Abstract
Background
Gallstone disease is one of the most common digestive disorders, affecting more than 30 million Americans. Previous twin studies suggest a heritability of 25% for gallstone formation. To date, one genome-wide association study (GWAS) has been performed in a population of European-descent. Several candidate gene studies have been performed in various populations, but most have been inconclusive. Given that gallstones consist of up to 80% cholesterol, we hypothesized that common genetic variants associated with high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) would also be associated with gallstone risk.
Methods
To test this hypothesis, the Epidemiologic Architecture for Genes Linked to Environment (EAGLE) study as part of the Population Architecture using Genomics and Epidemiology (PAGE) study performed tests of association between 49 GWAS-identified lipid trait SNPs and gallstone disease in non-Hispanic whites (446 cases and 1,962 controls), non-Hispanic blacks (179 cases and 1,540 controls), and Mexican Americans (227 cases and 1,478 controls) ascertained for the population-based Third National Health and Nutrition Examination Survey (NHANES III).
Results
At a liberal significance threshold of 0.05, five, four, and four SNP(s) were associated with disease risk in non-Hispanic whites, non-Hispanic blacks, and Mexican Americans, respectively. No one SNP was associated with gallstone disease risk in all three racial/ethnic groups. The most significant association was observed for ABCG5 rs6756629 in non-Hispanic whites [odds ratio (OR) = 1.89; 95% confidence interval (CI) = 1.44-2.49; p = 0.0001). ABCG5 rs6756629 is in strong linkage disequilibrium with rs11887534 (D19H), a variant previously associated with gallstone disease risk in populations of European-descent.
Conclusions
We replicated a previously associated variant for gallstone disease risk in non-Hispanic whites. Further discovery and fine-mapping efforts in diverse populations are needed to fully describe the genetic architecture of gallstone disease risk in humans.
Keywords: Gallstones, NHANES III, PAGE, EAGLE, ABCG5, Genetic association
Background
Gallstone disease has become one of the most common digestive disorders in the world. Gallstone disease affects more than 30 million Americans and accounts for 750,000 gallbladder removals in the United States annually and at least 190,000 gallbladder removals in European countries such as Germany [1]. The prevalence of gallstone disease varies by race/ethnicity with Native Americans and Hispanics having the highest reported prevalence compared with populations of European or African descent [2-7]. The incidence and prevalence is likely under-estimated given the fact that up to 80% of individuals with gallstones are asymptomatic or have non-traditional symptoms of disease [8].
There are a number of well-established gallstone disease risk factors including older age, female sex, increased body mass index, race/ethnicity, and certain dietary habits such as consumption of food rich in refined carbohydrates and lipids [1,9-13]. In addition to the established demographic and lifestyle risk factors, there is evidence that gallstone disease risk has a significant genetic component. Family history of gallstone disease increases an individual’s risk for the same disease [8,14,15]. Twin studies suggest that up to 25% of the risk of gallstone disease is due to genetic factors [16]. Indeed, recent candidate gene [17-19] and genome-wide association (GWA) [20] studies along with linkage studies [21] have identified common genetic variants associated with gallstone disease risk.
The majority of gallstones are composed of condensed bile components containing up to 80% cholesterol (termed “cholesterol gallstones”) [13]. Based on the composition of gallstones and recent results from genetic association studies, we hypothesized that common genetic variants associated with lipid profiles would be associated with gallstone disease risk. To test this hypothesis, we conducted a genetic association study for gallstone disease and 49 genetic variants previously associated with high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and triglycerides (TG) [22] in the diverse Third National Health and Nutrition Examination Survey (NHANES III) as part of the Epidemiologic Architecture for Genes Linked to Environment (EAGLE) study [23]. Overall, we observed thirteen associations between gallstone disease and lipid-associated variants among the three racial/ethnic groups at a liberal significance threshold of 0.05 with the most significant association observed for ABCG5 rs6756629 in non-Hispanic whites (OR = 1.89; 95% CI: 1.44-2.49). ABCG5 rs6756629 has been previously associated with lipid profiles (LDL-C, total cholesterol, and triglycerides) and is in strong linkage disequilibrium with ABCG5 rs112887534 in European-descent populations [24], a variant recently associated with gallstone disease [20]. Collectively, our data support the hypothesis that genetic variation associated with lipid trait variation is also associated with gallstone disease risk.
Methods
Study population and phenotypes
The study population described here includes phase 2 participants ascertained between 1991 and 1994 as part of the Third National Health and Nutrition Examination Survey (NHANES III). NHANES is now conducted yearly by the National Center on Health Statistics (NCHS) at the Center for Disease Control and Prevention (CDC) with the major goal of assessing the health and nutrition status of Americans regardless of health status. Beginning with NHANES III phase 2, CDC began collecting bio-specimens from consenting participants for genetic studies. NHANES is a complex survey design that oversamples specific age groups (such as the elderly) and racial/ethnic groups (such as non-Hispanic blacks).
All eligible participants were given an approximately hour long interview. Participants also underwent a health examination by the Mobile Exam Centers (MEC), and blood and urine samples were also obtained [25]. Participants were eligible for NHANES at two months of age or older, while participants consenting for bio-specimen collection for DNA extraction had to be at least twelve years of age. Non-eligible individuals included military personnel and institutionalized civilians.
Genetic NHANES III includes 7,159 participants, of which 2,631 are self-described non-Hispanic white, 2,108 are self-described non-Hispanic black, and 2,073 are self-described Mexican American. All procedures were approved by the CDC Ethics Review Board and written informed consent was obtained from all participants. Because no identifying information was accessed by the investigators, Vanderbilt University’s Institutional Review Board determined that this study met the criteria of “non-human subjects.”
