Abstract
Partial injury to the corticospinal tract (CST) causes sprouting of intact axons at their targets, and this sprouting correlates with functional improvement. Electrical stimulation of motor cortex augments sprouting of intact CST axons and promotes functional recovery when applied soon after injury. We hypothesized that electrical stimulation of motor cortex in the intact hemisphere after chronic lesion of the CST in the other hemisphere would restore function through ipsilateral control. To test motor skill, rats were trained and tested to walk on a horizontal ladder with irregularly spaced rungs. Eight weeks after injury, produced by pyramidal tract transection, half of the rats received forelimb motor cortex stimulation of the intact hemisphere. Rats with injury and stimulation had significantly improved forelimb control compared with rats with injury alone and achieved a level of proficiency similar to uninjured rats. To test whether recovery of forelimb function was attributable to ipsilateral control, we selectively inactivated the stimulated motor cortex using the GABA agonist muscimol. The dose of muscimol we used produces strong contralateral but no ipsilateral impairments in naive rats. In rats with injury and stimulation, but not those with injury alone, inactivation caused worsening of forelimb function; the initial deficit was reinstated. These results demonstrate that electrical stimulation can promote recovery of motor function when applied late after injury and that motor control can be exerted from the ipsilateral motor cortex. These results suggest that the uninjured motor cortex could be targeted for brain stimulation in people with large unilateral CST lesions.
Introduction
Hemiparesis attributable to unilateral brain injury is the primary impairment causing long-term disability in the United States (Roger et al., 2012). The severity of hemiparesis correlates strongly with injury to the corticospinal tract (CST; Stinear, 2010). After large CST lesion, primary motor cortex (M1) on the uninjured side is activated with movement of the impaired hand, and this activation is critical for the early stage of functional recovery (Nishimura et al., 2007). We demonstrated previously that M1 on the uninjured side can be electrically stimulated to promote brainstem and spinal axon outgrowth (Brus-Ramer et al., 2007; Carmel et al., 2013) and functional recovery after acute injury (Carmel et al., 2010). However, the extent to which the uninjured M1 can participate in functional recovery after chronic injury, when the injured M1 usually resumes control of the impaired hand or forelimb (Nishimura et al., 2007; Clarkson et al., 2010), is unknown.
We demonstrated previously that unilateral CST injury alone and CST stimulation alone each promote sprouting of functional spinal connections.(Brus-Ramer et al., 2007) Stimulation of the intact CST after injury produced axon outgrowth and spinal connectivity equivalent to the sum of injury only and stimulation only. It might be that electrical stimulation stabilizes injury-induced plasticity with increased activity and therefore would best be applied soon after injury when injury-induced plasticity is highest. Alternatively, if the effects of stimulation and injury are independent, then stimulation would be as effective after chronic injury as acute injury. We also investigated which circuits mediate the recovery of motor control. We asked whether we could create ipsilateral control of the impaired forelimb after large CST lesion, a critical issue in systems neuroscience and restorative neurology (Jankowska and Edgley, 2006).
We addressed the question of whether electrical stimulation could improve function after chronic injury by applying epidural stimulation over M1 in the uninjured hemisphere 8 weeks after CST lesion on the other side. By 4 weeks after stimulation, rats with injury and electrical stimulation had significantly fewer errors in skilled locomotion than those with injury alone. Thus, activity can be applied late after injury and still promote recovery of function. To determine whether the stimulated M1 had assumed control over the impaired forelimb, we pharmacologically inactivated it. In rats with injury and stimulation, but not those with injury alone, inactivation caused a transient worsening of function in the forelimb ipsilateral to stimulation. This indicates that the uninjured and stimulated hemisphere was responsible for restoring motor control after chronic injury.
