Abstract
Rhabdomyolysis is a syndrome involving the breakdown of skeletal muscle that causes myoglobin and other intracellular proteins to leak into the circulatory system, resulting in organ injury including acute kidney injury. We report a case of statin-induced rhabdomyolysis and acute kidney injury that developed in a 63-year-old woman with previously undiagnosed hypothyroidism. Untreated hypothyroidism may have caused her hypercholesterolemia requiring statin treatment, and it is postulated that statin-induced muscle injury was aggravated by hypothyroidism resulting in her full-blown rhabdomyolysis. Although this patient was successfully treated with continuous venovenous hemofiltration and L-thyroxin replacement, rhabdomyolysis with acute kidney injury is a potentially life-threatening disorder. Physicians must pay special attention to the possible presence of subclinical hypothyroidism when administering statins in patients with hypercholesterolemia.
Keywords: Hypothyroidism, Rhabdomyolysis, Statins
INTRODUCTION
Rhabdomyolysis is a disease characterized by myalgia, myasthenia, and myoglobinuria as well as increased muscle-related enzymes, such as serum creatine kinase. These findings occur as intracellular components of damaged cells are released into the blood stream due to necrosis of damaged myocytes.
Acute renal failure is a significant complication that can occur in about 15% to 50% of patients with rhabdomyolysis. Muscle injury may be severe enough to cause compartment syndrome or disseminated intravascular coagulation. The most commonly known causes of rhabdomyolysis are alcohol and substance abuse, epileptic seizure, and coma-induced muscle compression. Other causes include arterial thrombosis, extreme physical exercise, electric shock, hyperthermia, metabolic myopathy, muscle infections, and electrolyte disturbances [1]. In particular, use of statins has emerged as another significant cause [2].
In most cases of statin-induced muscle injury, clinical features are relatively mild, and patients may present with only increased muscle enzymes or symptoms of mild myalgia and myasthenia. These symptoms usually improve after discontinuation of statins [3]. However, the authors have experienced a case of severe rhabdomyolysis associated with oliguric acute renal failure requiring continuous renal replacement therapy after using high-dose statins in a patient with subclinical hypothyroidism. Therefore, we report the case together with a literature review.
CASE REPORT
A 63-year-old female patient presented to our hospital complaining of edema, fatigue, and numbness in the right femoral region. She had normal appetite, but her urine output had gradually decreased over the course of the 15 days prior to visiting the hospital. Oliguria began 8 to 9 days after making Kimchi in squat position. Her systemic edema had worsened over the three days prior to presentation, and she was experiencing asthenia and numbness of the right femoral region, which prompted her to visit the hospital through the emergency room. She did not complain of any respiratory distress or chest pain.
She had a medical history of hypertension and cerebral hemorrhage 7 years previously and her current medications were aspirin 100 mg, clinidipine 10 mg, valsartan 160 mg, thiazide 12.5 mg, and bisoprolol 5 mg. During the regular follow-up at the cardiology department of our hospital, hyperlipidemia (total cholesterol, 273 mg/dL; low density lipoprotein cholesterol [LDL-C], 167 mg/dL) was developed, and she was treated with rosuvastatin 20 mg starting 40 days before presentation. There were no specific findings in her family history.
Blood pressure at the time of presentation was 120/90 mm Hg, pulse rate was 54 beats per minute, and body temperature was 35℃. Heart and breath sounds were normal. The skin tugor was decreased, the femoral region was tender to palpation, and she had systemic nonpitting edema especially on the face and periocular region. There was no sign of goiter. Muscle strength was slightly decreased in the bilateral lower extremities, but the remainder of the motor exam was unremarkable. There were no sensory abnormalities in the lower extremities, and the results of straight leg raise test, patellar subluxation test, tarsal test, and ankle clonus reflex test were normal. The pulse on both dorsalis pedis and popliteal arteries was palpated normally.
