Abstract
Background
It is not clear whether microangiopathies are associated with subclinical atherosclerosis in type 2 diabetes mellitus (T2DM). We investigated the relation of cardiac autonomic neuropathy (CAN) and other microangiopathies with carotid atherosclerosis in T2DM.
Methods
A total of 131 patients with T2DM were stratified by mean carotid intima-media thickness (CIMT) ≥ or <1.0 mm and the number of carotid plaques. CAN was assessed by the five standard cardiovascular reflex tests according to the Ewing's protocol. CAN was defined as the presence of at least two abnormal tests or an autonomic neuropathy points ≥2. Diabetic microangiopathies were assessed.
Results
Patients with CAN comprised 77% of the group with mean CIMT ≥1.0 mm, while they were 29% of the group with CIMT <1.0 mm (P=0.016). Patients with diabetic retinopathy (DR) comprised 68% of the group with CIMT ≥1.0 mm, while they were 28% of the group without CIMT thickening (P=0.003). Patients with CAN comprised 51% of the group with ≥2 carotid plaques, while they were 23% of the group with ≤1 carotid plaque (P=0.014). In multivariable adjusted logistic regression analysis, the patients who presented with CAN showed an odds ratio [OR] of 8.6 (95% confidence interval [CI], 1.6 to 44.8) for CIMT thickening and an OR of 2.9 (95% CI, 1.1 to 7.5) for carotid plaques. Furthermore, patients with DR were 3.8 times (95% CI, 1.4 to 10.2) more likely to have CIMT thickening.
Conclusion
These results suggest that CAN is associated with carotid atherosclerosis, represented as CIMT and plaques, independent of the traditional cardiovascular risk factors in T2DM. CAN or DR may be a determinant of subclinical atherosclerosis in T2DM.
Keywords: Cardiac autonomic neuropathy; Diabetic angiopathies; Carotid intima-media thickness; Carotid plaque; Diabetes mellitus, type 2
INTRODUCTION
The leading cause of death in type 2 diabetes mellitus (T2DM) is cardiovascular disease (CVD) [1]. Recent studies have shown that diabetic microangiopathies are associated with macroangiopathies and suggest that microangiopathies may have a prominent role in the pathogenesis of microangiopathy development [2]. Although the precise underlying mechanisms linking the diabetic microangiopathies and macroangiopathies are unclear, some evidence suggests that the effects of microangiopathies may be linked to subclinical atherosclerosis [3-5].
Carotid atherosclerosis as estimated by carotid intima-media thickness (CIMT) and plaques is considered to reflect an early stage of atherosclerotic disease and is therefore used as a surrogate marker for diabetic macroangiopathies [6-8]. So far, studies on the relationship between diabetic microangiopathies and carotid atherosclerosis have yielded inconsistent results.
Cardiac autonomic neuropathy (CAN) is one of the most common complications of diabetes, and represents a significant cause of morbidity and mortality in diabetic patients. Although the pathophysiological mechanism of this increased mortality is unclear, it may be related to silent myocardial ischemia and subclinical coronary atherosclerosis [9,10]. However, studies on the relationship between CAN and carotid atherosclerosis are few [11,12].
Aassociations between carotid atherosclerosis and both urine albumin excretion (UAE) and glomerular filtration rate (GFR) in diabetic patients have been investigated, but these studies showed only inconsistent results [5,13,14]. Additionally, it is known that the presence of diabetic retinopathy (DR) is a marker for an increased risk of clinical CVD events [15,16]. Several studies have investigated the association between CIMT and DR. Some studies have reported that the presence of DR was associated with high CIMT [17,18], whereas other studies did not find any significant association between DR and CIMT [19]. However, these associations have not been adequately examined, particularly in the Korean population.
Therefore, the aim of this study was to investigate the relationship between CAN and carotid atherosclerosis in Korean T2DM patients. We also examined the relationship of other diabetic microangiopathies to carotid atherosclerosis.
METHODS
Study subjects
A total of 131 T2DM patients (mean age, 56 years) who visited Soonchunhyang University Bucheon Hospital from January 2009 to February 2011 were enrolled. Exclusion criteria were: malignancy, hepatic failure, acute infection, acute metabolic complications, fatal arrhythmia, and CVD such as acute coronary syndrome and previous myocardial infarction. We retrospectively reviewed detailed demographic data and clinical and treatment history using medical records. Because this study was a retrospective study based on the analysis of existing data from medical records, we did not need to obtain institutional review board approval.
Measurement of carotid atherosclerosis
Carotid atherosclerosis was assessed by the use of high-resolution B-mode ultrasonography (SSA-660A, Toshiba, Tokyo, Japan) performed with an ultrasound scanner equipped with a 12-MHz linear-array transducer. Intima-media thickness (IMT) measurements were performed on the right and left common carotid arteries 1.0 cm proximal to the origin of the bulb and the mean IMT values were calculated. Carotid IMT thickening was defined as mean CIMT ≥1.0 mm [20,21]. Protrusions into the lumen that were 100% thicker than the nearest area were defined as plaques. The plaque was dichotomized according to the number of carotid plaques (≤2 or >2).
Cardiac autonomic function test
Autonomic function tests were performed by the same operator. Patients were advised to abstain from alcohol and tobacco during the 24 hours prior to the test. Patients who underwent testing had also fasted for at least 3 hours prior to the test, with no coffee or caffeine. In addition, medications such as antihistamines, antidepressants, and β-blockers were withheld for 12 hours prior to the test [22].
CAN was assessed by five standard cardiovascular (CV) reflex tests according to Ewing's protocol [23,24]. Three of these measurements assess parasympathetic function: heart rate responses to deep breathing (beat to beat variation), to standing (30:15 ratio), and to the Valsalva maneuver. The other two tests assess sympathetic function: blood pressure responses to standing and a sustained handgrip. The heart rate response to deep breathing, standing, and the Valsalva maneuver were assessed automatically from electrocardiography recordings using the DICAN evaluation system (Medicore Co., Ltd., Seoul, Korea). The severity of CAN was quantified by summing the points obtained from each of the five tests, where each test scored with 0, 0.5, or 1 points depending on whether it yielded normal, borderline, or abnormal values, respectively (Table 1). Consequently, the minimum and maximum autonomic neuropathy points were 0 and 5, respectively. CAN was defined as the presence of at least two abnormal tests or autonomic neuropathy points ≥2 [25].
Table 1.
