Abstract
Background
Increased vascularity is a crucial event in the tumor progression and has prognostic significance in various cancers. However, the ultimate role of angiogenesis in the pathogenesis and clinical outcome of vulvar carcinoma patients is still not settled.
Methods
Tumor vascularity using CD34 stained slides measured by Chalkley counting method as well as hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF) immunoexpression was examined in 158 vulvar squamous cell carcinomas. Associations between vascular Chalkley count, HIF-1α and VEGF expression and clinicopathological factors and clinical outcome were evaluated.
Results
High CD34 Chalkley count was found to correlate with larger tumor diameter (P = 0.002), deep invasion (P < 0.001) and HIF-1α (P = 0.04), whereas high VEGF expression correlate significantly with poor tumor differentiation (P = 0.007). No significant association between CD34 Chalkley counts and VEGF expression and disease-specific survival was observed. High HIF-1α expression showed better disease specific survival in both univariate and multivariate analyses (P = 0.001).
Conclusions
A significant association between high tumor vascularity and larger tumor size as well as deeper tumor invasion suggests an important role of angiogenesis in the growth and progression of vulvar carcinomas. HIF-1α expression in vulvar carcinomas was a statistically independent prognostic factor.
Keywords: Vulvar squamous cell carcinoma, HIF-1α, Immunohistochemistry, Tumor vascularity, Chalkley method
Background
Vulvar carcinoma is accounting for 3-5% of all gynecological cancer and with an incidence ranging from 1 to 2 per 100 000 person-years worldwide [1,2]. The median age of these patients has been about 70 years. However, recently vulvar carcinomas are seen more frequently in younger patients [3,4]. The prognostic evaluation and treatment of vulvar carcinoma patients have been primarily guided by the lymph node status, the size of the tumor, depth of invasion, stage of the disease and grades [5-7]. Radical surgery is the most common treatment, but is often accompanied with physical and psychological adverse effects [5,8]. In an attempt to reduce severe complications, a change to individualized therapy has been reported [9]. Thus, identification of new markers which indicate the tumor behavior would be important to guide treatment decisions.
Angiogenesis is a crucial event for tumor growth and progression beyond a tumor size of 1–2 mm. Therefore, tumor neovasculature makes an important target for antiangiogenic therapy [10,11]. Increased tumor vascularity has been shown to have prognostic significance in various cancers including vulvar cancer [12-15]. The role of increased tumor vascularity in disease progression of various malignant gynecologic lesions, including malignant vulvar lesions, has been described [16,17]. Its importance in vulvar cancer has been emphasized by the increased vascularity in preinvasive lesions and invasive vulvar carcinomas [15-21]. Vulvar carcinoma patients with increased vascularity were reported to have poor prognosis in some studies [6,15,19], whereas other showed no significance [20].
Hypoxia-inducible factor (HIF)-1α, a transcription factor, is a key regulator of angiogenesis when a growing tumor experiences hypoxic stress and acts through various intracellular signalling pathways. Such activation results in the secretion of vascular endothelial growth factor (VEGF) and other factors related to tumor metabolism necessary for hypoxia compensation and tumor cell survival [22]. It is known to be expressed in various solid tumors including vulvar squamous cell carcinomas [23-30]. The relation between primary tumor vascularity and HIF-1α expression in head and neck and oesophageal squamous cell carcinoma has been reported [24,25], and the prognostic impact of HIF-1α expression in cancer is varied [26,27,29-32]. HIF-1α expression investigated recently in normal epithelium, intraepithelial neoplasia and invasive carcinoma of vulva did not show significant differences [28]. To our knowledge, no study of HIF-1α expression and its connection with prognosis in vulvar carcinoma patients has been reported. VEGF, a potent angiogenic molecule over-expressed in a hypoxic state, is crucial to induce tumor angiogenesis and acts through the receptors VEGFR1 and VEGFR2 [22,33]. It is expressed in various human cancers including vulvar malignancy [21,28,34,35]. A significant variation in expression of VEGF in nonneoplastic epithelium, preneoplastic lesions and invasive squamous cell carcinoma of vulva has been described [21,28,35]. Its expression in vulvar cancer and relationship with vascularity has been reported [19]. The prognostic impact of VEGF expression in invasive vulvar carcinoma is still not settled [19,36].
In the present study, we have evaluated a large series of primary vulvar squamous cell carcinomas for primary tumor vascularity and expression of HIF-1α and VEGF and elucidated their relationships with various clinicopathological parameters and clinical outcome.
Methods
Patient materials
A retrospective study was performed on a cohort of 158 patients with vulvar squamous cell carcinoma. All patients had undergone a resection at The Norwegian Radium Hospital between 1977 and 2006. The median age at diagnosis was 75 years (range, 41–92 years). In 108 (68%) of these cases radical surgery (a total vulvectomy plus a bilateral inguinal lymphadenectomy) had been performed, whereas the remaining 50 (32%) patients had non-radical surgery. Postoperative therapy had been administered to 44 patients including irradiation in 40 (25%) cases and irradiation/chemotherapy in four (3%) cases. Seventy-four (47%) of the patients died as a result of their vulvar cancer. All patients were followed up from the time of their confirmed diagnosis until death or 1. September, 2009. The median follow-up time for patients still alive was 108 months (range, 43 to 347 months). All tumors were staged based on the new International Federation of Gynecology and the Obstetrics (FIGO) classification from 2009 [37]. The Regional Committee for Medical Research Ethics South of Norway (S-06012), The Social and Health Directorate (04/2639 and 06/1478) and The Data Inspectorate (04/01043) approved the current study protocol. In this study we have used paraffin embedded tumor tissue from vulvar cancer patients diagnosed between 1977 and 2006. As many of these patients are dead or very old we did not have the opportunity to obtain patient consent. Permission to perform this study without patient consent was obtained from The Social and Health Directorate (04/2639).