Materials and Methods
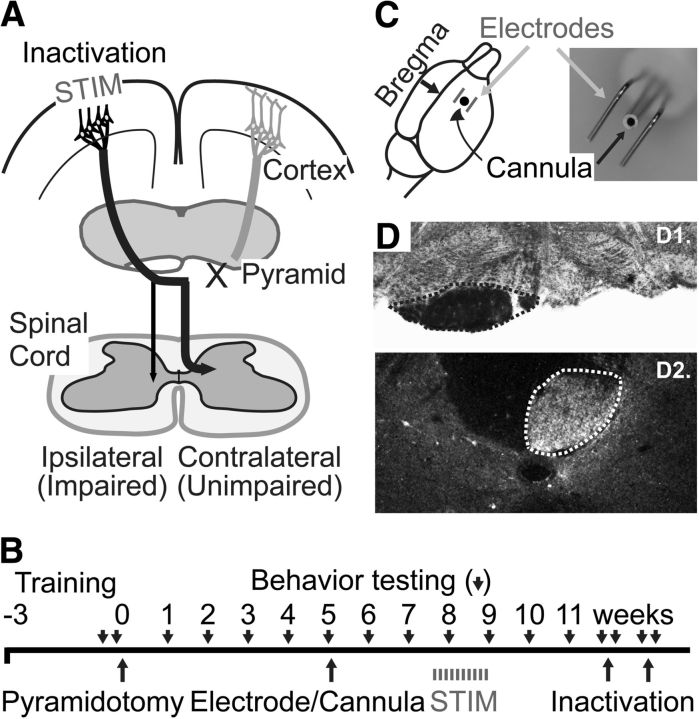
Figure 1A demonstrates the experimental paradigm and Figure 1B the timeline. Experiments were conducted on adult female Sprague Dawley rats (weighing 225–275 g; Charles River). A total of 13 rats were used: 10 for the therapeutic effects of epidural stimulation and three for inactivation controls. A power analysis with a predicted effect size of 33% relative reduction in error rate (β = 0.8, α = 0.05) and variance observed in our previous trial of electrical stimulation (Carmel et al., 2010) determined that a group size of five rats would be sufficient to detect statistically significant differences between groups. Surgeries (pyramidal lesion and electrode implantation) were performed under general anesthesia (90 mg/kg ketamine and 10 mg/kg xylazine via intraperitoneal injection) and aseptic conditions. All procedures were approved by the institutional animal care and use committee of City College of the City University of New York. All experiments comply with National Institutes of Health guidelines on the care and use of laboratory animals.
Figure 1.
Methods. A, Experimental schema. Eight weeks after CST lesion (X), the M1 in the uninjured hemisphere is electrically stimulated (STIM). M1 in the uninjured hemisphere was inactivated at the end of the efficacy trial. B, Experimental timeline. C, Position of stimulating electrodes and inactivation. Electrode wires were bent into an L shape and run parallel over the forelimb area of the motor cortex, as shown in the diagram. The anteroposterior position of the angle of the L-shaped electrodes was in line with bregma, and the lateral positions were 2 and 3.5 mm, respectively. The electrodes extended anterior from bregma to 4 mm anterior to bregma, which covers the caudal forelimb area of M1. A cannula for inactivation is located at the center between the stimulating electrodes, as pictured in the photograph. D, Pyramid lesions. D1, The injury site was examined with myelin-stained cross-sections through the lesion site. The intact pyramid in outlined; the opposite pyramid is absent. D2, Select lesions were verified with PKCγ staining of the CST below the lesion. The intact CST is outlined; the other half of the CST is absent.
Rats were trained and tested to walk on a horizontal ladder (Metz and Whishaw, 2002; Carmel et al., 2010). We trained rats until they crossed the ladder with a forelimb error rate 20%. During testing sessions, rats were run for 10 trials in each direction on the ladder. Rung positions were changed every five runs during the session to prevent rats from learning a particular pattern. All trials were video recorded (Canon ZR960), and scoring performed by a blinded observer. Videos were analyzed frame by frame, and the placement of the forepaws was measured only during active walking. Steps with placement of the palm of the forepaw, between the wrist and the digits, on the rung were scored as good. All other steps were recorded as errors, and the results were expressed as percentage errors. Errors were further classified as oversteps, understeps, or misses, as described previously (Carmel et al., 2010). Baseline error rates were established with at least two testing sessions within the 5 d before CST lesion. Testing was performed every 7 d after injury without additional training between. Each testing session requires ∼40 s of active ladder walking; this small amount of activity limits any therapeutic effect of testing.