Reference Values of the Five Autonomic Function Tests to Detect Cardiac Autonomic Neuropathy and the Scores Obtained for Each in the Patients Expressed as Points
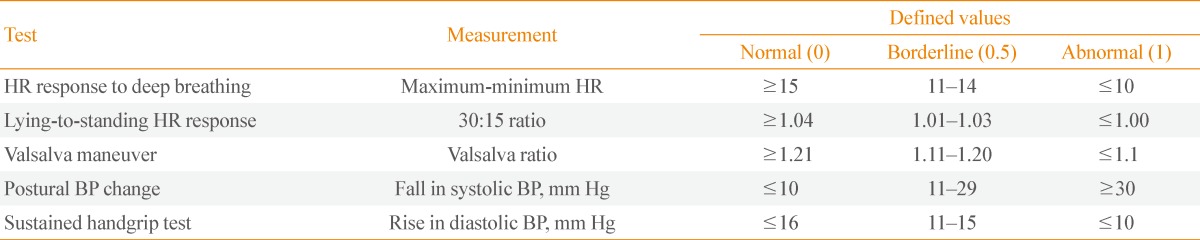
HR, heart rate; BP, blood pressure.
The CAN score was categorized as follows: CAN score 0 (total points 0), CAN score 1 (points 0.5 to 1.5), CAN score 2 (points 2 to 3), and CAN score 3 (points ≥3.5). CAN was considered absent, early, definite, or severe if the CAN scores were 0, 1, 2, or 3, respectively.
Evaluation of diabetic microvascular complications
DR was defined based on fundoscopy findings through dilated pupils by an ophthalmologist. DR was classified as normal, nonproliferative, and proliferative retinopathy. The patients were assigned to one of the two groups: no evidence of DR and presence of DR at any stage. Diabetic nephropathy was defined using albuminuria and urinary albumin was measured by radioimmunoassay. An albumin excretion rate (AER) <20 µg/min or urine albumin <30 mg/g creatinine was categorized as normoalbuminuria, AER in the range of 20 to 200 µg/min or urine albumin 30 to 300 mg/g creatinine as microalbuminuria, and >200 µg/min or urine albumin ≥300 mg/g creatinine as overt proteinuria. Kidney function was assessed using the estimated glomerular filtration rate (eGFR), which was calculated by the simplified Modification of Diet in Renal Disease (MDRD) formula. MDRD is a noninvasive method used to calculate eGFR, which is an established parameter used to analyze renal function in epidemiological settings [26]. We calculated the MDRD formula as follows: 186.3×(serum creatinine-1.154)×(age-0.203)×0.742 (if female), with the serum creatinine concentration expressed in mg/dL.
Patients were considered to have nephropathy if they showed microalbuminuria or overt proteinuria. Assessment of peripheral neuropathy was based on typical symptoms and a current perception threshold or nerve conduction study.
An automated device (VP-1000, Colin, Komaki, Japan) was used to measure brachial-ankle pulse wave velocity (baPWV), which we defined as the mean baPWV of both sides, and ankle-brachial index. The insulin resistance status was evaluated by the homeostasis model assessment-insulin resistance (HOMA-IR) index, which was calculated by the formula: (fasting insulin [µIU/mL]×fasting blood glucose [mmol/L])/22.5. Serum C-peptide levels were measured by radioimmunoassay (Immunotech, Prague, Czech Republic).
Statistical analysis
Statistical analysis was performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). Descriptive data are shown as mean±SD for continuous variables, number and percentage for categorical variables, and median for nonnormal distributed variables. The categorical variables of the groups were compared using the chi-square test. The significance of the mean differences between patients with mean CIMT ≥1.0 and <1.0 mm were evaluated with Student t test or the nonparametric Mann-Whitney U test. The significance of the mean differences between patients with number of plaque <2 and ≥ 2 was also evaluated with Student t test or the nonparametric Mann-Whitney U test. Correlation between levels of mean CIMT and other clinical parameters was analyzed by Spearman rank correlation analysis. Differences of the mean levels of CIMT according to the severity of CAN were evaluated with one-way analysis of variance (ANOVA) and post hoc analysis using the Bonferroni test.
The association between diabetic microvascular complications (CAN and other variables) and the presence of carotid atherosclerosis (CIMT thickening or carotid plaques) was analyzed using multivariate logistic regression. The selection of variables for use in the multivariate model was based on univariate analysis. However, only eGFR was used in the multiple logistic regression analysis model because of its close collinearity with serum creatinine using Pearson correlation. Also, we selected the following variables for the multiple logistic regression analysis, which are well known as factors associated with carotid atherosclerosis: age, hypertension (HTN), smoking, duration of diabetes, and use of statin or angiotensin converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB). A P<0.05 was considered significant.
RESULTS
Clinical characteristics of the subjects according to the mean CIMT
The mean age and duration of diabetes of all subjects were 55.6±11.3 and 7.4±6.8 years, respectively. The mean CIMT was 0.8 mm and 24.7% of the subjects showed the CIMT thickening (≥1.0 mm). The proportion of patients with ≥2 carotid plaques was 28.2%. A total of 85 (65%) of the 131 study participants were using a statin and 66 (50%) were using ACEI or/and ARB. Table 2 shows the clinical characteristics of the subjects according to the mean CIMT. The patients with mean CIMT ≥1.0 mm showed significantly older ages and a higher prevalence of HTN compared with patients with CIMT <1.0 mm (P=0.002 and P=0.01, respectively). Patients with CAN comprised 77% of the group with mean CIMT ≥1.0 mm, but only 29% of the group with CIMT <1.0 mm (P=0.016). Patients with DR comprised 68% of the group with CIMT ≥1.0 mm, but only 28% of the group without CIMT thickening (P=0.003). The patients with mean CIMT ≥1.0 mm comprised 31% of the group with DR, but only 11% of the group without DR (P=0.011). The patients with mean CIMT ≥1.0 mm comprised 23% of the group with CAN, but only 6% of the group without CAN (P=0.031) (data not shown). However, there were no differences in the prevalence of nephropathy, peripheral neuropathy and statin use, or nephropathy and neuropathy between subjects with mean CIMT ≥1.0 or <1.0 mm. No differences in other variables such as duration of diabetes, hemoglobin A1c (HbA1c), lipid profiles, eGFR, HOMA-IR, or baPWV were observed between the two groups.
Table 2.