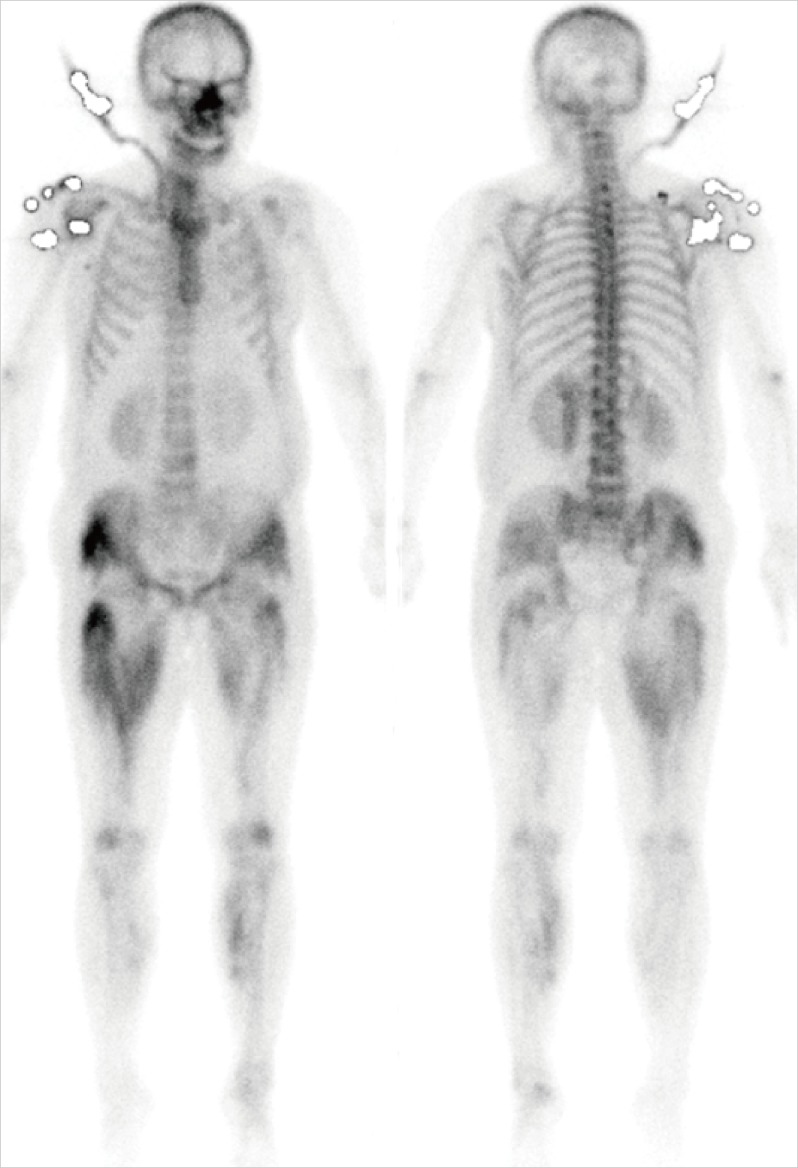
Blood tests performed at presentation demonstrated white blood cell, hemoglobin, and platelets of 11,890/mm3, 15.5 g/dL, and 249,000/mm3, respectively. Blood urea nitrogen and serum creatinine were 89.1 and 9.8 mg/dL. Serum Na/K/Cl/total CO2, serum calcium/phosphate, plasma total cholesterol, triglycerides, and LDL-C were 131/4.1/91/17.5 mmol/L, 7.7/7.6 mg/dL, 111, 158, and 42 mg/dL. Aspartate aminotransferase/alanine aminotransferase, creatine kinase, and lactate dehydronase (LDH) were increased to 1,521/360, 72,850, and 1,974 IU/L, respectively. A whole-body bone scan showed diffuse isotope uptake in the muscle of right pelvis and both lower extremities, consistent with rhabdomyolysis (Fig. 1). Urinalysis revealed hematuria and 2+ proteinuria but urine myoglobin was negative.
Fig. 1.
Bone scans show a diffuse soft tissue uptake in right pelvis and both lower legs.
To rule out hypothyroidism, as a possible risk factor for statin-induced muscle injury and as a cause of nonpitting edema and hypercholesterolemia, thyroid function test was performed. T3, free T4, and thyroid-stimulating hormone (TSH) were <25 ng/dL (normal range, 58 to 159), <0.4 ng/dL (normal range, 0.7 to 1.48), and 100 µIU/mL (normal range, 0.35 to 4.94), respectively. In addition, the patient had high titer of thyroid peroxidase autoantibody (1:6,400) and thyroglobulin autoantibodies (1:6,400). Based on all of the aforementioned findings, the patient was diagnosed as hypothyroidism associated with Hashimoto thyroiditis.