Gallstones were defined by a positive “yes” response to an administered questionnaire exam asking, “Has the doctor ever told you that you had gallstones?” or a positive ultrasound reading (presence of scar tissue (surgical removal of gallbladder or cholecystectomy) or demonstration of gallstones present in the gallbladder) from trained technicians. Controls were defined as gallstone-free participants who answered “no” response to the administered questionnaire or had no history of gallstone removal surgeries. To assess the potential for misclassification, we examined participants with both ultrasound and questionnaire data to validate asymptomatic cases and to identify misclassified controls.
SNP selection and genotyping
A total of 49 GWAS-identified SNPs were selected for study based on evidence of being previously associated with at least one lipid profile (HDL-C, LDL-C, TG) in published candidate gene and genome-wide association studies [10,12,18,20,22]. The SNP selection criteria meet the genome-wide significance of p < 10-8 in previous published studies [22]. SNP data was generated by genotyping using Sequenom and Illumina BeadXpress platforms. Thorough genotyping and SNP details (gene region, physical location, coding type, etc.) have been previously published in Text S1 of [22]. CDC quality control metrics were implemented on the 49 SNPs and included tests of Hardy-Weinberg Equilibrium (threshold of p > 0.0001 in at least two of the three race/ethnicities) as well as concordance with blinded duplicates supplied by CDC (at least 95%). Also, as part of the PAGE study [23], we genotyped these lipid-associated SNPs in 360 HapMap samples for network-wide quality control assessment.
Statistical methods
Participants less than 18 years of age were excluded from this study. Single SNP tests of association were performed using logistic regression. Gallstone status (yes/no) was the dependent variable and each SNP assuming an additive genetic model was the independent variable. All models were adjusted for age, sex, and body mass index. Logistic regressions were performed stratified by self-reported race/ethnicity, and genetic effect estimates were expressed as odds ratios (ORs) with 95% confidence intervals (95% CIs). All analyses were conducted in SAS v9.2 (SAS Institute, Cary, NC) using the Analytic Data Research by Email (ANDRE) portal of the CDC Research Data Center in Hyattsville, MD. Results were plotted using Synthesis-View [26]. Linkage disequilibrium was assessed using SNP Annotation and Proxy Search (SNAP) [27].
Results
Study population characteristics
Study population characteristics by self-described race/ethnicity and gallstone disease status are given in Table 1. Overall, we identified 446, 179, and 227 cases of gallstone disease among non-Hispanic whites, non-Hispanic blacks, and Mexican Americans, respectively. As expected, based on the known epidemiology of gallstone disease [3,13], cases tended to be female, older, and have a higher BMI compared with controls (Table 1). Also, overall, there were proportionally fewer cases among non-Hispanic blacks compared with non-Hispanic whites and Mexican Americans, which is consistent with the lower prevalence of this disease among this group [3].
Table 1.
Study population characteristics by gallstone disease status stratified by race/ethnicity
|
Non-hispanic whites |
Non-hispanic blacks |
Mexican Americans |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
n = 2,408 |
n = 1,719 |
n = 1,708 |
|||||||
| Cases | Controls | P-value | Cases | Controls | P-value | Cases | Controls | P-value | |
|
n |
446 |
1,962 |
- |
179 |
1,540 |
- |
227 |
1,478 |
- |
|
% Female |
71.13% |
58.10% |
5.24E-07 |
76.54% |
56.17% |
3.66E-07 |
73.29% |
45.81% |
5.55E-16 |
|
Age in years |
61.74 |
51.91 |
6.49E-23 |
49.67 |
40.02 |
1.11E-16 |
53.80 |
39.27 |
2.59E-33 |
| (16.45) |
(20.61) |
(16.71) |
(16.48) |
(15.16) |
(16.95) |
||||
|
BMI |
28.51 |
26.24 |
5.13E-14 | 30.77 |
27.96 |
2..32E-07 | 30.27 |
27.22 |
3.33E-16 |
| (kg/m 2 ) | (6.1) | (5.35) | 6.67) | (6.61) | (5.86) | (5.2) | |||
Abbreviations: BMI body mass index.
Means (and standard deviations) are given unless otherwise noted. P-values were calculated based on the Wald test using unweighted survey data.
Among the 49 lipid trait-associated SNPs tested for association with gallstone disease, five, four, and four SNPs were associated with disease risk at a liberal significance threshold of 0.05 among non-Hispanic whites, non-Hispanic blacks, and Mexican Americans, respectively (Table 2; Additional file 1: Table S1). Among all groups, the most significant finding was observed in non-Hispanic whites (OR = 1.89; 95% CI = 1.44-2.49; p = 0.0001) for non-synonymous ABCG5 rs6756629, a variant whose minor allele (A) was previously associated with decreased LDL-C and total cholesterol and increased triglycerides among European-descent populations [28]. This associated SNP is in strong linkage disequilibrium with ABCG5 rs11887534 identified in a genome-wide association within a European-descent population for gallstone disease [20,24]. The second association identified for gallstone disease in non-Hispanic whites involved ABCG8 rs6544713 (OR = 1.35; 95% CI = 1.14-1.61; p = 0.0007), a variant previously associated with LDL-C levels in European-descent populations [29]. ABCG8 rs6544713 is not in linkage disequilibrium with rs6756629 or rs11887534 (both r2 = 0.047 in CEU 1000 Genomes Project) and therefore most likely represents an independent association with gallstone disease risk.
Table 2.