After training, we cut one pyramid, transecting the CST from one hemisphere at the rostral medulla (Brus-Ramer et al., 2007, 2009; Carmel et al., 2010, 2013). Lesions were examined using Kluver–Barrera staining (Kluver and Barrera, 1953) or dark-field microscopy of the medulla at the site of injury and reconstructed (Neurolucida; MicroBrightField; Fig. 1C). In rats whose lesions could not be confirmed by lesion reconstruction because we could not determine whether there was any spared tissue of the pyramidal tract, we immunostained the CST below the lesion site with PKCγ to verify loss of CST axons (rabbit anti-PKCγ, 1:500; Santa Cruz Biotechnology; Tan et al., 2012).
Five weeks after CST lesion, we implanted electrodes for stimulation and a cannula for inactivation. These are incorporated in a single connector (Plastics One; Fig. 1C), which allows stimulation and inactivation at the same location. This device was implanted over the caudal forelimb area of motor cortex, 1.5 mm anterior and 2.5 mm lateral to bregma (Fig. 1C), as in our previous studies (Carmel et al., 2010, 2013). Briefly, rats were anesthetized and head fixed in a stereotactic frame. Through a craniotomy, parallel stainless steel stimulating electrodes attached to a connector were placed over the dura mater (Fig. 1C). To confirm placement, we used a constant-current stimulator (A-M Systems) to deliver trains of stimuli (0.2 ms biphasic pulse, 333 Hz, 45 ms duration) at the minimal current (i.e., threshold) to provoke a movement, which was 0.9–1.7 mA. This stimulation produced selective contralateral forelimb movement, never hindlimb or whisker movements. Beginning 8 weeks after CST lesion, we used the electrodes to give five randomly selected rats therapeutic stimulation. Rats were attached via freely swiveling cables to a constant-current stimulator; five received active stimulation. We used the same trains of stimuli used for testing of the electrode at motor threshold every 2 s, 6 h/d, for 10 d as described previously (Carmel et al., 2010, 2013). Stimulation was performed during the day. At the end of 6 h of active (injury and stimulation) or sham (injury only) stimulation, rats were untethered and returned to their home cage. These stimulation parameters are well tolerated and do not injure the underlying brain (Carmel et al., 2013). The locomotor performance of the groups was compared using repeated-measures ANOVA (Kaleidograph).
To determine the locus of control for the gain in function, we developed the methods to transiently inactivate M1 in the awake rat, as we have in the cat (Martin and Ghez, 1999). The indwelling cannula for inactivation was placed as described above. For inactivation, an inner cannula was inserted through the outer cannula, through dura, and 1.5 mm into cortex. A syringe pump infused 1 μl of muscimol (catalog #M1523; Sigma) solution over 5 min, and the cannula was removed 5 min later. Rats were tested on the ladder task before and 1 h after inactivation. Two days after inactivation, rats were tested again to ensure that muscimol had washed out and error rates had returned to preinactivation values. Saline infusion was used to verify the specificity of muscimol actions. Effects of inactivation were tested with a paired Student's t test (Excel).