Histological specimens were reviewed by the co-author J.M.N. without access to any clinical information on the patients. The tumors were classified according to the World Health Organization recommendations [38]. All 158 tumors were classified as keratinizing/non-keratinizing squamous cell carcinomas.
Immunohistochemistry
Three micrometer sections were processed for immunohistochemistry using the Dako EnVision™ Flex+ System (K8012; Dako, Glostrup, Denmark) and the Dako Autostainer. Deparaffinization and the unmasking of epitopes were performed using PT-Link (Dako) and EnVision™ Flex target retrieval solution at a high pH. After treatment with 0.03% hydrogen peroxide (H2O2) for 5 min to block endogenous peroxidase activity, the sections were incubated with monoclonal antibodies raised against CD34 (30 min at room temperature, clone QBEND-10, 1:1000, 1μg IgG1/ml) purchased from Monosan (Uden, The Netherlands), HIF-1α (over night at 4°C, clone 54/HIF-1α, 1:100, 2.5 μg IgG1/ml) purchased from BD Transduction Laboratories™ (San Jose, CA, USA) and VEGF (over night at 4°C, clone VG1, 1:100, 0.45 μg IgG1/ml) purchased from Dako. Then the slides were incubated with EnVision™ Flex+ mouse linker (15 min), EnVision™ Flex/HRP enzyme (30 min) and 3’3-diaminobenzidine tetrahydrochloride (DAB) (10 min). After counterstaining with hematoxylin the samples were dehydrated and mounted in Richard-Allan Scientific Cyto seal XYL (Thermo Scientific, Waltham, MA, USA). All of the sample series included positive controls known to be positive for CD34, HIF-1α and VEGF. As negative controls, the primary antibodies were replaced with mouse myeloma protein IgG1 at the equivalent concentration.
Quantification of tumor vascularity
Chalkley method was used for quantification of tumor vascularity as recommended in a consensus meeting [39]. The method has been described in detail earlier [14]. Three most vascularized areas in the CD34 stained tumor section known as “hotspots” were identified under the low power magnification after scanning first at ×40 and then ×100 magnification following the Weidner’s method of selection of vascular hotspots [40]. Then a 25 point Chalkley eyepiece graticule fixed in one of the eyepieces of the microscope was applied to each vascular hotspot at ×200 magnification [Chalkley grid area of 0.1886 mm2 (Nikon microscope, Eclipse E400)] in such a way that maximum number of black dots in Chalkley graticule fell on or within immunostained microvessels. The number of these dots that have fallen on or within the immunostained microvessels were counted in each selected hotspot area and recorded as Chalkley count. Sclerotic and necrotic area was avoided and count was done in only invasive carcinoma including margin. The highest count among the 3 hotspots counts from each tumor was used for further analyses. Measurement of vascularity was performed without the knowledge of clinicopathological data or clinical outcome.
Evaluation for HIF-1α and VEGF expression
Expression of HIF-1α was evaluated on immunostained slides semiquantitatively into four classes and only nuclear immunoreactivity of the tumor cells was taken into account. Due to similar staining intensity of the HIF-1α positive cases we did not consider the intensity of immunostaining. Based on the number of HIF-1α positively stained tumor cells, tumors were grouped into: 0% of the cells; < 10% of the cells; 10-50% of the cells and > 50% of the cells. For further analyses, HIF-1α expression in nucleus in more than 50% of the tumor cells was considered as high. VEGF positive cases showed different staining intensity and both intensity and number of positive tumor cells were evaluated. Cytoplasmic expression of VEGF was categorized semiquantitatively on the basis of intensity of the signal (absent, 0; weak, 1; moderate, 2; strong, 3) and the percentage of positive tumor cells (absent, 0; < 10%, 1; 10-50%, 2; > 50%, 3). The composite score was calculated as fraction of positive tumor cells score multiplied by intensity score, and range from 0 to 9. For further analyses, cytoplasmic VEGF immunostaining with a composite score ≥ 6 was classified as high expression. Examination of immunostaining was performed in a blinded fashion with no knowledge of the clinicopathological variables and patient outcomes.
Statistical analyses
The associations between the HIF-1α and VEGF expression and CD34 Chalkley counts of primary tumor vascularity and the clinicopathological variables were evaluated by the Pearson chi-square (χ2), Fisher’s exact test and linear-by-linear association as required. The disease-specific survival analysis, based on death from vulvar cancer only, was performed using the Kaplan Meier method and P value computed by log-rank test. A Cox proportional hazards regression model was used for both univariate and multivariate evaluation of survival rates. In the multivariate analysis, a backward regression was performed and variables with a P ≤ 0.05 in univariate survival analysis were included in the model. The vulvar carcinoma tissues in our cohort have been collected over an extensive period from 1977–2006. Due to the large variation in storage time and given that the fixation protocol for these tissues up to 1987 was acid formalin, whereas from 1987–2006 was buffered formalin, Mann–Whitney U test was performed to evaluate whether this has any influence on the CD34, HIF-1α and VEGF immunostaining. The Mann–Whitney U test showed that the distribution of CD34, HIF-1α and VEGF expression was the same between samples processed before and after 1987. All analyses were processed using the SPSS 18.0 statistical software package (SPSS, Chicago, IL). Statistical significance was considered for P < 0.05.