Significant tests of association for gallstone disease by population
| SNPs | Gene | OAT |
Non-Hispanic Whites |
Non-Hispanic Blacks |
Mexican Americans |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case/ Control CAF | OR (95% C.I.) | P | Case/ Control CAF | OR (95% C.I.) | P | Case/ Control CAF | OR (95% C.I.) | P | |||
| rs10401969 |
SUGP1 |
TG |
§ |
1.37 (1.05, 1.78) |
1.89E-02
|
§ |
1.01 (0.74, 1.38) |
9.61E-01 |
§ |
0.69 (0.41, 1.15) |
1.52E-01 |
| rs10889353 |
DOCK7 |
TG |
0.64/ 0.67 |
0.90 (0.76, 1.05) |
1.83E-01 |
0.61/ 0.62 |
0.84 (0.67, 1.06) |
1.50E-01 |
0.66/ 0.62 |
1.32 (1.05, 1.67) |
1.73E-02
|
| rs17216525 |
CILP2 |
TG |
§ |
0.76 (0.58, 0.99) |
4.45E-02
|
§ |
1.26 (0.64, 2.46) |
5.02E-01 |
§ |
0.88 (0.53, 1.45) |
6.15E-01 |
| rs1883025 |
ABCA1 |
HDL |
0.27/ 0.26 |
1.09 (0.92, 1.30) |
3.36E-01 |
0.30/ 0.33 |
0.84 (0.66, 1.09) |
1.86E-01 |
0.26/ 0.28 |
0.78 (0.62, 0.98) |
3.30E-02
|
| rs2650000 |
HNF1A LEF1 |
LDL |
0.66/ 0.65 |
1.146 (0.97, 1.35) |
1.02E-01 |
§ |
1.50 (1.02, 2.21) |
3.73E-02
|
0.63/ 0.63 |
0.976 (0.79, 1.20) |
8.21E-01 |
| rs28927680 |
BUD13 |
HDL TG |
0.09/ 0.07 |
1.23 (0.93, 1.62) |
1.53E-01 |
0.22/ 0.17 |
1.40 (1.06, 1.86) |
1.92E-02
|
0.16/ 0.14 |
1.07 (0.81, 1.42) |
6.27E-01 |
| rs3764261 |
CETP |
HDL |
0.68/ 0.68 |
0.95 (0.80, 1.12) |
5.15E-01 |
0.63/ 0.69 |
0.71 (0.56, 0.90) |
4.70E-03
|
0.66/ 0.66 |
1.06 (0.85, 1.33) |
5.89E-01 |
| rs4149268 |
ABCA1 |
HDL |
0.38/ 0.38 |
1.10 (0.94, 1.30) |
2.34E-01 |
0.69/ 0.65 |
1.03 (0.81, 1.31) |
8.21E-01 |
0.30/ 0.33 |
0.75 (0.60, 0.95) |
1.53E-02
|
| rs4939883 |
LIPG |
HDL |
0.83/ 0.83 |
1.28 (1.03, 1.59) |
2.71E-02
|
0.56/ 0.54 |
0.94 (0.75, 1.19) |
6.17E-01 |
§ |
0.88 (0.65, 1.19) |
4.14E-01 |
| rs6544713 |
ABCG8 |
LDL |
0.71/ 0.68 |
1.35 (1.14, 1.61) |
7.00E-04
|
§ |
1.19 (0.88, 1.62) |
2.65E-01 |
0.85/ 0.82 |
1.22 (0.92, 1.60) |
1.66E-01 |
| rs6756629 |
ABCG5 |
LDL |
0.09/ 0.06 |
1.89 (1.44, 2.49) |
1.00E-04
|
0.13/ 0.08 |
1.24 (0.82, 1.87) |
3.03E-01 |
0.12/ 0.09 |
1.19 (0.85, 1.67) |
3.04E-01 |
| rs754523 |
APOB |
LDL |
0.33/ 0.32 |
1.02 (0.86, 1.20) |
8.34E-01 |
0.27/ 0.22 |
1.36 (1.04, 1.78) |
2.41E-02
|
0.24/ 0.29 |
0.86 (0.68, 1.08) |
1.82E-01 |
| rs7679 | PCIF1 | HDL | 0.19/ 0.16 | 0.97 (0.79, 1.19) | 7.45E-01 | § | 1.18 (0.75, 1.84) | 4.73E-01 | 0.14/ 0.11 | 1.51 (1.13, 2.01) | 4.80E-03 |
Single SNP tests of association were performed using logistic regression assuming an additive genetic model. Only results for SNPs associated at p < 0.05 in any one race/ethnicity are shown (bolded and italicized). Results displayed here were adjusted for age, sex, and body mass index. §Per data use agreement, coded allele frequencies are not outputted by CDC for cells with ≤5 counts.
Abbreviations: Original associated Trait (OAT); High density lipoprotein cholesterol (HDL-C); low density lipoprotein cholesterol (LDL-C); triglycerides (TG); coded allele frequency (CAF); odds ratio (OR); confidence interval (CI); p-value (P).
Interestingly, despite the smaller sample size, four associations at p < 0.05 were observed among non-Hispanic blacks. The most significant finding among non-Hispanic blacks was BUD13 rs28927680 (OR = 1.40; 95% CI = 1.06-1.86, p = 0.019) previously associated with HDL-C and TG in European-descent populations [29]. Other nominal associations observed in non-Hispanic blacks included genetic variants previously associated with HDL-C (CETP rs3764261 (OR = 0.71; 95% CI 0.56-0.9)) and LDL-C (HNF1A/LEF1 rs2650000 (OR = 1.50; 95% CI 1.02-2.21) and APOB rs754523 (OR = 1.36; 95% CI 1.04-1.78)).
Among Mexican Americans, four associations were nominally associated with gallstone disease risk: HDL-C (ABCA1 rs1883025 (OR = 0.78; 95% CI 0.62-0.98; p = 0.033), ABCA1 rs4149268 (OR = 0.75; 95% CI 0.60-0.95; p = 0.015), and PCIF1 rs7679 (OR = 1.51; 95% CI 1.13-2.01; p = 0.048)) and TG (DOCK7 rs10889353 (OR = 1.23; 95% CI 1.05-1.67; p = 0.017). No association was associated at p < 0.05 across all three racial/ethnicity groups (Table 2).
