system elicited by stress. In turn, changes in CRF and N/OFQ functions may contribute to the setpoint shift of the brain stress system leading to maladaptive responses to stress. Finally, our results indicate the potential of NOP agonism as treatment for psychiatric conditions associated with heightened CRF system activity or triggered by stress.
Footnotes
This work was supported by National Institutes of Health Grants AA017447, AA015566, AA06420, AA016985, AA014351, AA013498, and AA021491 and the Pearson Center for Alcoholism and Addiction Research. A.C.H. was supported by Deutsche Forschungsgemeinschaft HA 6102/1. We thank Dr Marian Logrip for helpful comments on the manuscript.
The authors declare no competing financial interests.
References
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict Biol. 2013;18:467–479. doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12:1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M. Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64–6198. Psychopharmacology (Berl) 2002;161:113–119. doi: 10.1007/s00213-002-1020-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced γ-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- and alcohol-related behaviors. Alcohol. 2012;46:329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavin GB, Paré WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994;18:223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Sommer W, Rimondini R, Andbjer B, Strömberg I, Fuxe K. c-fos reduces corticosterone-mediated effects on neurotrophic factor expression in the rat hippocampal CA1 region. J Neurosci. 2003;23:6013–6022. doi: 10.1523/JNEUROSCI.23-14-06013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BN, Jeppesen LJ, Mortensen BB, Kjaergaard B, Andreasen H, Glavind K. The superiority of rectal thermometry to oral thermometry with regard to accuracy. J Adv Nurs. 1994;20:660–665. doi: 10.1046/j.1365-2648.1994.20040660.x. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/S0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547. doi: 10.1002/(SICI)1096-9861(19990419)406:43.0.CO%3B2-P. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther. 1994;271:1566–1573. [PubMed] [Google Scholar]
- Papadopoulou N, Chen J, Randeva HS, Levine MA, Hillhouse EW, Grammatopoulos DK. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1alpha receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol Endocrinol. 2004;18:624–639. doi: 10.1210/me.2003-0365. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Effects of maternal separation on brain nociceptin/orphanin FQ peptide levels in male Wistar rats. Pharmacol Biochem Behav. 2002;73:123–129. doi: 10.1016/S0091-3057(02)00778-5. [DOI] [PubMed] [Google Scholar]
- Reiss D, Wolter-Sutter A, Krezel W, Ouagazzal AM. Effects of social crowding on emotionality and expression of hippocampal nociceptin/orphanin FQ system transcripts in mice. Behav Brain Res. 2007;184:167–173. doi: 10.1016/j.bbr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala γ-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl) 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, Peeters BW, Kozicz T. Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res. 2012;90:179–192. doi: 10.1002/jnr.22737. [DOI] [PubMed] [Google Scholar]
- Uchiyama H, Toda A, Hiranita T, Watanabe S, Eyanagi R. Role of amygdaloid nuclei in the anxiolytic-like effect of nociceptin/orphanin FQ in rats. Neurosci Lett. 2008a;431:66–70. doi: 10.1016/j.neulet.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Uchiyama H, Yamaguchi T, Toda A, Hiranita T, Watanabe S, Eyanagi R. Involvement of the GABA/benzodiazepine receptor in the anxiolytic-like effect of nociceptin/orphanin FQ. Eur J Pharmacol. 2008b;590:185–189. doi: 10.1016/j.ejphar.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res. 1998;794:188–198. doi: 10.1016/S0006-8993(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Veinante P, Stoeckel ME, Freund-Mercier MJ. GABA- and peptide-immunoreactivities co-localize in the rat central extended amygdala. Neuroreport. 1997;8:2985–2989. doi: 10.1097/00001756-199709080-00035. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66:76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]