General Characteristics of the Subjects according to the Carotid Intima-Media Thickness
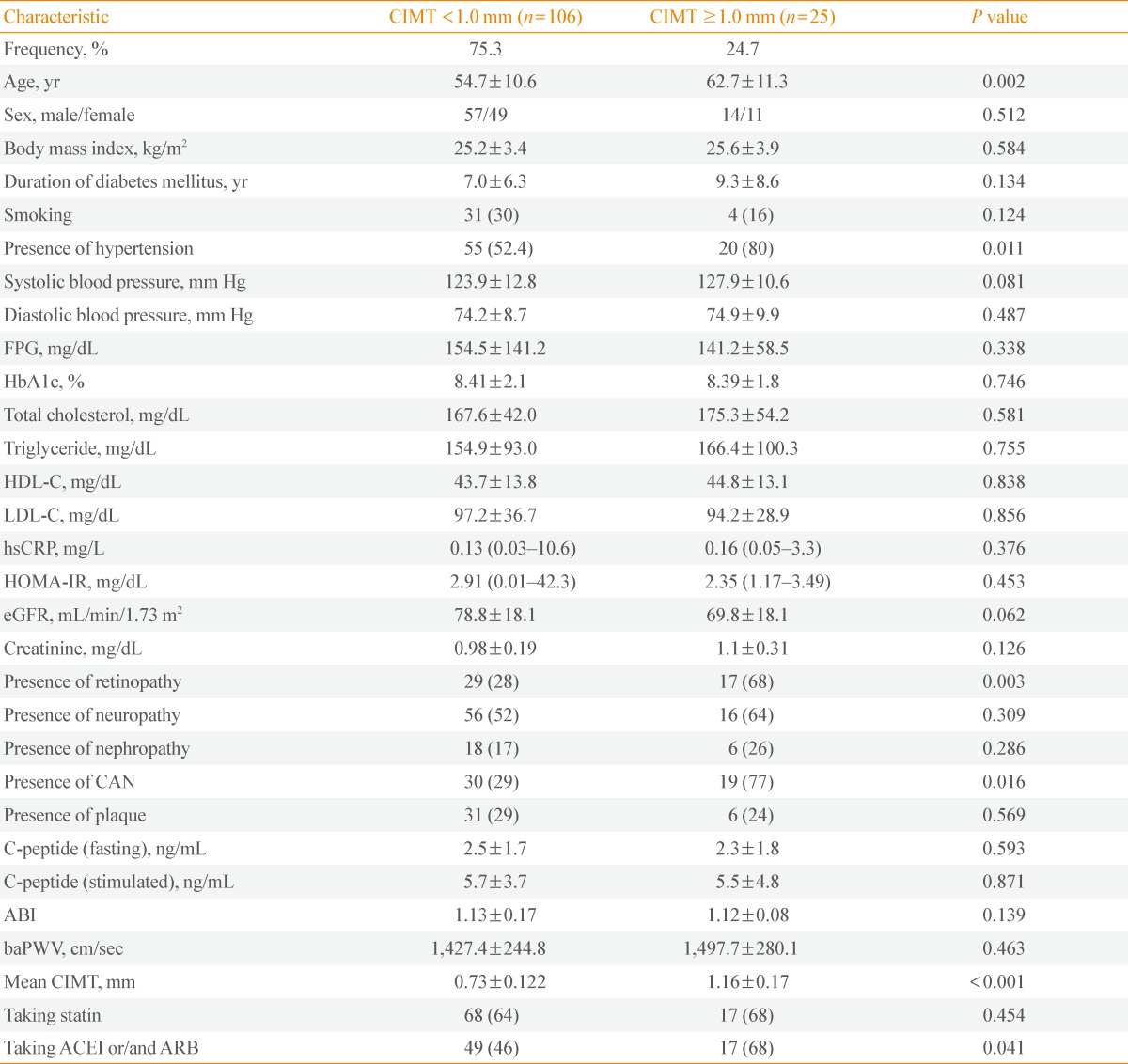
Values are expressed as mean±SD, number (%), or mean (95% confidence interval).
CIMT, carotid intima-media thickness; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment-insulin resistance; eGFR, estimated glomerular filtration rate; CAN, cardiac autonomic neuropathy; ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Clinical characteristics of the subjects according to amount of carotid plaques
The patients with ≥2 carotid plaques were significantly older and had significantly lower body mass index (BMI), lower diastolic blood pressure, lower high density lipoprotein cholesterol, and lower fasting C-peptide (P=0.007, P=0.003, P=0.008, P=0.048, P=0.014, respectively) (Table 3). Patients with CAN comprised 51% of the group with ≥2 carotid plaque, but only 23% of the group with ≤1 carotid plaque (P=0.014) (Table 3). The patients with ≥2 carotid plaques comprised 53% of the group with CAN, but only 24% of the group without CAN (P=0.01). However, the prevalence of patients with plaques ≥2 according to the presence of DR was not significantly different (P=0.079) (data not shown).
Table 3.
The General Characteristics of the Subjects according to the Plaques
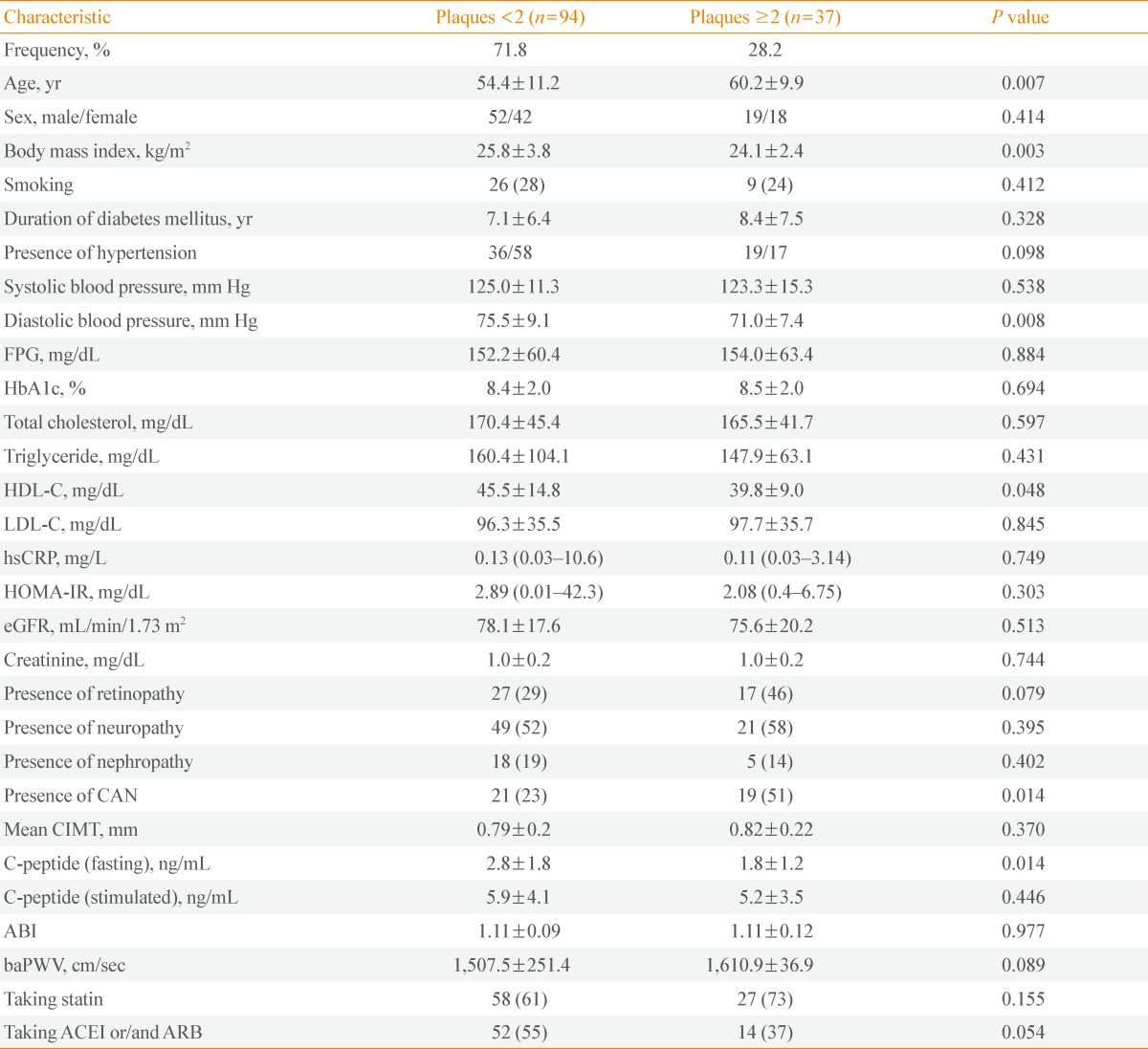
Values are expressed as mean±SD, number (%), or mean (95% confidence interval).
FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment-insulin resistance; eGFR, estimated glomerular filtration rate; CAN, cardiac autonomic neuropathy; CIMT, carotid intima-media thickness; ABI, ankle-brachial index; baPWV, brachial-ankle pulse wave velocity; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Correlation analyses between CIMT and clinical variables
Table 4 shows the correlation between CIMT, carotid plaques, and clinical variables, including the CAN score. Mean CIMT levels positively correlated with serum creatinine, baPWV, and CAN score and negatively correlated with eGFR. The number of carotid plaques positively correlated with age and CAN score and negatively correlated with BMI, diastolic blood pressure, and fasting C-peptide (data not shown).
Table 4.
Correlation Analysis between Carotid Intima-Media Thickness and Clinical Variables Including Diabetic Microvascular Complications
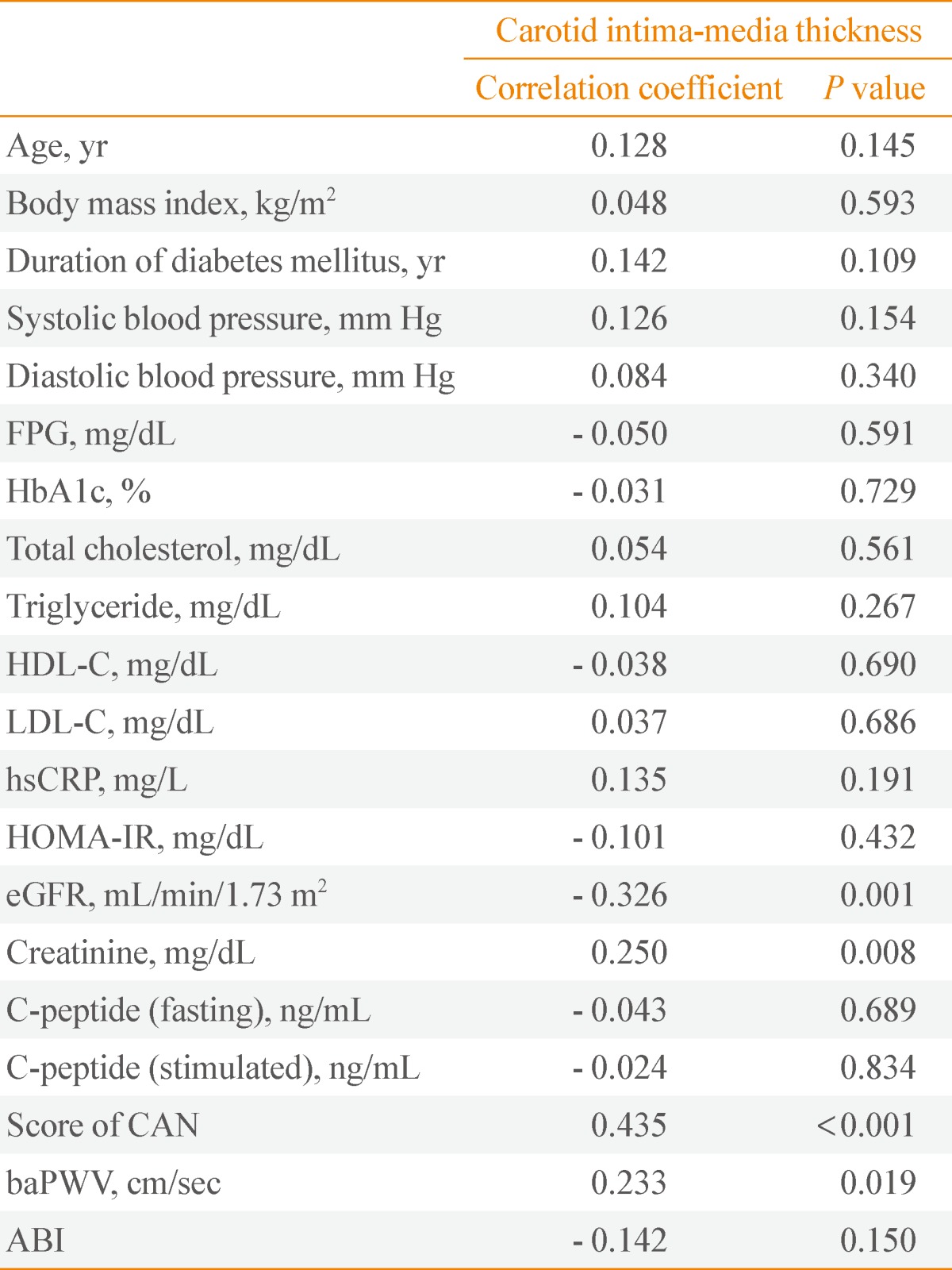
FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment-insulin resistance; eGFR, estimated glomerular filtration rate; CAN, cardiac autonomic neuropathy; baPWV, brachial-ankle pulse wave velocity; ABI, ankle-brachial index.