In addition to examining genetic associations for gallstone disease risk by race/ethnicity, we also examined the associations by associated lipid trait (Figures 1, 2 and 3; Additional file 2: Figure S1). That is, among the 49 SNPs tested here, 24, 14, and 17 SNPs were reported to be associated with HDL-C, LDL-C, and TG levels, respectively. As previously mentioned, the most significant finding involved an LDL-C associated variant, and, overall, approximately one-third (4 out of 14 or 29%) of LDL-C associated SNPs were nominally associated with gallstone disease risk in any one population. The other nominal findings reported at p < 0.05 were associated with HDL-C (6 out of 24 or 25%) or triglycerides (4 out of 17 or 24%) associated SNPs.
Figure 1.
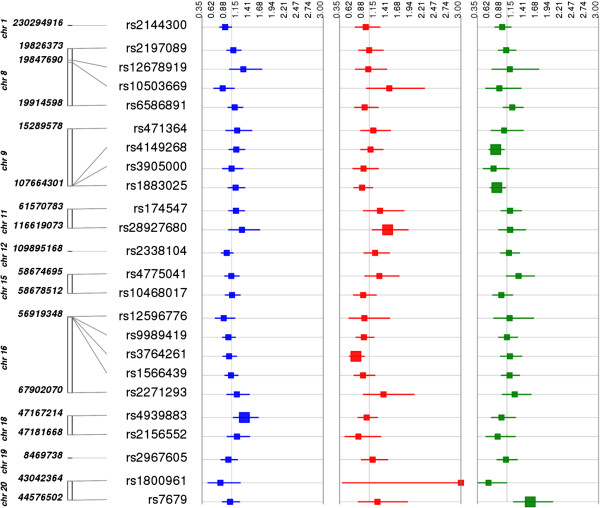
Associations between HDL-C associated SNPs and gallstone disease by population. Each high-density lipoprotein (HDL)-associated SNP was tested for an association with gallstone disease (yes/no) assuming an additive genetic model adjusted for age, sex, and body mass index [kg/m2]. The odds ratio and 95% confidence intervals are plotted by race-ethnicity using Synthesis-View [26]. SNP locations (genome build 37.5) are given on the y-axis. Each square represents an odds-ratio and each line represents a 95% confidence interval for each population. The larger square represents a significantly associated SNP at p < 0.05. Populations are color-coded as follows: non-Hispanic whites (blue), non-Hispanic blacks (red), and Mexican Americans (green). The grey vertical line represents the 1.0 threshold for odds-ratio values.
Figure 2.
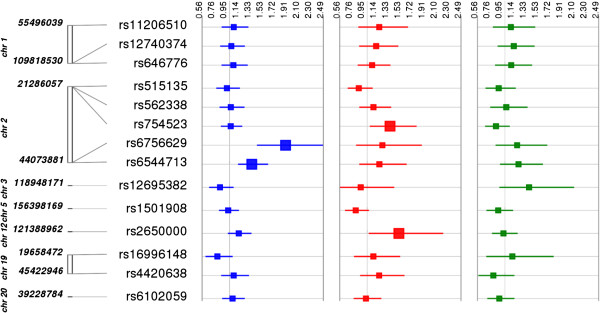
Associations between LDL-C associated SNPs and gallstone disease by population. Each low-density lipoprotein (LDL)-associated SNP was tested for an association with gallstone disease (yes/no) assuming an additive genetic model adjusted for age, sex, and body mass index [kg/m2]. The odds ratio and 95% confidence intervals are plotted by race-ethnicity using Synthesis-View [26]. SNP locations (genome build 37.5) are given on the y-axis. Each square represents an odds-ratio and each line represents a 95% confidence interval for each population. The larger square represents a significantly associated SNP at p < 0.05. Populations are color-coded as follows: non-Hispanic whites (blue), non-Hispanic blacks (red), and Mexican Americans (green). The grey vertical line represents the 1.0 threshold for odds-ratio values.
Figure 3.
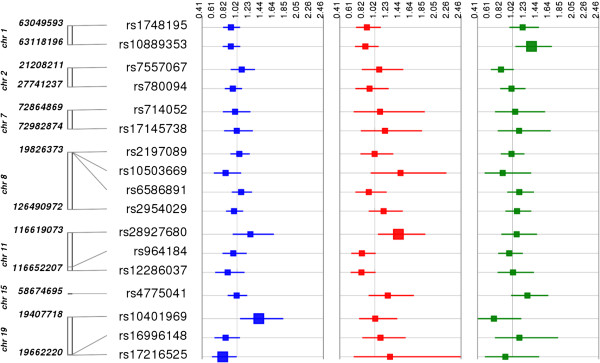
Associations between TG associated SNPs and gallstone disease by population. Each triglyceride (TG)-associated SNP was tested for an association with gallstone disease (yes/no) assuming an additive genetic model adjusted for age, sex, and body mass index [kg/m2]. The odds ratio and 95% confidence intervals are plotted by race-ethnicity using Synthesis-View [26]. SNP locations (genome build 37.5) are given on the y-axis. Each square represents an odds-ratio and each line represents a 95% confidence interval for each population. The larger square represents a significantly associated SNP at p < 0.05. Populations are color-coded as follows: non-Hispanic whites (blue), non-Hispanic blacks (red), and Mexican Americans (green). The grey vertical line represents the 1.0 threshold for odds-ratio values.
Discussion
Among the 49 lipid-trait associated SNPs tested here, thirteen were associated with gallstone disease risk at a liberal significance threshold of 0.05 in at least one racial/ethnic group. Most (5/13; 38%) associations were observed among non-Hispanic whites; conversely, four associations each were identified among non-Hispanic blacks and Mexican Americans. None of the associations were observed in all three racial/ethnic groups. The strongest association for gallstone disease risk was observed in non-Hispanic whites (OR = 1.89; 95% CI: 1.44-2.49) for ABCG5 rs6756629; although not significant (p = 0.30), this association trended in the same direction in non-Hispanic blacks (OR = 1.24; 95% CI 0.82-1.87) and Mexican Americans (OR = 1.26; 95% CI 0.89-1.79).