Results
First, we tested whether electrical stimulation of M1 in the uninjured hemisphere could improve motor function after chronic CST injury. Unilateral CST lesion (Fig. 1D) completely (n = 4 per group) or nearly completely (n = 1 per group) severed the CST; in all rats there was little to no extension into the underlying medulla. Eight weeks after CST lesion, we gave daily epidural M1 electrical stimulation for 10 d in five randomly chosen rats (injury plus stimulation group) and sham stimulation to the five rats in the injury-only group. We measured locomotor performance for 4 weeks after the start of electrical stimulation. At the end of this period, we began inactivation experiments in a subset of rats (n = 3 from each group) to determine whether the stimulated and ipsilateral M1 gained motor control over the impaired forelimb. The GABA agonist muscimol was infused through a cannula centered on the caudal forelimb area of M1 at the site of stimulation (Fig. 1C).
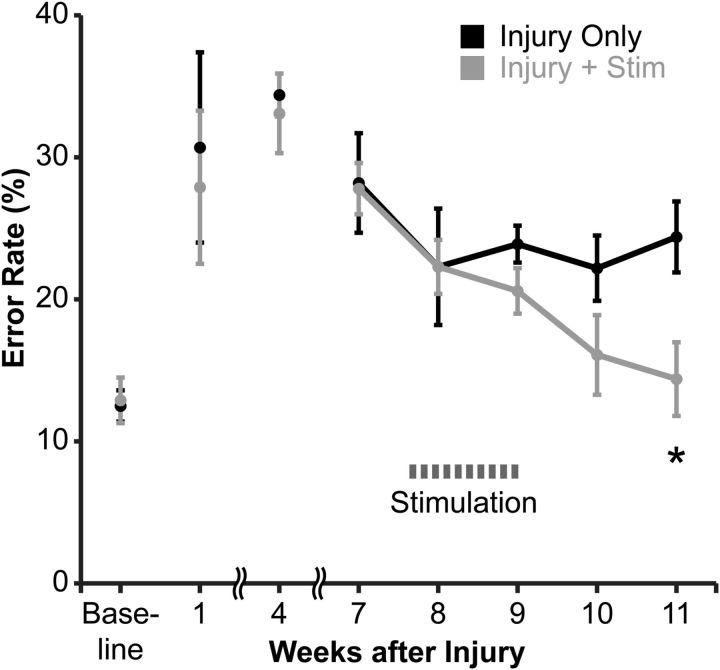
Figure 2 demonstrates the error rates of the forelimb affected by CST lesion in rats with injury alone (black) and rats with injury and epidural stimulation (gray). The error rates of the two groups did not differ between baseline and just before stimulation, at week 7 (repeated-measures ANOVA; F(1,8) = 0.54, p = 0.48). The week before stimulation, the error rates in the rats with injury only (28.2 ± 3.5%) were nearly identical to those of rats with injury and stimulation (27.8 ± 1.8%). We next asked whether the groups differed after the start of electrical stimulation (weeks 8–11). The error rates of rats with injury and stimulation steadily declined after the start of stimulation, and the groups were different from one another over this time (repeated-measures ANOVA, F(1,4) = 5.8, p = 0.04). At 11 weeks, the error rate was significantly lower in rats with injury and stimulation (14.4 ± 2.6%) than rats with injury only (24.4 ± 2.5%; Bonferroni's post hoc test, p = 0.03). The error rate of rats with injury and stimulation declined to baseline (12.9 ± 1.6%; not different from preinjury performance, paired t test, p = 0.19). Thus, epidural M1 electrical stimulation promoted recovery of skilled locomotion in rats after chronic CST injury.
Figure 2.
M1 electrical stimulation after chronic injury improves promotes recovery of skilled walking in the impaired forelimb. Rats were trained to cross a horizontal ladder with irregularly spaced rungs until they achieved a baseline error rate n = 5 per group). Until the start of stimulation (weeks 1–7), the error rates in the two groups were not different. After the start of stimulation (weeks 8–11), the groups differed significantly (repeated-measures ANOVA, with Bonferroni's post hoc correction, *p = 0.03).