Results
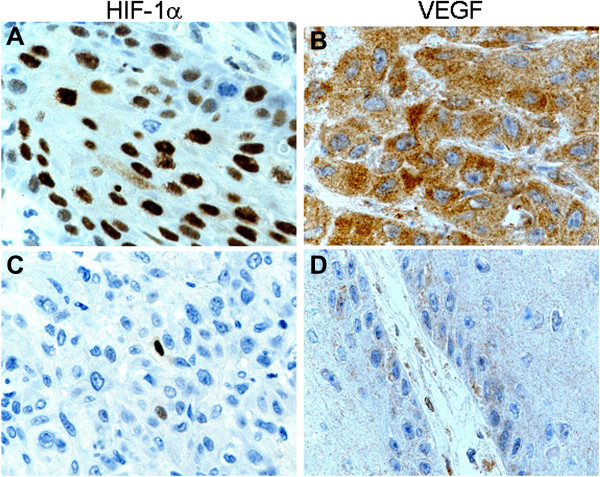
Vascularization in vulvar squamous cell carcinoma was heterogenously distributed. Microvessels were located in the tumor stroma lying between the islands of tumor cells and the size and shape of the vessels greatly varied. The CD34 Chalkley counts for the vulvar carcinoma vascularity ranged from 3–14 (mean, 7.92; median, 8; SD, 2.29). Predefined cutoff value of 8 (median value) was used to dichotomize the tumor into high and low vascular groups. Low (Chalkley counts < 8) and high (Chalkley counts ≥ 8) vascularity was identified in 67 (42%) and 91 (58%) of the vulvar carcinomas, respectively (Figure 1A and B). In vulvar carcinomas, high HIF-1α immunostaining (> 50% tumor cells) in the nucleus was observed in 57 (36%) and low levels (≤ 50% tumor cells) in 101 (64%) cases (Figure 2A and B), whereas high VEGF expression (score ≥ 6) in the cytoplasm was identified in 63 (40%) and low low level (score < 6) in 95 (60%) cases (Figure 2C and D).
Figure 1.
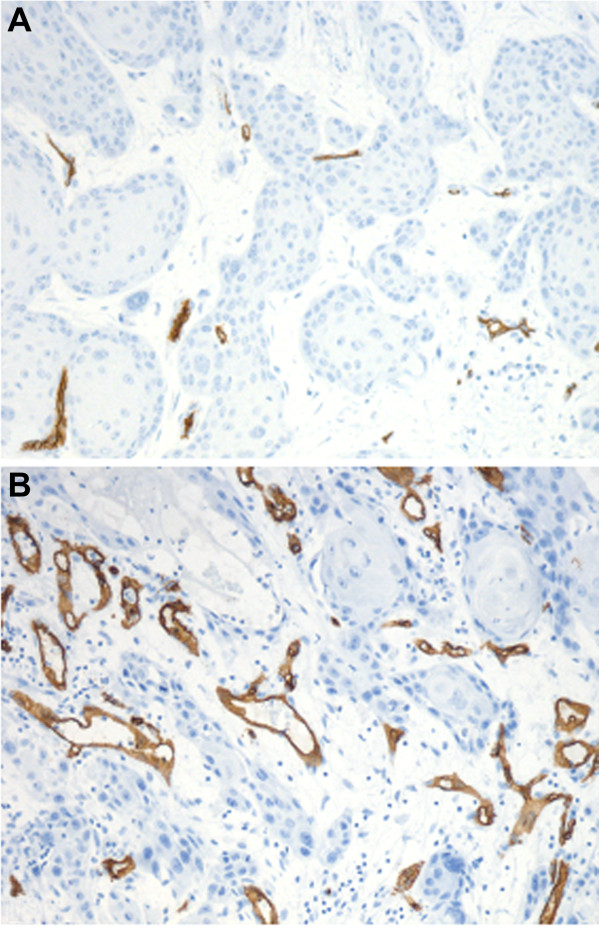
Representative images of CD34 staining of primary vulvar carcinoma vascularization. (A) Low vascularity (low Chalkley count) and (B) High vascularity (high Chalkley count). Images were taken by a Leica DFC 320 digital camera with a Plan-neofluar 10× objective lens in Axiophot microscope (Zeiss Germany).
Figure 2.
Representative images of HIF-1α and VEGF immunoexpression in primary vulvar carcinoma. (A) high HIF-α nuclear expression and (B) low HIF-α nuclear expression (C) high VEGF cytoplasmic staining and (D) low VEGF cytoplasmic staining. 40× objective lens.
CD34 Chalkley count, HIF-1α and VEGF expression in relation to clinicopathological parameters are shown in Table 1. High CD34 Chalkley count was found to correlate significantly with larger tumor diameter (P = 0.002) and deeper invasion (P < 0.001), whereas high VEGF expression correlate significantly with poor tumor differentiation (P = 0.007). High level of HIF-1α was significantly correlated to high CD34 Chalkley counts (P = 0.04). VEGF expression did not show any association with CD34 Chalkley count and HIF-1α levels.
Table 1.