Replication in European-descent populations
ABCG5 rs6756629 (R50C) is in strong linkage disequilibrium with rs11887534 (D19H), a variant previously associated with gallstone disease risk in populations of European-descent [20]. Pairwise linkage disequilibrium (r2) was 1.00 in CEU from the 1000 Genomes Project Pilot Study [30] and 0.95 in a German study population [24]. Thus, the association observed here with rs6756629 likely represents a replication of the association identified in the original GWAS [20]. The magnitude of effect (OR = 1.89) is somewhat smaller compared with the original (OR = 2.2) [20] and subsequent reports [18,21,24,31], but the confidence intervals overlap suggesting similar overall genetic effect sizes for this population.
Associations among non-European-descent populations
Very few genetic association studies and no GWA studies of gallstone disease risk have been performed in populations of non-European descent. A few studies have examined the association between ABCG5 rs11887534 and gallstone disease in populations from China [24], Taiwan [32], and India [24]. To our knowledge, no study has examined ABCG5 rs11887534 or tagged rs6756629 in African Americans or Mexican Americans for gallstone disease risk. As expected based on the known epidemiology of gallstone disease, the sample sizes available for non-Hispanic blacks (n = 179 cases) and Mexican Americans (n = 227 cases) were smaller compared with non-Hispanic whites (n = 446 cases). ABCG5 rs6756629 was not associated with gallstone disease risk in non-Hispanic blacks (Table 2). The ABCG5 variants rs11887534 and rs6756629 have lower pair-wise linkage disequilibrium in African-descent populations (YRI r2 = 0.613) compared with European-descent populations (CEU r2 = 1.000) based on data from the 1000 Genomes Project Pilot Study [30]. Recent statistical and functional data suggest that ABCG5 rs11887534 (D19H) is likely the functional variant responsible for the association with gallstone disease risk while rs6756629 is likely a tagSNP [24]. The smaller sample sizes available for non-European descent populations coupled with differences in linkage disequilibrium between the genotyped rs6756629 and putative functional rs11887534 likely resulted in low statistical power to detect this association in non-Hispanic blacks and Mexican Americans. Further ABCG5 fine-mapping and functional studies are needed to fully catalogue gallstone disease risk variants in diverse populations.
Limitations and strengths
This study has several limitations and strengths. A major limitation is sample size and power. Although genetic NHANES III is large overall (n = 7,159), the number of cases and controls available for this gallstone disease study is small when stratified by race/ethnicity. More recent NHANES did not collect survey or ultrasound data; therefore, large sample sizes or independent replication datasets for genetic associations for gallstone disease risk are not available in NHANES. To maximize the power of the present study within NHANES III, we used both questionnaire and ultrasound data (where ultrasound data represented the “truth” or gold standard when both were available per participant) to define cases and controls. Indeed, limiting the dataset to participants with both data types would have resulted in a 14% reduction in sample size. A trade-off of this approach to maximize sample size, however, is that we may have misclassified participants who only had questionnaire data but no corroborating ultrasound data. To examine the possible extent of misclassification, we compared our case/control definition (questionnaire or ultrasound data) with case/control status among participants with both data types (excluding those participants with questionnaire data only). Overall, we found little evidence for misclassification using questionnaire data only (positive predictive value = 99%, negative predictive value = 100%, sensitivity = 100%, and specificity = 99%). We also compared results of tests of association between the two definitions and found little difference (Additional file 3: Figure S2).
Another limitation to the study is that we did not adjust our significance level for multiple statistical tests. Our liberal significance threshold coupled with the lack of an independent dataset can be cause for concerns for false positive associations. However, the most significant association reported here replicated an association previously reported in a GWAS in a population of similar race/ethnicity. The association between rs6756629 and gallstone disease risk in non-Hispanic whites, along with the association involving ABCG5 rs6544713, would be considered significant with a conservative Bonferroni correction. The facts that the associations represent a replication of a previous report [20], that rs6756629 tags a likely functional SNP associated with gallstone disease risk [24], and that both survive correction for multiple testing support the conclusion that these are not a false positive findings.
A major strength of the current study is its diversity. Little to no data exist for the genetics of gallstone disease risk in African Americans and Mexican Americans. Further discovery studies are needed to identify the full genetic architecture of gallstone disease risk in diverse populations [33]. Another major strength of this current study is the combination of questionnaire and ultrasound data available in NHANES III. For participants with overlapping questionnaire and ultrasound data, we were able to identify asymptomatic cases of gallstones that would have otherwise been misclassified using questionnaire data only. Misclassification of case/control status lowers power to detect genetic associations; therefore, careful phenotyping is one of many essential components in the conduction of genetic association studies [34]. Combination of these data types maximized sample size for this study whereas restricting eligible cases and controls to participants with ultrasound data only would have reduced the sample size by 14% and therefore reduced power.
Conclusions
In summary, we demonstrate here that lipid-trait associated genetic variants such as ABCG5 rs6756629 are associated with gallstone disease risk. Larger studies of diverse populations are needed to determine the full spectrum of the genetic variants that contribute to gallstone disease risk in humans.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RG and DCC designed the study, interpreted the data, and drafted and edited the manuscript. RG and KB-G conducted the analyses. NBG, HJ, PM, MA, CS, NS-B, and HHD generated the genetic data and performed quality control analyses. BM and JB developed the analysis pipeline and maintained the study databases. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
All tests of association for gallstone disease by population. Single SNP tests of association were performed using logistic regression assuming an additive genetic model. Results displayed here were adjusted for age, sex, and body mass index. SNP position is based on genome build 37.5.