The mean CIMT levels according to CAN severity showed significant increases by one-way ANOVA analysis (P<0.001) (Table 5). According to the post hoc analysis using the Bonferroni test, mean CIMT levels between the CAN-absent group and the early CAN group were not different (P=0.94). Additionally, mean CIMT levels between the definite and severe CAN groups showed insignificant differences (P=0.4). Except in the above cases of two, remaining cases among four groups showed statistically significant different results (data not shown).
Table 5.
The Mean Levels of Carotid Intima-Media Thickness according to the Severity of the Cardiac Autonomic Neuropathy
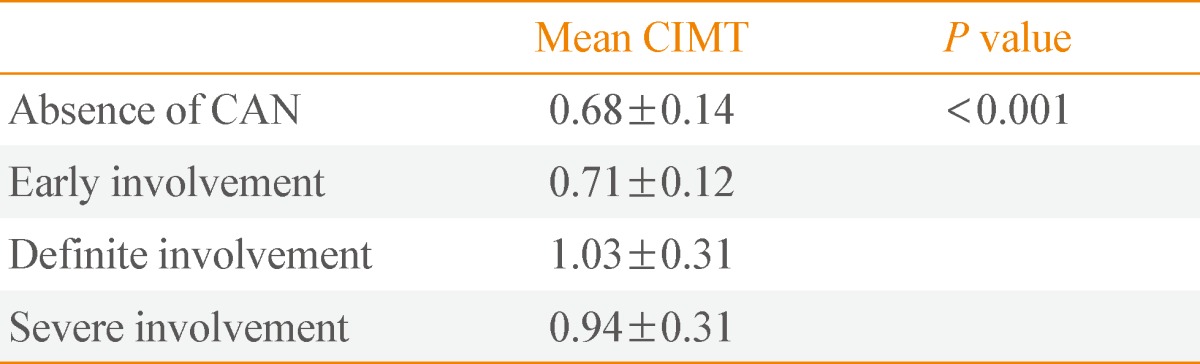
Values are expressed as mean±SD.
IMT, carotid intima-media thickness; CAN, cardiac autonomic neuropathy.
Multivariate logistic regression analysis: the adjusted effect of variables on carotid atherosclerosis
Multivariate logistic regression analysis was performed with carotid atherosclerosis (CIMT thickening, carotid plaques) as the dependent variable (Table 6). In multivariate adjusted logistic regression analysis, the patients with CAN exhibited an odds ratio (OR) of 8.6 (95% confidence interval [CI], 1.6 to 44.8) for CIMT thickening and an OR of 2.9 (95% CI, 1.1 to 7.5) for carotid plaques. Moreover, patients with DR were 3.8 (95% CI, 1.4 to 10.2) times more likely to have CIMT thickening after multivariate adjustment.
Table 6.
Multivariate Logistic Regression Analysis on the Various Risk Factors for Carotid Intima-Media Thickness Thickening and Cartoid Plaques
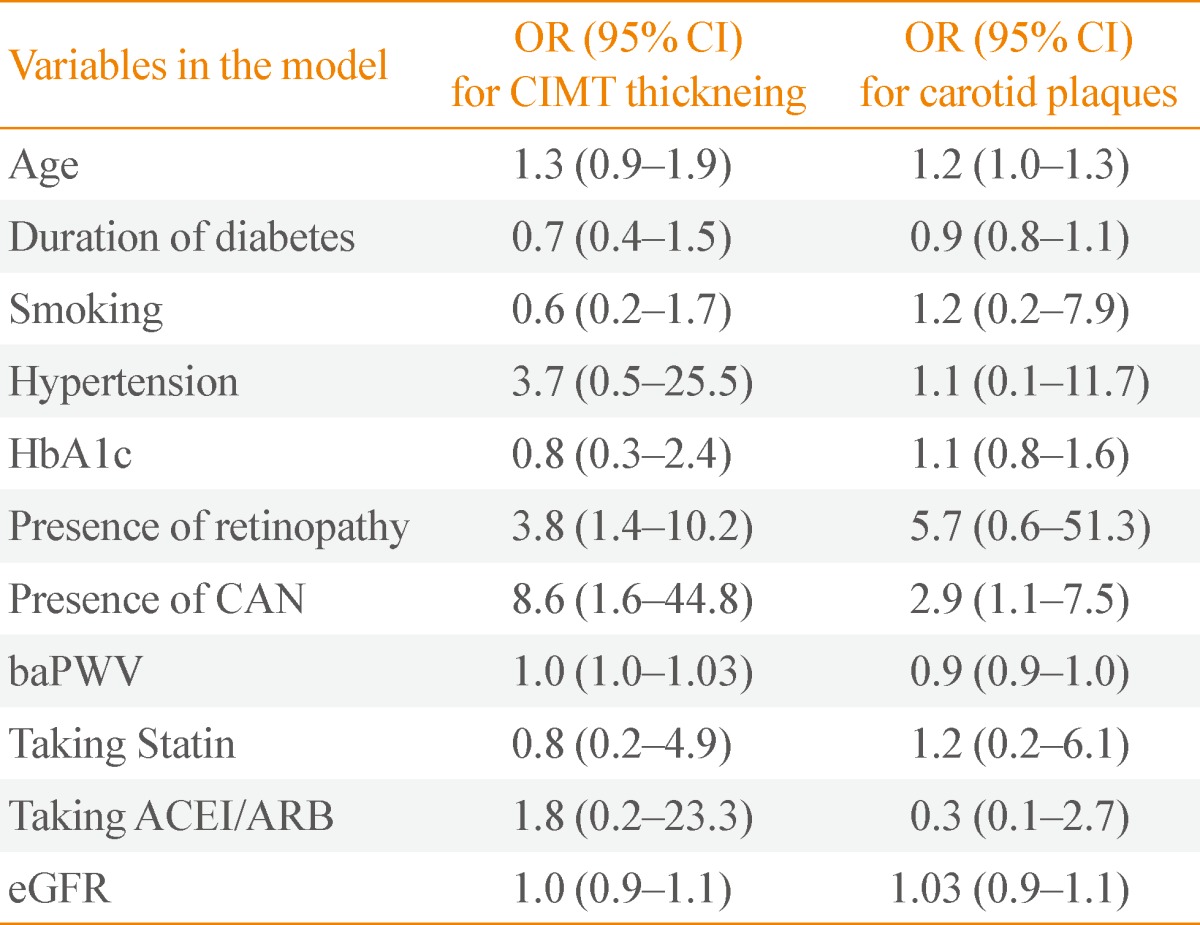
OR, odds ratio; CI, confidence interval; CIMT, carotid intima-media thickness; HbA1c, hemoglobin A1c; CAN, cardiac autonomic neuropathy; baPWV, brachial-ankle pulse wave velocity; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate.