CD34 Chalkley count, HIF-1α and VEGF expression in relation to clinicopathological variables in vulvar carcinomas
|
Variable |
Total |
CD34 Chalkley count |
HIF-1α |
VEGF |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Low | High (%) | P value | Low | High (%) | P value | Low | High (%) | P value | |
| Age |
|
|
|
0.251 |
|
|
0.281 |
|
|
0.681 |
| 25–69 |
59 |
30 |
29 (49) |
|
34 |
25 (42) |
|
36 |
23 (39) |
|
| 70–84 |
81 |
29 |
52 (64) |
|
55 |
26 (32) |
|
46 |
35 (43) |
|
| 85+ |
18 |
8 |
10 (56) |
|
12 |
6 (33) |
|
13 |
5 (28) |
|
| FIGO |
|
|
|
0.672 |
|
|
0.222 |
|
|
0.082 |
| Ia |
0 |
0 |
0 (0) |
|
0 |
0 (0) |
|
0 |
0 (0) |
|
| Ib |
77 |
35 |
42 (55) |
|
51 |
26 (34) |
|
48 |
29 (38) |
|
| II |
7 |
2 |
5 (71) |
|
4 |
3 (43) |
|
7 |
0 (0) |
|
| IIIa |
30 |
14 |
16 (53) |
|
13 |
17 (57) |
|
20 |
10 (33) |
|
| IIIb |
26 |
8 |
18 (69) |
|
18 |
8 (31) |
|
11 |
15 (58) |
|
| IIIc |
7 |
2 |
5 (71) |
|
5 |
2 (29) |
|
4 |
3 (43) |
|
| IVa |
1 |
1 |
0 (0) |
|
1 |
0 (0) |
|
0 |
1 (100) |
|
| IVb |
7 |
3 |
4 (57) |
|
6 |
1 (14) |
|
4 |
3 (43) |
|
| Not available |
3 |
|
|
|
|
|
|
|
|
|
| Lymph node metastasis |
|
|
|
0.213 |
|
|
0.543 |
|
|
0.113 |
| None |
87 |
39 |
48 (55) |
|
58 |
29 (33) |
|
55 |
31 (37) |
|
| Unilateral |
44 |
19 |
25 (57) |
|
25 |
19 (43) |
|
29 |
15 (34) |
|
| Bilateral |
24 |
6 |
18 (75) |
|
15 |
9 (38) |
|
10 |
14 (58) |
|
| Not available |
3 |
|
|
|
|
|
|
|
|
|
| Tumor diameter (cm) |
|
|
|
0.0021 |
|
|
0.951 |
|
|
0.981 |
| 0.3–2.5 |
32 |
19 |
13 (41) |
|
19 |
13 (41) |
|
20 |
12 (38) |
|
| 2.6–4.0 |
51 |
24 |
27 (53) |
|
33 |
16 (31) |
|
30 |
21 (41) |
|
| 4.1–20.0 |
72 |
21 |
51 (71) |
|
44 |
28 (39) |
|
44 |
28 (39) |
|
| Not available |
3 |
|
|
|
|
|
|
|
|
|
| Tumor differentiation |
|
|
|
0.073 |
|
|
0.233 |
|
|
0.0073 |
| Well |
35 |
19 |
16 (46) |
|
26 |
9 (26) |
|
19 |
16 (46) |
|
| Moderate |
92 |
32 |
60 (65) |
|
54 |
38 (41) |
|
64 |
28 (30) |
|
| Poor |
31 |
16 |
15 (48) |
|
21 |
10 (32) |
|
12 |
19 (61) |
|
| Depth of invasion (mm) |
|
|
|
<0.0011 |
|
|
0.581 |
|
|
0.781 |
| 0.0–4.0 |
26 |
19 |
7 (27) |
|
17 |
9 (35) |
|
15 |
11 (42) |
|
| 4.1–8.0 |
56 |
26 |
30 (54) |
|
37 |
19 (34) |
|
37 |
19 (34) |
|
| 8.1–40.0 |
74 |
20 |
54 (73) |
|
45 |
29 (39) |
|
43 |
31 (42) |
|
| Not available |
2 |
|
|
|
|
|
|
|
|
|
| Infiltration of vessel |
|
|
|
0.553 |
|
|
0.063 |
|
|
0.733 |
| No |
116 |
51 |
65 (56) |
|
70 |
46 (40) |
|
72 |
44 (38) |
|
| Yes |
39 |
15 |
24 (62) |
|
30 |
9 (23) |
|
23 |
16 (41) |
|
| Not available | 3 | |||||||||
1Linear-by-linear association.
2Fisher’s exact test.
3Pearson chi-square.
Low: CD34 Chalkley count < 8; High: CD34 Chalkley count ≥ 8.
Low: HIF-1α in ≤ 50% tumor cells; High: HIF-1α in > 50% tumor cells.
Low: VEGF score < 6, High: VEGF score ≥ 6.
In univariate survival analysis, high HIF-1α expression was associated with better disease-specific survival (P = 0.001) (Figure 3), whereas no significant association between CD34 Chalkley counts and VEGF expression and disease-specific survival (P = 0.16 and P = 0.45, respectively) was observed. In multivariate analysis, lymph node metastases, age and HIF-1α expression retained independent prognostic significance (Table 2).
Figure 3.
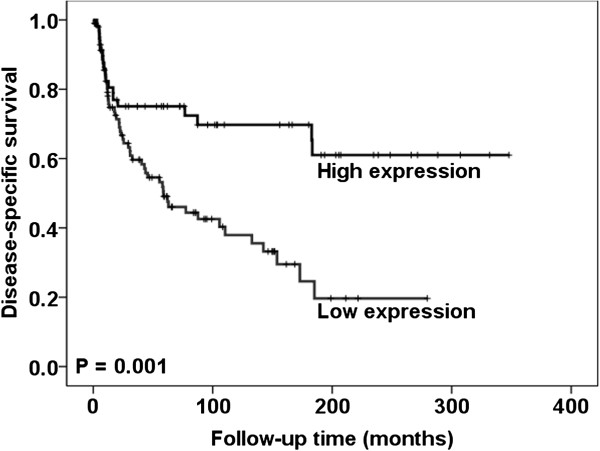
Survival curves using the Kaplan-Meier method. The Kaplan-Meier curve of disease-specific survival in relation to the HIF-1α showed that patients whose tumors expressed low levels of HIF-1α had a worse prognosis than those with high levels.