All associations between lipid trait-associated SNPs and gallstone disease by population. A total of 49 lipid-trait associated SNPs were tested for an association with gallstone disease using logistic regression adjusted for age, sex, and body mass index [kg/m2]. Synthesis-View [26] was used to display the results. SNP location (genome build 37.5) is given on the x-axis and p-values (-log10 transformed) are plotted along the y-axis at the top of the figure, while coded allele frequencies (CAF) are plotted along the y-axis at the bottom of the figure. Each triangle represents a p-value and each circle represents a CAF for each race-ethnicity. Populations are color-coded as follows: non-Hispanic whites (blue), non-Hispanic blacks (red), and Mexican Americans (green). The direction of the arrows corresponds to the direction of the beta coefficient. The significance threshold is indicated by the red bar at p = 0.05.
Assessment of the impact of including questionnaire data to maximize sample size versus potential misclassification. A total of 49 lipid-trait associated SNPs were tested for an association with gallstone disease using logistic regression adjusted for age, sex, and body mass index [kg/m2]. The analysis was performed twice where cases and controls were 1) defined by questionnaire or ultrasound data and 2) defined by questionnaire and ultrasound data. Synthesis-View [26] was used to display the results. SNP location (genome build 37.5) is given on the x-axis and p-values (-log10 transformed) are plotted along the y-axis for the top of the figure, while case/control totals are plotted along the y-axis for the bottom of the figure. Each triangle represents a p-value and each full circle represents a case while each empty circle represents a control. Triangles are color-coded such that red represents results from cases and controls defined by questionnaire or ultrasound data and blue represents results from cases and controls defined by questionnaire and ultrasound data. The direction of the arrows corresponds to the direction of the beta coefficient. The significance threshold is indicated by the red bar at p = 0.05.
Contributor Information
Robert Goodloe, Email: robert.j.goodloe@vanderbilt.edu.
Kristin Brown-Gentry, Email: brownkd1908@yahoo.com.
Niloufar B Gillani, Email: nila.gillani@chgr.mc.vanderbilt.edu.
Hailing Jin, Email: hailing.jin@chgr.mc.vanderbilt.edu.
Ping Mayo, Email: mayo@chgr.mc.vanderbilt.edu.
Melissa Allen, Email: melissa.allen@chgr.mc.vanderbilt.edu.
Bob McClellan, Jr, Email: bob.mcclellan@chgr.mc.vanderbilt.edu.
Jonathan Boston, Email: boston@chgr.mc.vanderbilt.edu.
Cara Sutcliffe, Email: cara.b.sutcliffe@vanderbilt.edu.
Nathalie Schnetz-Boutaud, Email: nathalie.schnetz-boutaud@chgr.mc.vanderbilt.edu.
Holli H Dilks, Email: holli.dilks@chgr.mc.vanderbilt.edu.
Dana C Crawford, Email: crawford@chgr.mc.vanderbilt.edu.
Acknowledgements
Genotyping in NHANES was supported in part by The Population Architecture using Genomics and Epidemiology (PAGE) study, which is funded by the National Human Genome Research Institute (NHGRI). Data included in this report were resulted from the Epidemiologic Architecture for Genes Linked to Environment (EAGLE) Study, as part of the NHGRI PAGE study (U01HG004798). Genotyping services for select EAGLE NHANES III SNPs presented here were also provided by the Johns Hopkins University under federal contract number (N01-HV-48195) from NHLBI. We at EAGLE would like to thank Dr. Geraldine McQuillan and Jody McLean for their help in accessing the Genetic NHANES data. The Vanderbilt University Center for Human Genetics Research, Computational Genomics Core provided computational and/or analytical support for this work. The NHANES DNA samples are stored and plated by the Vanderbilt DNA Resources Core. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes for Health or the Centers for Disease Control and Prevention.
References
- Krawczyk M, Rusticeanu M, Grunhage F, Mahler M, Portincasa P, Acalovschi M, Lammert F. Analysis of the common vasoactive intestinal peptide receptor 1 polymorphism in gallstone patients. J Gastrointest Liver Dis. 2010;19:273–277. [PubMed] [Google Scholar]
- Mendez-Sanchez N, King-Martinez AC, Ramos MH, Pichardo-Bahena R, Uribe M. The Amerindian's Genes in the Mexican Population are Associated with Development of Gallstone Disease. Am J Gastroenterol. 2004;99:2166–2170. doi: 10.1111/j.1572-0241.2004.40159.x. [DOI] [PubMed] [Google Scholar]
- Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the United States. Gastroenterology. 1999;117:632–639. doi: 10.1016/S0016-5085(99)70456-7. [DOI] [PubMed] [Google Scholar]
- Everhart JE, Yeh F, Lee ET, Hill MC, Fabsitz R, Howard BV, Welty TK. Prevalence of gallbladder disease in American Indian populations: findings from the strong heart study. Hepatology. 2002;35:1507–1512. doi: 10.1053/jhep.2002.33336. [DOI] [PubMed] [Google Scholar]
- Maurer KR, Everhart JE, Ezzati TM, Johannes RS, Knowler WC, Larson DL, Sanders R, Shawker TH, Roth HP. Prevalence of gallstone disease in Hspanic populations in the United States. Gastroenterology. 1989;96:487–492. doi: 10.1016/0016-5085(89)91575-8. [DOI] [PubMed] [Google Scholar]
- Everhart JE. Gallstones and ethnicity in the Americas. J Assoc Acad Minor Phys. 2001;12:137–143. [PubMed] [Google Scholar]