Age was weakly associated with presence of carotid plaques. Duration of diabetes, smoking, HTN, HbA1c, statin use, ACEI/ARB use, and eGFR were not associated with carotid atherosclerosis.
DISCUSSION
In this study, the presence of CAN is associated with carotid atherosclerosis representative as CIMT and carotid plaque independently of traditional CV risk factors. Our results suggest that CAN may be an important determinant of subclinical atherosclerosis in T2DM. This finding of the association between CAN and vascular dysfunction may help to explain the excess CV mortality seen in those with T2DM subjects with CAN.
CIMT is an established marker for subclinical atherosclerotic disease in general population and diabetes [6,27]. In this study, the mean levels of CIMT showed significantly positive correlation with score of CAN. Also, although severe CAN group showed lower mean CIMT levels than definite CAN group, the mean CIMT levels according to the severity of the CAN were increased significantly. The reasons why the mean CIMT levels in severe CAN group are lower than those of definite CAN group are unclear.
Although several studies have reported that association between CIMT and other diabetic microangiopathies, only a few studies reported the relationship between CAN and CIMT in our knowledge. Majority of previous studies to examine association between CAN and carotid atherosclerosis were those by Gottsater's group. They assessed CAN by expiration/inspiration (E/I) ratio or age-adusted acceleration index (AI) [12,28,29]. They reported that patients with abnormal E/I ratio or AI showed increased degree of stenosis in the common carotid artery (CCA). Also, they reported that low frequency (LF) heart rate variability (HRV) was negatively associated with the extent and progression of carotid atherosclerosis. This present study showed that LF/high frequency (HF) upright HRV power was correlated with the mean CIMT (r=-0.234, P=0.042) and HF supine was correlated with number of plaque (r=-0.270, P=0.023) (data not shown). To the best of my knowledge, only one study was reported using CAN score for assessment of CAN like this present study [11]. Contrary to our study, in the study by Meyer et al. [11] CIMT and CAN score showed no significant correlation.
Several studies about DR as an independent risk factor for the presence of atherosclerosis in T2DM were reported [15,16]. In our present study, DR was associated with increased CIMT. This result is consistent with other previous studies [4,17]. Malecki et al. [4] found that IMT was higher in patients with DR than those without DR and DR was among the predictors of increased IMT in multiple regression analysis. However, the Carodiovascular Health Study (CHS) didn't show a relationship between CIMT or plaque and DR [30]. In the present study, the frequency of patients with the plaque ≥2 according to the presence of DR was not significantly different. Miyamoto et al. [31] reported that mean CIMT and plaque score were not significantly correlated with DR. Rema et al. [18] explained that one of the causes showing discrepancy about association DR with carotid atherosclerosis is by the differences in the ages of the populations studied (Atherosclerosis Risk in Communities study, 51 to 72 years of age; CHS, 69 to 102 years of age; our present study, 31 to 86 years of age). The other potential reasons for the discrepant results may include different sample sizes and population characteristics. The exact mechanisms underlying the association between carotid atherosclerosis and DR are poorly understood. One possible explanation is that atherosclerosis and DR share common risk factors in the causal pathway. The retinal ischemia can be compensated by retinal blood vessels dilated via a local chan ge of the capillary circulation, and recent evidence on DR suggests the involvement of the circulation of larger vessels, including the CCA [4,18]. Malecki et al. [4] suggested that a potential common denominator beyond the classical risk factors of both DR and increased IMT is endothelial dysfunction.
Other factors that are relatively well-known to correlate with carotid atherosclerosis, such as smoking, HTN, and duration of diabetes, didn't show significant association with carotid atherosclerosis in our study. HTN was associated with CIMT thickening in univariate analysis, but its significant association disappeared after multivariate analysis. These results are inconsistent with those of previous studies, which generally showed that these factors are associated with carotid atherosclerosis [32,33]. The evidence relating glucose level to carotid atherosclerosis is less clear and inconclusive. Only a few studies demonstrated relationship with fasting glucose [34].
It is well-known that statin affects carotid atherosclerosis [35]. The proportions of patients taking statin were not significantly different between groups with or without CIMT thickening and plaque. Also, in multivariate adjusted logistic regression analysis, statin use was not associated with carotid atherosclerosis. Also, several reports suggest that drugs inhibiting renin-angiotensin system may have inhibitory effects on atherosclerotic process [36]. However, in our present study, ACEI/ARB did not have any association on carotid atherosclerosis. Wang et al. [37] suggested by meta-analysis that drugs inhibiting RAS may not have any significant effect on IMT at least for a relatively short period such as 3 months or 1 year. Therefore, duration of taking drugs may affect progression of atherosclerosis.
Our study showed that the CIMT was not different according to the presence or absence of the diabetic nephropathy, but that it was negatively correlated with the eGFR. Our finding is in agreement with previous studies [13,38]. Although the predictive value of microalbuminuria for atherosclerotic vascular disease in T2DM patients has been in several studies, an association between the IMT and diabetic nephropathy based on the UAE showed a discrepancy [5,13,39]. The reasons for this discrepancy are not clear; in a part, this might be explained by the differences in the patients factor such as age and duration of DM. Further studies are needed to know the reasons.
Also, the CIMT was not different between the patients with and without diabetic neuropathy in this study. This result is in accordance with the study by Ito et al. [13].
In this study, nor carotid plaque or CIMT have significant association with HOMA-IR as marker of insulin resistance. Results in previously published studies on the relationship between IR and early carotid atherosclerotic lesions are equivocal [40,41]. In addition, few studies have examined the possible relation between carotid plaque and IR. Shinozaki et al. [42] reported that HOMA-IR was associated with carotid plaque but not with CIMT. It is not known the exact reason why HOMA-IR was associated with only carotid plaque, but a possible explanation may be that different risk factors may affect only carotid plaque or CIMT if the pathological features differ, at least in part [43].
Also, consistent with other studies, the CIMT positively correlated with the arterial stiffness representing as baPWV [5,13]. PWV reflects arterial stiffness and is a marker of atherosclerotic vascular disease in patients with diabetes [44]. Although the measures of IMT and PWV may assess the different aspects of the atherosclerotic process, both an CIMT thickening and an increased baPWV are likely to reflect the early atherosclerosis [45,46].