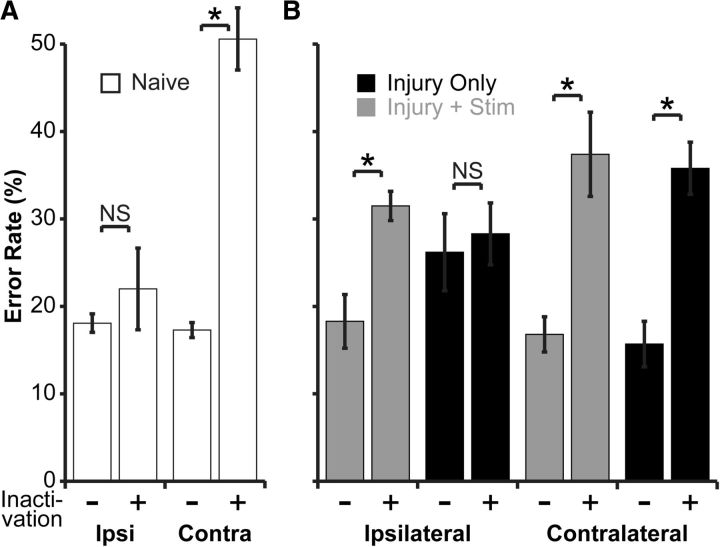
We next developed the methods to transiently inactivate M1 using the GABA agonist muscimol. We first determined in naive rats (n = 3) the dose of muscimol that produced substantial contralateral impairment but no ipsilateral impairment, as measured by performance on the horizontal ladder task. We started with 1.0 μg in 1.0 μl of normal saline and gradually decreased the dose. We found that 0.1 μg in 1.0 μl (0.88 mm) of normal saline produced a contralateral impairment as large or larger than pyramidal tract lesion but no ipsilateral impairment. As shown in Figure 3A, at baseline and before inactivation, naive rats had error rates of 17.3 ± 0.9% in the contralateral forelimb. During inactivation, the error rates increased to 50.6 ± 3.6%; paired t test, p = 0.02). The ipsilateral forelimb had a baseline error rate of 18.1 ± 1.0%, and this did not increase significantly during inactivation (22.0 ± 4.7%; paired t test, p = 0.45). After washout of muscimol, the error rate came back to baseline in the contralateral forelimb (18.7 ± 0.5%; paired t test vs baseline, p = 0.53) and remained unchanged in the ipsilateral forelimb (17.2 ± 0.6; paired t test, p = 0.65). This effect was attributable to pharmacological inactivation and not to any physical effects of cortical infusion because saline infusion did not increase the error rate in either the contralateral (16.6 ± 1.6 vs 17.8 ± 1.2; p = 0.37) or the ipsilateral (17.2 ± 0.5 vs 14.7 ± 0.3, a decrease) forelimb. Thus, in naive rats, M1 inactivation produced a strong and selective contralateral deficit.
Figure 3.
Inactivation experiments. Performance on the horizontal ladder is measured before (−) and during (+) inactivation. For experimental schema, see Figure 1A. A, Inactivation of the motor cortex in naive rats produces a strong and selective impairment in the contralateral (Contra) forelimb (paired t test, p = 0.02); the ipsilateral (Ipsi) forelimb is not affected. B, Loss of behavioral recovery with ipsilateral motor cortex inactivation. A subset of rats from the study of brain stimulation for motor recovery (n = 3 per group) was subjected to motor cortex inactivation (n = 6 inactivations per group). In the rats with injury only, inactivation did not change the error rate in the impaired forelimb. However, in rats with injury and stimulation, inactivation of the stimulated motor cortex reinstated their initial deficit in the ipsilateral forelimb (paired t test, p = 0.01). There was no difference between the two groups in the effect of inactivation on the contralateral forelimb. This demonstrates that improved motor performance with electrical stimulation was attributable to control from the ipsilateral and stimulated motor cortex. *p < 0.05.