- Diehl AK, Stern MP, Ostrower VS, Friedman PC. Prevalence of clinical gallbladder disease in Mexican-American, Anglo, and black women. South Med J. 1980;73:438–441. doi: 10.1097/00007611-198004000-00012. [DOI] [PubMed] [Google Scholar]
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6:172–187. doi: 10.5009/gnl.2012.6.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch S, Schafmayer C, Volzke H, Seeger M, Miquel JF, Sookoian SC, Egberts JH, Arlt A, Pirola CJ, Lerch MM, John U, Franke A, Von KO, Brosch M, Nothnagel M, Kratzer W, Boehm BO, Broring DC, Schreiber S, Krawczak M, Hampe J. Loci from a genome-wide analysis of bilirubin levels are associated with gallstone risk and composition. Gastroenterology. 2010;139:1942–1951. doi: 10.1053/j.gastro.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Kavousi M, Smith AV, Chen MH, Dehghan A, Aspelund T, Lin JP, van Duijn CM, Harris TB, Cupples LA, Uitterlinden AG, Launer L, Hofman A, Rivadeneira F, Stricker B, Yang Q, O'Donnell CJ, Gudnason V, Witteman JC. Genome-wide association meta-analysis for total serum bilirubin levels. Hum Mol Genet. 2009;18:2700–2710. doi: 10.1093/hmg/ddp202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Wang DQ, Portincasa P, Lammert F. Dissecting the genetic heterogeneity of gallbladder stone formation. Semin Liver Dis. 2011;31:157–172. doi: 10.1055/s-0031-1276645. [DOI] [PubMed] [Google Scholar]
- Renner O, Harsch S, Schaeffeler E, Winter S, Schwab M, Krawczyk M, Rosendahl J, Wittenburg H, Lammert F, Stange EF. A variant of the SLC10A2 gene encoding the apical sodium-dependent bile acid transporter is a risk factor for gallstone disease. PLoS One. 2009;4:e7321. doi: 10.1371/journal.pone.0007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes CS, Krawczyk M, Lammert F. Gallstones: environment, lifestyle and genes. Dig Dis. 2011;29:191–201. doi: 10.1159/000323885. [DOI] [PubMed] [Google Scholar]
- Gilat T, Feldman C, Halpern Z, Dan M, Bar-Meir S. An increased familial frequency of gallstones. Gastroenterology. 1983;84:242–246. [PubMed] [Google Scholar]
- Attili AF, De Santis A, Attili F, Roda E, Festi D, Carulli N. Prevalence of gallstone disease in first-degree relatives of patients with cholelithiasis. World J Gastroenterol. 2005;11:6508–6511. doi: 10.3748/wjg.v11.i41.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, Marschall HU. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology. 2005;41:1138–1143. doi: 10.1002/hep.20654. [DOI] [PubMed] [Google Scholar]
- Kurzawski M, Juzyszyn Z, Modrzejewski A, Pawlik A, Wiatr M, Czerny B, Adamcewicz R, Drozdzik M. Apolipoprotein B (APOB) gene polymorphism in patients with gallbladder disease. Arch Med Res. 2008;38:360–363. doi: 10.1016/j.arcmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Katsika D, Magnusson P, Krawczyk M, Grunhage F, Lichtenstein P, Einarsson C, Lammert F, Marschall HU. Gallstone disease in Swedish twins: risk is associated with ABCG8 D19H genotype. J Intern Med. 2010;268:279–285. doi: 10.1111/j.1365-2796.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- Juvonen T, Savolainen MJ, Kairaluoma MI, Lajunen LH, Humphries SE, Kes + ñniemi YA. Polymorphisms at the apoB, apoA-I, and cholesteryl ester transfer protein gene loci in patients with gallbladder disease. J Lipid Res. 1995;36:804–812. [PubMed] [Google Scholar]
- Buch S, Schafmayer C, Volzke H, Becker C, Franke A, von Eller-Eberstein H, Kluck C, Bassmann I, Brosch M, Lammert F, Miquel JF, Nervi F, Wittig M, Rosskopf D, Timm B, Holl C, Seeger M, ElSharawy A, Lu T, Egberts J, Fandrich F, Folsch UR, Krawczak M, Schreiber S, Nurnberg P, Tepel J, Hampe J. A genome-wide association scan identifies the hepatic cholesterol transporter ABCG8 as a susceptibility factor for human gallstone disease. Nat Genet. 2007;39:995–999. doi: 10.1038/ng2101. [DOI] [PubMed] [Google Scholar]
- Grunhage F, Acalovschi M, Tirziu S, Walier M, Wienker TF, Ciocan A, Mosteanu O, Sauerbruch T, Lammert F. Increased gallstone risk in humans conferred by common variant of hepatic ATP-binding cassette transporter for cholesterol. Hepatology. 2007;46:793–801. doi: 10.1002/hep.21847. [DOI] [PubMed] [Google Scholar]
- Dumitrescu L, Carty CL, Taylor K, Schumacher FR, Hindorff LA, Ambite J-L, Anderson G, Best LG, Brown-Gentry K, Buzkova P, Carlson CS, Cochran B, Cole SA, Devereux RB, Duggan D, Eaton CB, Fornage M, Franceschini N, Haessler J, Howard BV, Johnson KC, Laston S, Kolonel LN, Lee ET, MacCluer JW, Manolio TA, Pendergrass SA, Quibrera M, Shohet RV, Wilkens LR, Haiman CA, Le Marchand L, Buyske S, Kooperberg C, North KE, Crawford DC. Genetic determinants of lipid traits in diverse populations from the Population Architecture using Genomics and Epidemiology (PAGE) study. PLoS Genet. 2011;7:e1002138. doi: 10.1371/journal.pgen.1002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise TC, Ambite JL, Buyske S, Carlson CS, Cole SA, Crawford DC, Haiman CA, Heiss G, Kooperberg C, Le Marchand L, Manolio TA, North KE, Peters U, Ritchie MD, Hindorff LA, Haines JL. The next PAGE in understanding complex traits: design for the analysis of Population Architecture Using genetics and Epidemiology (PAGE) study. Am J Epidemiol. 