The reason why previous published studies have been inconsistent concerning the association carotid atherosclerosis and microangiopathies is not clear. Some possible explanations can be suggested. First, the discrepancy may be due to differences of study populations according to the various diagnostic criteria about carotid atherosclerosis. Moreover, definition for the presence of the significant CIMT thickness and plaque were different each study. Also, it depends on using a certain method among several methods such as IMT, plaque, and carotid artery diameter to assessing carotid atherosclerosis. Which ultrasonic parameter may be superior than other parameters for evaluating the carotid atherosclerosis with regard to their clinical relevance will need to be confirmed in further studies. Second, different distributions of prevalence and severity of microangiopathies in the study participants may be another reason. Differences between studies with regard to eligibility criteria, i.e., whether patients with advanced or mild complications have been excluded or not.
Limitations of our study should be addressed. First, because of the cross-sectional design, we cannot determine the causative relationship between carotid atherosclerosis, DR and CAN. Prospective studies are required to address this important question. Second, because our study population consisted of individuals who received comprehensive examinations for the complications of diabetes, we can assume that these patients had relatively good compliance. Therefore, some characteristics of the present study population may be substantially different from those of other patients who did not undergo studies of complication. Therefore, the generalizability of our study may be limited. Third, the present study included small numbers of subjects with advanced microangiopathies. If the study was performed in a larger number of patients with microangiopathies, the results could have more impact in support of the association between diabetic microangiopathies and carotid atherosclerosis.
However, our study is meaningful in that this is the first study in Korean T2DM patients for the evaluation of relationship between carotid atherosclerosis and all diabetic microangiopathies including CAN.
In conclusion, this present study showed that the presence of CAN is associated with carotid atherosclerosis representative as CIMT and carotid plaque. Also, DR is associated with increased CIMT. A further prospective follow up of our cohort with increasing duration of T2DM will reveal whether the carotid atherosclerosis associated with CAN or DR will translate into an increased risk for clinically relevant atherosclerotic events such as stroke and coronary heart disease.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Geiss LS, Herman WH, Smith PJ National Diabetes Data Group. Diabetes in America. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney diseases; 1995. pp. 233–257. [Google Scholar]
- 2.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S54–S64. doi: 10.1007/pl00002940. [DOI] [PubMed] [Google Scholar]
- 3.de Kreutzenberg SV, Coracina A, Volpi A, Fadini GP, Frigo AC, Guarneri G, Tiengo A, Avogaro A. Microangiopathy is independently associated with presence, severity and composition of carotid atherosclerosis in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:286–293. doi: 10.1016/j.numecd.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Malecki MT, Osmenda G, Walus-Miarka M, Skupien J, Cyganek K, Mirkiewicz-Sieradzka B, damek-Guzik TA, Guzik TJ, Sieradzki J. Retinopathy in type 2 diabetes mellitus is associated with increased intima-media thickness and endothelial dysfunction. Eur J Clin Invest. 2008;38:925–930. doi: 10.1111/j.1365-2362.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama H, Aoki T, Imahori M, Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 2004;66:448–454. doi: 10.1111/j.1523-1755.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 6.Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. doi: 10.1161/01.cir.96.5.1432. [DOI] [PubMed] [Google Scholar]
- 7.Fleg JL, Mete M, Howard BV, Umans JG, Roman MJ, Ratner RE, Silverman A, Galloway JM, Henderson JA, Weir MR, Wilson C, Stylianou M, Howard WJ. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008;52:2198–2205. doi: 10.1016/j.jacc.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyman RA, Fraizer MC, Keevil JG, Busse KL, Aeschlimann SE, Korcarz CE, Stein JH. Ultrasound-detected carotid plaque as a screening tool for advanced subclinical atherosclerosis. Am Heart J. 2005;150:1081–1085. doi: 10.1016/j.ahj.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Maser RE, Lenhard MJ. Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab. 2005;90:5896–5903. doi: 10.1210/jc.2005-0754. [DOI] [PubMed] [Google Scholar]
- 10.Gerritsen J, Dekker JM, TenVoorde BJ, Kostense PJ, Heine RJ, Bouter LM, Heethaar RM, Stehouwer CD. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care. 2001;24:1793–1798. doi: 10.2337/diacare.24.10.1793. [DOI] [PubMed] [Google Scholar]
- 11.Meyer C, Milat F, McGrath BP, Cameron J, Kotsopoulos D, Teede HJ. Vascular dysfunction and autonomic neuropathy in Type 2 diabetes. Diabet Med. 2004;21:746–751. doi: 10.1111/j.1464-5491.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- 12.Gottsater A, Ahlgren AR, Taimour S, Sundkvist G. Decreased heart rate variability may predict the progression of carotid atherosclerosis in type 2 diabetes. Clin Auton Res. 2006;16:228–234. doi: 10.1007/s10286-006-0345-4. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Komatsu Y, Mifune M, Antoku S, Ishida H, Takeuchi Y, Togane M. The estimated GFR, but not the stage of diabetic nephropathy graded by the urinary albumin excretion, is associated with the carotid intima-media thickness in patients with type 2 diabetes mellitus: a cross-sectional study. Cardiovasc Diabetol. 2010;9:18. doi: 10.1186/1475-2840-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomura M, Kasami R, Ohashi M, Yamada Y, Abe H. Significantly higher incidence of carotid atherosclerosis found in Japanese type 2 diabetic patients with early nephropathy. Diabetes Res Clin Pract. 2004;66(Suppl 1):S161–S163. doi: 10.1016/j.diabres.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Retinopathy predicts cardiovascular mortality in type 2 diabetic men and women. Diabetes Care. 2007;30:292–299. doi: 10.2337/dc06-1747. [DOI] [PubMed] [Google Scholar]
- 16.Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2007;30:1742–1746. doi: 10.2337/dc07-0264. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, Brancati FL, Hubbard LD, Couper D ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes: the atherosclerosis risk in communities study. Ophthalmology. 2002;109:1225–1234. doi: 10.1016/s0161-6420(02)01074-6. [DOI] [PubMed] [Google Scholar]