We next tested whether improvement in motor control was directed by the stimulated M1 ipsilateral to the impaired forelimb. Five weeks after initiation of stimulation (Fig. 1B), rats were subject to pharmacological inactivation of M1. In two rats, the acrylic implants became loose, and in two other rats, the cannulae became irreversibly clogged. So three rats from each group were subjected to inactivation. The inactivations were repeated for a total of six inactivations per group. We predicted that, for rats with injury and stimulation, inactivation of stimulated M1 would cause increases in forelimb errors on the initially impaired side, i.e., the initial deficit would be reinstated. This was the case. As shown in Figure 3B, in rats with injury and stimulation, the ipsilateral error rate was 18.3 ± 3.1% at the end of the stimulation period. During muscimol inactivation, this error rate went to 31.5 ± 1.7% (p = 0.01). After washout, the error rates returned to baseline (19.6 ± 2.5%; p = 0.56). In contrast, in rats with injury only, the ipsilateral error rate did not change during inactivation (26.2 ± 4.4 to 28.3 ± 3.5%; p = 0.35). There was also no change in the subtypes of errors the rats with injury only made during inactivation, whether oversteps (21.2% before vs 25.2% during inactivation), understeps (5.0 vs 2.1%), or misses (0 vs 1.1%; all p > 0.05). The difference in effects on the ipsilateral forelimb in the two groups was not attributable to differences in the degree of M1 inactivation, because the error rate in the contralateral and unimpaired forelimb increased to similar degrees (36.5 ± 3.2% for rats with injury and stimulation vs 35.8 ± 3.0% for rats with injury only; unpaired t test, p = 0.8). Thus, epidural stimulation after chronic injury promotes recovery of motor function by establishing functional control from the stimulated M1 to the ipsilateral and impaired forelimb.
Discussion
This study had two main findings: (1) electrical stimulation promoted recovery of function after chronic injury; and (2) recovery was mediated by ipsilateral M1. Primarily because most rodents recover function within the first few weeks after injury and humans within the first few months, the capacity for recovery is presumed to diminish after this time (Murphy and Corbett, 2009; Krakauer et al., 2012). Although injury-induced plasticity may wane, the capacity to respond to activity-based treatments persists. The specific targeting of intact motor circuits with electrical stimulation may allow profound activity-dependent plasticity even when injury-induced plasticity is low.
The behavioral effects of stimulation were similar to those that we observed when the same stimulation paradigm was initiated the day after injury (Carmel et al., 2010). A striking similarity between the acute and chronic electrical stimulation studies is that the behavioral improvement did not begin until after the end of the 10 d stimulation period and became progressively greater. Our previous anatomical analyses in stimulation after acute injury support the idea that electrical stimulation promotes growth of CST axon terminations within the cervical spinal cord gray matter that continue beyond the stimulation period. In different groups of animals, we measured axon length in the ipsilateral half of the cervical spinal cord either 1 d after the end of stimulation or 1 month after stimulation. We compared rats with CST lesion and stimulation and rats with CST lesion only. Whereas 1 d after the end of stimulation (day 12 after injury) there was a 35% increase in axon length (Brus-Ramer et al., 2007), 1 month after the end of stimulation (day 42 after injury) we observed a 460% increase in axon length (Carmel et al., 2013). Thus, M1 stimulation may spur a growth program in axon terminations that continues beyond the stimulation period, and this could explain the delayed behavioral improvement.
The inactivation experiment demonstrates that recovery of motor control is established by M1 ipsilateral to the impaired forelimb. Ipsilateral control does not appear to be an important pathway for control in rats with CST lesion alone because inactivation of M1 did not raise the error rate in that group. Our finding of a minimal role in ipsilateral control in nonstimulated animals is not attributable to an inability to further increase errors during inactivation (i.e., a ceiling effect) because errors rose to 50% in naive rats using the same muscimol dose. The ipsilateral M1 can be targeted for recovery of function after corticospinal lesion in this study and others (Liu et al., 2008; Maier et al., 2008; Reitmeir et al., 2011). This indicates flexibility in the motor systems that can be targeted for functional benefit. In humans, ipsilateral control can be established after large, perinatal brain injury (Staudt et al., 2002, 2004) or after hemispherectomy in children (Jonas et al., 2004). However, in adults, severe injury to the CST is associated with poor recovery (Stinear et al., 2007), and like the rats with injury only, the uninjured hemisphere does not spontaneously assume control of the impaired hand (Bradnam et al., 2012). However, electrical stimulation may promote sufficient plasticity in the uninjured hemisphere that the stimulated M1 gains adaptive ipsilateral control, as it does after developmental injury.