2011;174:849–859. doi: 10.1093/aje/kwr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kampen O, Buch S, Nothnagel M, Azocar L, Molina H, Brosch M, Erhart W, von Schonfels W, Egberts J, Seeger M, Arlt A, Balschun T, Franke A, Lerch MM, Mayerle J, Kratzer W, Boehm BO, Huse K, Schniewind B, Tiemann K, Jiang ZY, Han TQ, Mittal B, Srivastava A, Fenger M, Jorgensen T, Schirin-Sokhan R, Tonjes A, Wittenburg H, Stumvoll M, Kalthoff H, Lammert F, Tepel J, Puschel K, Becker T, Schreiber S, Platzer M, Volzke H, Krawczak M, Miquel JF, Schafmayer C, Hampe J. Genetic and functional identification of the likely causative variant for cholesterol gallstone disease at the ABCG5/8 lithogenic locus. Hepatology. 2013;57:2407–2417. doi: 10.1002/hep.26009. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. Hyattsville, MD: National Center for Health Statistics; 1994. Vital and Health Statistics, Series 1: Programs and Collection Procedures, no. 32) (DHHS publication no. (PHS) 94-1308) (GPO no. 017-022-01260-0. [PubMed] [Google Scholar]
- Pendergrass S, Dudek S, Crawford D, Ritchie M. Synthesis-View: visualization and interpretation of SNP association results for multi-cohort, multi-phenotype data and meta-analysis. Bio Data Mining. 2010;3:10. doi: 10.1186/1756-0381-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Handsaker RE, Pulit S, Nizzari MM, ODonnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulchenko YS, Ripatti S, Lindqvist I, Boomsma D, Heid IM, Pramstaller PP, Penninx BW, Janssens AC, Wilson JF, Spector T, Martin NG, Pedersen NL, Kyvik KO, Kaprio J, Hofman A, Freimer NB, Jarvelin MR, Gyllensten U, Campbell H, Rudan I, Johansson A, Marroni F, Hayward C, Vitart V, Jonasson I, Pattaro C, Wright A, Hastie N, Pichler I, Hicks AA, Falchi M, Willemsen G, Hottenga JJ, de Geus EJ, Montgomery GW, Whitfield J, Magnusson P, Saharinen J, Perola M, Silander K, Isaacs A, Sijbrands EJ, Uitterlinden AG, Witteman JC, Oostra BA, Elliott P, Ruokonen A, Sabatti C, Gieger C, Meitinger T, Kronenberg F, Döring A, Wichmann HE, Smit JH, McCarthy MI, van Duijn CM, Peltonen L. ENGAGE Consortium. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stender S, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Sterol transporter adenosine triphosphate-binding cassette transporter G8, gallstones, and biliary cancer in 62,000 individuals from the general population. Hepatology. 2011;53:640–648. doi: 10.1002/hep.24046. [DOI] [PubMed] [Google Scholar]
- Kuo KK, Shin SJ, Chen ZC, Yang Y-HC, Yang JF, Hsiao PJ. Significant association of ABCG5 604Q and ABCG8 D19H polymorphisms with gallstone disease. Br J Surg. 2008;95:1005–1011. doi: 10.1002/bjs.6178. [DOI] [PubMed] [Google Scholar]
- Rosenberg NA, Huang L, Jewett EM, Szpiech ZA, Jankovic I, Boehnke M. Genome-wide association studies in diverse populations. Nat Rev Genet. 2010;11:356–366. doi: 10.1038/nrg2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Cardon LR. Designing candidate gene and genome-wide case–control association studies. Nat Protocols. 2007;2:2492–2501. doi: 10.1038/nprot.2007.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All tests of association for gallstone disease by population. Single SNP tests of association were performed using logistic regression assuming an additive genetic model. Results displayed here were adjusted for age, sex, and body mass index. SNP position is based on genome build 37.5.
All associations between lipid trait-associated SNPs and gallstone disease by population. A total of 49 lipid-trait associated SNPs were tested for an association with gallstone disease using logistic regression adjusted for age, sex, and body mass index [kg/m2]. Synthesis-View [26] was used to display the results. SNP location (genome build 37.5) is given on the x-axis and p-values (-log10 transformed) are plotted along the y-axis at the top of the figure, while coded allele frequencies (CAF) are plotted along the y-axis at the bottom of the figure. Each triangle represents a p-value and each circle represents a CAF for each race-ethnicity. Populations are color-coded as follows: non-Hispanic whites (blue), non-Hispanic blacks (red), and Mexican Americans (green). The direction of the arrows corresponds to the direction of the beta coefficient. The significance threshold is indicated by the red bar at p = 0.05.
Assessment of the impact of including questionnaire data to maximize sample size versus potential misclassification. A total of 49 lipid-trait associated SNPs were tested for an association with gallstone disease using logistic regression adjusted for age, sex, and body mass index [kg/m2]. The analysis was performed twice where cases and controls were 1) defined by questionnaire or ultrasound data and 2) defined by questionnaire and ultrasound data. Synthesis-View [26] was used to display the results. SNP location (genome build 37.5) is given on the x-axis and p-values (-log10 transformed) are plotted along the y-axis for the top of the figure, while case/control totals are plotted along the y-axis for the bottom of the figure. Each triangle represents a p-value and each full circle represents a case while each empty circle represents a control. Triangles are color-coded such that red represents results from cases and controls defined by questionnaire or ultrasound data and blue represents results from cases and controls defined by questionnaire and ultrasound data. The direction of the arrows corresponds to the direction of the beta coefficient. The significance threshold is indicated by the red bar at p = 0.05.