- 18.Rema M, Mohan V, Deepa R, Ravikumar R Chennai Urban Rural Epidemiology Study-2. Association of carotid intima-media thickness and arterial stiffness with diabetic retinopathy: the Chennai Urban Rural Epidemiology Study (CURES-2) Diabetes Care. 2004;27:1962–1967. doi: 10.2337/diacare.27.8.1962. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki R, Cheung N, Islam FM, Klein R, Klein BE, Cotch MF, Sharrett AR, O'Leary D, Wong TY Multi-Ethnic Study of Atherosclerosis. Is diabetic retinopathy related to subclinical cardiovascular disease? Ophthalmology. 2011;118:860–865. doi: 10.1016/j.ophtha.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G. Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151:478–487. doi: 10.1093/oxfordjournals.aje.a010233. [DOI] [PubMed] [Google Scholar]
- 21.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 22.Valensi P, Paries J, Attali JR French Group for Research and Study of Diabetic Neuropathy. Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications: the French multicenter study. Metabolism. 2003;52:815–820. doi: 10.1016/s0026-0495(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 23.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 24.Bellavere F, Bosello G, Fedele D, Cardone C, Ferri M. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1983;287:61. doi: 10.1136/bmj.287.6384.61-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien IA, O'Hare JP, Lewin IG, Corrall RJ. The prevalence of autonomic neuropathy in insulin-dependent diabetes mellitus: a controlled study based on heart rate variability. Q J Med. 1986;61:957–967. [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 28.Gottsater A, Ryden-Ahlgren A, Szelag B, Hedblad B, Persson J, Berglund G, Wroblewski M, Sundkvist G. Cardiovascular autonomic neuropathy associated with carotid atherosclerosis in Type 2 diabetic patients. Diabet Med. 2003;20:495–499. doi: 10.1046/j.1464-5491.2003.00956.x. [DOI] [PubMed] [Google Scholar]
- 29.Gottsater A, Szelag B, Berglund G, Wroblewski M, Sundkvist G. Changing associations between progressive cardiovascular autonomic neuropathy and carotid atherosclerosis with increasing duration of type 2 diabetes mellitus. J Diabetes Complications. 2005;19:212–217. doi: 10.1016/j.jdiacomp.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Marino EK, Kuller LH, Polak JF, Tracy RP, Gottdiener JS, Burke GL, Hubbard LD, Boineau R. The relation of atherosclerotic cardiovascular disease to retinopathy in people with diabetes in the Cardiovascular Health Study. Br J Ophthalmol. 2002;86:84–90. doi: 10.1136/bjo.86.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto M, Kotani K, Okada K, Fujii Y, Konno K, Ishibashi S, Taniguchi N. The correlation of common carotid arterial diameter with atherosclerosis and diabetic retinopathy in patients with type 2 diabetes mellitus. Acta Diabetol. 2012;49:63–68. doi: 10.1007/s00592-011-0287-8. [DOI] [PubMed] [Google Scholar]
- 32.Bonithon-Kopp C, Touboul PJ, Berr C, Leroux C, Mainard F, Courbon D, Ducimetiere P The Vascular Aging (EVA) Study. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. Arterioscler Thromb Vasc Biol. 1996;16:310–316. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 33.Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke. 1999;30:841–850. doi: 10.1161/01.str.30.4.841. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Lin CH, Lu CJ, Yip PK, Chen RC. Carotid atherosclerosis, intima media thickness and risk factors: an analysis of 1781 asymptomatic subjects in Taiwan. Atherosclerosis. 2002;164:89–94. doi: 10.1016/s0021-9150(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 35.Bedi US, Singh M, Singh PP, Bhuriya R, Bahekar A, Molnar J, Khosla S, Arora R. Effects of statins on progression of carotid atherosclerosis as measured by carotid intimal: medial thickness: a meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2010;15:268–273. doi: 10.1177/1074248410369110. [DOI] [PubMed] [Google Scholar]
- 36.Kalinowski L, Matys T, Chabielska E, Buczko W, Malinski T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension. 2002;40:521–527. doi: 10.1161/01.hyp.0000034745.98129.ec. [DOI] [PubMed] [Google Scholar]
- 37.Wang JG, Staessen JA, Li Y, Van Bortel LM, Nawrot T, Fagard R, Messerli FH, Safar M. Carotid intima-media thickness and antihypertensive treatment: a meta-analysis of randomized controlled trials. Stroke. 2006;37:1933–1940. doi: 10.1161/01.STR.0000227223.90239.13. [DOI] [PubMed] [Google Scholar]
- 38.Taniwaki H, Nishizawa Y, Kawagishi T, Ishimura E, Emoto M, Okamura T, Okuno Y, Morii H. Decrease in glomerular filtration rate in Japanese patients with type 2 diabetes is linked to atherosclerosis. Diabetes Care. 1998;21:1848–1855. doi: 10.2337/diacare.21.11.1848. [DOI] [PubMed] [Google Scholar]
- 39.Mattock MB, Morrish NJ, Viberti G, Keen H, Fitzgerald AP, Jackson G. Prospective study of microalbuminuria as predictor of mortality in NIDDM. Diabetes. 1992;41:736–741. doi: 10.2337/diab.41.6.736. [DOI] [PubMed] [Google Scholar]
- 40.Park SW, Kim SK, Cho YW, Kim DJ, Song YD, Choi YJ, Huh BW, Choi SH, Jee SH, Cho MA, Lee EJ, Huh KB. Insulin resistance and carotid atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2009;205:309–313. doi: 10.1016/j.atherosclerosis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Ishizaka N, Ishizaka Y, Takahashi E, Unuma T, Tooda E, Nagai R, Togo M, Tsukamoto K, Hashimoto H, Yamakado M. Association between insulin resistance and carotid arteriosclerosis in subjects with normal fasting glucose and normal glucose tolerance. Arterioscler Thromb Vasc Biol. 2003;23:295–301. doi: 10.1161/01.atv.0000050142.09911.0b. [DOI] [PubMed] [Google Scholar]
- 42.Shinozaki K, Hattori Y, Suzuki M, Hara Y, Kanazawa A, Takaki H, Tsushima M, Harano Y. Insulin resistance as an independent risk factor for carotid artery wall intima media thickening in vasospastic angina. Arterioscler Thromb Vasc Biol. 1997;17:3302–3310. doi: 10.1161/01.atv.17.11.3302. [DOI] [PubMed] [Google Scholar]
- 43.Zureik M, Touboul PJ, Bonithon-Kopp C, Courbon D, Ruelland I, Ducimetiere P. Differential association of common carotid intima-media thickness and carotid atherosclerotic plaques with parental history of premature death from coronary heart disease: the EVA study. Arterioscler Thromb Vasc Biol. 1999;19:366–371. doi: 10.1161/01.atv.19.2.366. [DOI] [PubMed] [Google Scholar]
- 44.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 45.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 46.Taniwaki H, Kawagishi T, Emoto M, Shoji T, Kanda H, Maekawa K, Nishizawa Y, Morii H. Correlation between the intima-media thickness of the carotid artery and aortic pulse-wave velocity in patients with type 2 diabetes. Vessel wall properties in type 2 diabetes. Diabetes Care. 1999;22:1851–1857. doi: 10.2337/diacare.22.11.1851. [DOI] [PubMed] [Google Scholar]