Electrical stimulation of the motor cortex is a promising therapeutic approach for recovery of motor function, but it must be properly targeted. Following promising preclinical studies of epidural stimulation in rodents (Adkins et al., 2006, 2008), clinical trials were initiated that targeted perilesional cortex in the stroke hemisphere. Early stage trials showed benefit of this approach, but the phase 3 trial did not meet its primary endpoint; patients receiving physical therapy and M1 stimulation were not different from physical therapy alone (Plow et al., 2009). A post hoc analysis revealed that, when the stimulation was directed to intact corticospinal circuits in perilesional cortex, function was restored (Nouri and Cramer, 2011). Thus, epidural stimulation can restore function, by either targeting the perilesional cortex that is responsible for endogenous recovery (Starkey et al., 2012; Zeiler et al., 2013) or creating bilateral control from the uninjured hemisphere, as shown here. In addition, spared motor circuits can be strengthened with non-invasive brain stimulation, such as transcranial magnetic stimulation (Reis et al., 2008) or transcranial direct current stimulation (Vines et al., 2008). This study identifies motor cortex in the uninjured hemisphere as an attractive target for electrical brain stimulation to promote recovery of motor function after large, unilateral corticospinal injury.
Footnotes
This work was supported by National Institutes of Health Grants K08NS073796, (J.B.C.) and R01NS064004 (J.H.M.), a Travis Roy Foundation grant administered by the Christopher and Dana Reeve Foundation (J.B.C.), and the Burke Foundation (J.B.C.). We thank Xiuli Wu for histochemistry and histology.
The authors declare no competing financial interests.
References
- Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006;200:356–370. doi: 10.1016/j.expneurol.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212:14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22:2662–2671. doi: 10.1093/cercor/bhr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29:6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Kimura H, Berrol LJ, Martin JH. Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury. Eur J Neurosci. 2013;37:1090–1102. doi: 10.1111/ejn.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas R, Nguyen S, Hu B, Asarnow RF, LoPresti C, Curtiss S, de Bode S, Yudovin S, Shields WD, Vinters HV, Mathern GW. Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology. 2004;62:1712–1721. doi: 10.1212/01.wnl.0000127109.14569.c3. [DOI] [PubMed] [Google Scholar]
- Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953;12:400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26:923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang X, Savant-Bhonsale S, Chopp M. Contralesional axonal remodeling of the corticospinal system in adult rats after stroke and bone marrow stromal cell treatment. Stroke. 2008;39:2571–2577. doi: 10.1161/STROKEAHA.107.511659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/S0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/S0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77:1076–1083. doi: 10.1212/WNL.0b013e31822e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586:325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitmeir R, Kilic E, Kilic U, Bacigaluppi M, ElAli A, Salani G, Pluchino S, Gassmann M, Hermann DM. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134:84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey ML, Bleul C, Zörner B, Lindau NT, Mueggler T, Rudin M, Schwab ME. Back seat driving: hindlimb corticospinal neurons assume forelimb control following ischaemic stroke. Brain. 2012;135:3265–3281. doi: 10.1093/brain/aws270. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56:854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci. 2012;32:12896–12908. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O'Brien RJ, Krakauer JW. Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke. 2013;44:483–489. doi: 10.1161/STROKEAHA.112.676940. [DOI] [PMC free article] [PubMed] [Google Scholar]