Table 2.
Relative risk (RR) of dying from vulvar cancer
| Variables |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| RR | 95% CI a | p | RR | 95% CI a | p | |
| Lymph node metastasis |
1.99 |
1.49–2.65 |
<0.001 |
2.28 |
1.69–3.07 |
<0.001 |
| Infiltration of vessel |
2.20 |
1.36–3.58 |
0.001 |
- |
- |
- |
| Age |
1.70 |
1.20–2.41 |
0.003 |
1.92 |
1.31–2.81 |
0.001 |
| Tumor diameter |
1.40 |
1.03–1.91 |
0.03 |
- |
- |
- |
| HIF-1αb | 2.49 | 1.45–4.28 | 0.001 | 2.53 | 1.46–4.37 | 0.001 |
a95% confidence interval.
bLow: ≤ 50% tumor cells and high: > 50% tumor cells.
Discussion
We observed that primary tumor vascularity, quantified by Chalkley method, had a significant association with tumor size and depth of invasion in invasive vulvar carcinomas. Tumor size has been reported to predict local lymph node metastasis [41] and is an important prognostic marker in vulvar cancer patients. Tumor size is at present used to stratify patients into different risk groups and acts as a determinant for surgical treatment [6,7]. In vulvar carcinomas, depth of tumor invasion is also indicative of the aggressiveness of primary tumor and is reported to be associated with lymph node metastases [41] and reduced survival [6]. Inguinofemoral lymph node status is the most powerful indicator of poor prognosis in vulvar cancer [42-44] and a significantly reduced survival in the current study has been confirmed. In the present study, no prognostic significance of tumor vascularity was observed for patients with vulvar carcinoma. This is in accordance with an earlier study on vulvar cancer [20], but in contrast to others [6,15,19]. These conflicting reports on primary tumor vascularity and prognosis might be due to methodological differences, different study cohort or biological factors [6,19,20]. We used the Chalkley counting method for vascular quantification which measures the relative vascular area [45], as recommended in a consensus meeting for quantification of vascularity in solid tumors [39], whereas in other studies microvessels have been counted manually [15,19,20] or using image analyses [6]. Moreover, other studies [6,15,19,20] had analysed smaller number of cases compared to our large series of vulvar carcinomas. Thus, our results of high tumor vascularity associated with larger tumor size and deeper invasion (known pathological markers for tumor aggressiveness) indicates angiogenesis as a marker for the aggressive behaviour of vulvar carcinoma.
HIF-1α, is a crucial molecule in inducing angiogenesis in growing tumor under hypoxic stress [22] and several reports have been published on relation between HIF-1α expression and angiogenesis in head and neck and oesophageal squamous cell carcinoma [24,25]. In present study, we did observe a positive association between HIF-1α expression and CD34 Chalkey count of primary tumor vascularity similar to a report for head and neck squamous cell carcinoma patients [25]. This confirms the role of HIF-1α for the initiation and the promotion of angiogenesis in vulvar cancer. High tumoral HIF-1α expression is reported to be associated with reduced survival in oral, oropharyngeal and cervical cancers [29,32,46]. In contrast, in the present study, a significantly improved survival of vulvar carcinoma patients with high HIF-1α expression was observed as reported for the squamous cell caricnoma in head and neck region, oral cavity and uterine cervix [26,27,30,31]. Other did not find prognostic significance in oesophageal squamous cell carcinoma [47]. Various factors are thought to affect the impact of HIF-1α activation in tumor behaviour [48] including methodology, cut off(s) and treatment modalities [26,27,30-32,47]. Lack of CAIX and Glut-I expression along with high HIF-1α expression in squamous cell carcinoma indicates alternative mechanism for HIF-1α upregulation [26]. Furthermore, we have shown that the patients in good prognosis group had >50% HIF-1α positive tumor cells as reported for its strong expression in squamous cell carcinoma of oral cavity [27]. Diffuse HIF-1α expression based on tumor types and its nonhypoxic activation through various genetic alterations that might result in different outcomes [22,26] may also explain our observation. Vascularization was heterogenously distributed in the tumor including tumor fronts and in the stromal tissue between the islands of tumor cells. Perinecrotic tumor cells distant from the supplying vessels under hypoxic stress express HIF-1α, whereas nonnecrotic tumor shows diffuse expression throughout the tumor including the tumor cells close to the blood vessels [29]. Despite, the heterogenous distribution of vascularity, our observation of positive association between HIF-1α and tumor vascularity suggests the HIF-1α induced angiogenesis. HIF-1α is known to induce expression of various genes including genes linked to cell survival, apoptosis, cellular proliferation [22]. Perhaps, the better outcome in patients with high HIF-1α expressing tumors might be due to its inhibitory role on tumor cells through induction of proapoptotic pathway [49,50].
Hypoxia markers; HIF-1α, GLUT-1, CA IX and VEGF are expressed in both vulvar preneoplastic lesions and invasive squamous cell carcinoma [28]. An increasing expression of VEGF from normal epithelium to premalignant lesion to invasive squamous cell carcinoma was found in vulva [28]. In the present study, high VEGF expression was significantly associated with only poor tumor differentiation, however, an other study reported no such association in vulvar carcinomas [19]. We did not find that the VEGF levels demonstrated prognostic significance, a result being different from a report by Obermair and colleagues [19]. A lack of correlation of VEGF with vascularity observed in the present series which is different from an earlier report [19], might be due to its possible non-angiogenic effects and/or autocrine role on tumor cells [51,52]. Alternative mechanisms of VEGF independent neoangiogenesis by inducing other potent angiogenic molecules like basic fibroblast growth factor and size related proainherent angiogenic effect on the tumor [53] may have resulted in nonsignificant relationship. We noted no association between VEGF and HIF-1α expression possibly due to an alternative non HIF-1α mechanism of VEGF induction [54]. The proangiogenic effect of VEGF is closely related to the tumor size and no impact on angiogenesis is found when tumor reaches to certain size [53].
There are several pitfalls associated to immunohistochemical methods. In addition, the handling of tissue specimens, such as fixation and storage time, may influence the immunohistochemical results [55]. The lack of consideration for these limitations may reduce the usefulness of immunohistochemical studies. In the present study, the fixation and storage time of the tissues did not influence the CD34, HIF-1α and VEGF immunostaining. Both false positive and negative results may limit the outcome of immunohistochemical studies. To reduce the possibility of false negative results we have used the EnVision™ Flex+ detection system reported to have a high sensitivity [56]. Furthermore, we have included positive controls in each run to exclude the possibility of false negative result due to methodological problems. To avoid nonspecific staining we extensively optimalized the dilutions of the primary antibodies used. In addition, negative controls, replacing the primary antibodies with the mouse myeloma protein IgG1, were included to exclude the possibility of false positive results. Despite the effort to quality secure immunostaining processes there are major limitations connected to immunohistochemistry between the studies that are linked to methodological differences including immunostaining procedures and scoring systems [57]. In the future it is clearly needed a standarization of immunohistochemical methodology and scoring systems.
Conclusions
Our results show that high tumor vascularity in vulvar carcinoma is associated with larger tumor size and deeper invasion, indicating that it is a feature of aggressive tumor phenotype. High HIF-1α expression has favorable prognostic impact in vulvar carcinoma patients.
Competing interests
Authors declare that they have no competing interests.
Author’ contributions
HPD participated in the design of the study, quantified tumor vascularity and draft the manuscript. JMN participated in the design of the study, performed systematic pathologic review of vulvar carcinomas and revised the manuscript critically. MF carried out the immunohistochemistry and revised the manuscript critically. CGT collected clinical data, participated in interpretation of data and revised the manuscript critically. RH participated in the design of the study, protein, statistical and data analysis and helped to draft the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Hari Prasad Dhakal, Email: hari.dhakal19@gmail.com.
Jahn M Nesland, Email: j.m.nesland@medisin.uio.no.
Mette Førsund, Email: msq@ous-hf.no.
Claes G Trope, Email: c.g.trope@medisin.uio.no.
Ruth Holm, Email: ruth.holm@oslo-universitetssykehus.no.
Acknowledgements
This work was supported by the Inger and John Fredriksen Foundation for Ovarian Cancer Research and the Norwegian Cancer Society.
References
- Giles GG, Kneale BL. Vulvar cancer: the Cinderella of gynaecological oncology. Aust N Z J Obstet Gynaecol. 1995;35:71–75. doi: 10.1111/j.1479-828X.1995.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Coulter J, Gleeson N. Local and regional recurrence of vulval cancer: management dilemmas. Best Pract Res Clin Obstet Gynaecol. 2003;17:663–681. doi: 10.1016/S1521-6934(03)00050-6. [DOI] [PubMed] [Google Scholar]
- Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstet Gynecol. 1997;90:448–452. doi: 10.1016/S0029-7844(97)00298-6. [DOI] [PubMed] [Google Scholar]
- Messing MJ, Gallup DG. Carcinoma of the vulva in young women. Obstet Gynecol. 1995;86:51–54. doi: 10.1016/0029-7844(95)00101-V. [DOI] [PubMed] [Google Scholar]
- Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Maisonneuve P, Pecorelli S, Odicino F, Heintz AP. Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(1):S7–27. doi: 10.1016/S0020-7292(06)60028-3. [DOI] [PubMed] [Google Scholar]
- Nayha VV, Stenback FG. Increased angiogenesis is associated with poor prognosis of squamous cell carcinoma of the vulva. Acta Obstet Gynecol Scand. 2007;86:1392–1397. doi: 10.1080/00016340701674303. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trope CG, Florenes VA, Suo Z, Nesland JM, Holm R. Overexpression of CDC25B, CDC25C and phospho-CDC25C (Ser216) in vulvar squamous cell carcinomas are associated with malignant features and aggressive cancer phenotypes. BMC Cancer. 2010;10:233. doi: 10.1186/1471-2407-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring SK. Vulvar squamous cell carcinoma: guidelines for early diagnosis and treatment. Am J Obstet Gynecol. 2003;189:S17–S23. doi: 10.1067/S0002-9378(03)00792-0. [DOI] [PubMed] [Google Scholar]
- Stehman FB, Look KY. Carcinoma of the vulva. Obstet Gynecol. 2006;107:719–733. doi: 10.1097/01.AOG.0000202404.55215.72. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Vermeulen PB, Libura M, Libura J, O'Neill PJ, Van DP, Van ME, Van Oosterom AT, Dirix LY. Influence of investigator experience and microscopic field size on microvessel density in node-negative breast carcinoma. Breast Cancer Res Treat. 1997;42:165–172. doi: 10.1023/A:1005737524541. [DOI] [PubMed] [Google Scholar]
- Offersen BV, Borre M, Overgaard J. Quantification of angiogenesis as a prognostic marker in human carcinomas: a critical evaluation of histopathological methods for estimation of vascular density. Eur J Cancer. 2003;39:881–890. doi: 10.1016/S0959-8049(02)00663-9. [DOI] [PubMed] [Google Scholar]
- Dhakal HP, Naume B, Synnestvedt M, Borgen E, Kaaresen R, Schlichting E, Wiedswang G, Bassarova A, Giercksky KE, Nesland JM. Vascularization in primary breast carcinomas: its prognostic significance and relationship with tumor cell dissemination. Clin Cancer Res. 2008;14:2341–2350. doi: 10.1158/1078-0432.CCR-07-4214. [DOI] [PubMed] [Google Scholar]
- Hantschmann P, Jeschke U, Friese K. TGF-alpha, c-erbB-2 expression and neoangiogenesis in vulvar squamous cell carcinoma. Anticancer Res. 2005;25:1731–1737. [PubMed] [Google Scholar]
- Abulafia O, Triest WE, Sherer DM. Angiogenesis in malignancies of the female genital tract. Gynecol Oncol. 1999;72:220–231. doi: 10.1006/gyno.1998.5152. [DOI] [PubMed] [Google Scholar]
- Saravanamuthu J, Reid WM, George DS, Crow JC, Rolfe KJ, MacLean AB, Perrett CW. The role of angiogenesis in vulvar cancer, vulvar intraepithelial neoplasia, and vulvar lichen sclerosus as determined by microvessel density analysis. Gynecol Oncol. 2003;89:251–258. doi: 10.1016/S0090-8258(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Bancher-Todesca D, Obermair A, Bilgi S, Kohlberger P, Kainz C, Breitenecker G, Leodolter S, Gitsch G. Angiogenesis in vulvar intraepithelial neoplasia. Gynecol Oncol. 1997;64:496–500. doi: 10.1006/gyno.1996.4582. [DOI] [PubMed] [Google Scholar]
- Obermair A, Kohlberger P, Bancher-Todesca D, Tempfer C, Sliutz G, Leodolter S, Reinthaller A, Kainz C, Breitenecker G, Gitsch G. Influence of microvessel density and vascular permeability factor/vascular endothelial growth factor expression on prognosis in vulvar cancer. Gynecol Oncol. 1996;63:204–209. doi: 10.1006/gyno.1996.0307. [DOI] [PubMed] [Google Scholar]
- Qureshi F, Munkarah A, Banerjee M, Jacques SM. Tumor angiogenesis in vulvar squamous cell carcinoma. Gynecol Oncol. 1999;72:65–70. doi: 10.1006/gyno.1998.5218. [DOI] [PubMed] [Google Scholar]
- Raspollini MR, Asirelli G, Taddei GL. The role of angiogenesis and COX-2 expression in the evolution of vulvar lichen sclerosus to squamous cell carcinoma of the vulva. Gynecol Oncol. 2007;106:567–571. doi: 10.1016/j.ygyno.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- Kimura S, Kitadai Y, Tanaka S, Kuwai T, Hihara J, Yoshida K, Toge T, Chayama K. Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer. 2004;40:1904–1912. doi: 10.1016/j.ejca.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos C, Turley H, Talks K, Gatter KC, Harris AL. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192–1202. doi: 10.1016/S0360-3016(02)02848-1. [DOI] [PubMed] [Google Scholar]
- Fillies T, Werkmeister R, van Diest PJ, Brandt B, Joos U, Buerger H. HIF1-alpha overexpression indicates a good prognosis in early stage squamous cell carcinomas of the oral floor. BMC Cancer. 2005;5:84. doi: 10.1186/1471-2407-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos M, Mercante AM, Louro ID, Goncalves AJ, de Carvalho MB, da Silva EH, da Silva AM. HIF1-alpha expression predicts survival of patients with squamous cell carcinoma of the oral cavity. PLoS One. 2012;7:e45228. doi: 10.1371/journal.pone.0045228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YZ, Li SL, Li X, Wang LJ, Wang JL, Xu JW, Wu ZH, Gong L, Zhang XD. Expression of endogenous hypoxia markers in vulvar squamous cell carcinoma. Asian Pac J Cancer Prev. 2012;13:3675–3680. doi: 10.7314/APJCP.2012.13.8.3675. [DOI] [PubMed] [Google Scholar]
- Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, Semenza GL. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. [PubMed] [Google Scholar]
- Beasley NJ, Leek R, Alam M, Turley H, Cox GJ, Gatter K, Millard P, Fuggle S, Harris AL. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62:2493–2497. [PubMed] [Google Scholar]
- Hutchison GJ, Valentine HR, Loncaster JA, Davidson SE, Hunter RD, Roberts SA, Harris AL, Stratford IJ, Price PM, West CM. Hypoxia-inducible factor 1alpha expression as an intrinsic marker of hypoxia: correlation with tumor oxygen, pimonidazole measurements, and outcome in locally advanced carcinoma of the cervix. Clin Cancer Res. 2004;10:8405–8412. doi: 10.1158/1078-0432.CCR-03-0135. [DOI] [PubMed] [Google Scholar]
- Lin PY, Yu CH, Wang JT, Chen HH, Cheng SJ, Kuo MY, Chiang CP. Expression of hypoxia-inducible factor-1 alpha is significantly associated with the progression and prognosis of oral squamous cell carcinomas in Taiwan. J Oral Pathol Med. 2008;37:18–25. doi: 10.1111/j.1600-0714.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Jubb AM, Pham TQ, Hanby AM, Frantz GD, Peale FV, Wu TD, Koeppen HW, Hillan KJ. Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin Pathol. 2004;57:504–512. doi: 10.1136/jcp.2003.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doldi N, Origoni M, Bassan M, Ferrari D, Rossi M, Ferrari A. Vascular endothelial growth factor. Expression in human vulvar neoplastic and nonneoplastic tissues. J Reprod Med. 1996;41:844–848. [PubMed] [Google Scholar]
- Knopp S, Trope C, Nesland JM, Holm R. A review of molecular pathological markers in vulvar carcinoma: lack of application in clinical practice. J Clin Pathol. 2009;62:212–218. doi: 10.1136/jcp.2008.057240. [DOI] [PubMed] [Google Scholar]
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Wilkinson EJaTMR. Tumours of the vulva. Epithelial tumours. In: Tavassoli FA, Devilee P, editor. World Health Organization Classification of Tumours. Pathology and genetics of tumors of the breast and female genital organs. Lyon: ARC Press; 2003. pp. 316–325. [Google Scholar]
- Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Belien JA, de Waal RM, Van ME, Magnani E, Weidner N, Harris AL, Dirix LY. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564–1579. doi: 10.1016/S0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Binder SW, Huang I, Fu YS, Hacker NF, Berek JS. Risk factors for the development of lymph node metastasis in vulvar squamous cell carcinoma. Gynecol Oncol. 1990;37:9–16. doi: 10.1016/0090-8258(90)90298-Y. [DOI] [PubMed] [Google Scholar]
- der SS V, De Nieuwenhof HP, Massuger L, Bulten J, De Hullu JA. New FIGO staging system of vulvar cancer indeed provides a better reflection of prognosis. Gynecol Oncol. 2010;119:520–525. doi: 10.1016/j.ygyno.2010.08.036. [DOI] [PubMed] [Google Scholar]
- Woelber L, Eulenburg C, Choschzick M, Kruell A, Petersen C, Gieseking F, Jaenicke F, Mahner S. Prognostic role of lymph node metastases in vulvar cancer and implications for adjuvant treatment. Int J Gynecol Cancer. 2012;22:503–508. doi: 10.1097/IGC.0b013e31823eed4c. [DOI] [PubMed] [Google Scholar]
- Woelber L, Mahner S, Voelker K, Eulenburg CZ, Gieseking F, Choschzick M, Jaenicke F, Schwarz J. Clinicopathological prognostic factors and patterns of recurrence in vulvar cancer. Anticancer Res. 2009;29:545–552. [PubMed] [Google Scholar]
- Fox SB, Leek RD, Weekes MP, Whitehouse RM, Gatter KC, Harris AL. Quantitation and prognostic value of breast cancer angiogenesis: comparison of microvessel density, Chalkley count, and computer image analysis. J Pathol. 1995;177:275–283. doi: 10.1002/path.1711770310. [DOI] [PubMed] [Google Scholar]
- Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–4696. [PubMed] [Google Scholar]
- Shibata-Kobayashi S, Yamashita H, Okuma K, Shiraishi K, Igaki H, Ohtomo K, Nakagawa K. Correlation among 16 biological factors [p53, p21(waf1), MIB-1 (Ki-67), p16(INK4A), cyclin D1, E-cadherin, Bcl-2, TNF-alpha, NF-kappaB, TGF-beta, MMP-7, COX-2, EGFR, HER2/neu, ER, and HIF-1alpha] and clinical outcomes following curative chemoradiation therapy in 10 patients with esophageal squamous cell carcinoma. Oncol Lett. 2013;5:903–910. doi: 10.3892/ol.2013.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K, Searfoss G, Krolikowski D, Pagnoni M, Franks C, Clark K, Yu KT, Jaye M, Ivashchenko Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001;8:367–376. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–6673. [PubMed] [Google Scholar]
- Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ei G, Wang E, Pal K, Dutta SK, Bar-Sagi D, Mukhopadhyay D. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72:3912–3918. doi: 10.1158/0008-5472.CAN-11-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57:3924–3928. [PubMed] [Google Scholar]
- Cao Y, Li CY, Moeller BJ, Yu D, Zhao Y, Dreher MR, Shan S, Dewhirst MW. Observation of incipient tumor angiogenesis that is independent of hypoxia and hypoxia inducible factor-1 activation. Cancer Res. 2005;65:5498–5505. doi: 10.1158/0008-5472.CAN-04-4553. [DOI] [PubMed] [Google Scholar]
- Mighell AJ, Hume WJ, Robinson PA. An overview of the complexities and subtleties of immunohistochemistry. Oral Dis. 1998;4:217–223. doi: 10.1111/j.1601-0825.1998.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Skaland I, Nordhus M, Gudlaugsson E, Klos J, Kjellevold KH, Janssen EA, Baak JP. Evaluation of 5 different labeled polymer immunohistochemical detection systems. Appl Immunohistochem Mol Morphol. 2010;18:90–96. doi: 10.1097/PAI.0b013e3181b0eaad. [DOI] [PubMed] [Google Scholar]
- Warren MV, Chan WY, Ridley JM. Analysis of protein biomarkers in human clinical tumor samples: critical aspects to success from tissue acquisition to analysis. Biomark Med. 2011;5:227–248. doi: 10.2217/bmm.11.9. [DOI] [PubMed] [Google Scholar]