Despite fluid resuscitation with normal saline and administration of a diuretic, the patient failed to maintain adequate urine output of ≥30 mL/hr. Instead, she developed symptoms of fluid overload. Accordingly, she was treated by continuous venovenous hemofiltration (CVVH; Prisma, Gambro, Lund, Sweden) with 2 L per hour of filtration volume via internal jugular venous catheter. Her hypothyroidism was also treated with levothyroxine 50 µg once daily starting from the second day of hospitalization immediately after she was diagnosed with hypothyroidism. After the second day of CVVH, urine output increased to ≥100 mL per hour and renal replacement therapy was stopped on the third day of hospitalization. The patient was discharged from the hospital on the 19th day with the following biochemical values: creatine kinase 507 IU/L, LDH 445 IU/L, blood urea nitrogen 24 mg/dL, and creatinine 2.3 mg/dL. Serum creatinine recovered to the normal range of 1.2 mg/dL 2 months after discharge. Due to the diagnosis of statin induced rhabdomyolysis, rosuvastatin was discontinued at discharge; however, plasma total cholesterol and LDL-C were again elevated to 232 and 151 mg/dL, respectively, 2 months after discharge. Therefore, we began treatment with another type of hydrophilic statin, pravastatin, at a dose of 20 mg per day. At 6 months after discharge, the patient's kidney function remained stable and T3, free T4, and TSH were 89.3 ng/mL, 1.19 ng/dL, and 6.181 µIU/mL, respectively. We continued levothyroxine 50 µg/day and slowly adjusted the dose depending on thyroid function tests.
DISCUSSION
Myocyte necrosis in rhabdomyolysis is caused by an increase in the intramyocyte calcium concentration due to suppression of intracellular Na+-Ca+ exchange. This is a functional disorder of sarcolemma Na+-K+ ATPase resulting from sarcolemma changes induced by muscle injury [4]. Acute kidney injury is the most serious complication, and it is results from ischemic injury due to renal vasoconstriction, impaired perfusion, renal tubular obstruction by tubular casts, and direct renal toxicity from myoglobin released from the injured myocytes [1].
Recently as the usage of statins has rapidly increased due to their beneficial effects in cardiovascular disease prevention, concerns regarding statin-induced muscle injury are increasing [2]. The mechanisms of statin-induced muscle injury have not yet been clearly defined. But a wide range of mitochondrial injuries were identified from electronic microscopic observation of lovastatin-induced rhabdomyolysis and it is reported that these injuries may result from inappropriate synthesis of coenzyme Q 10 and heme A in the electron transport chains of the inner mitochondrial membrane [5]. Therefore, the risk of muscle injury may differ among the statins depending on permeability into the myocyte. Hydrophilic statins, such as pravastatin or rosuvastatin, have low myocyte permeability, lowering their risk of muscle injury [6]. In addition to hydrophilicity, the statin dosage and metabolic route are also significant risk factors for muscle injury. In particular, statins like lovastatin that are metabolized by the hepatic cytochrome P450 3A4 enzyme may be affected by drug-drug interactions, causing a relatively high risk of muscle injury [7]. In this case rosuvastatin was initiated at a relatively high dose. Rosuvastatin is hydrophilic and not metabolized by cytochrome P450 3A4, generally allowing it to be administered at a comparatively high dose. However, despite the use of the relatively safe rosuvastatin, severe rhabdomyolysis developed in this patients, which suggests that there could be other risk factors for the statin-induced rhabdomyolysis. In the case of this patient, it is assumed that underlying unrecognized hypothyroidism was the major contributing risk factor. A muscle injury from preparing Kimchi in squat position is also a possible risk factor; however, symptoms developed 8 to 9 days afterward, making this a less likely factor. In addition, urinary myoglobin, a characteristic of rhabdomyolysis, was not detected from the urine of this patient, implying that myoglobins with elimination half-life of 2 to 3 days were all metabolized and eliminated by the time of presentation.
The pathophysiology of hypothyroidism-induced muscle injuries is not well known. But it is postulated that thyroxine deficiency suppresses glycogenolysis, mitochondrial oxidation, and conversion of triglycerides into an energy source, which cause muscle dysfunction and under condition of oxidative stress, finally result in muscle injury and even rhabdomyolysis [8]. Clinical relevance of hypothyroidism as an important cause of muscle injury is significantly fortified as it inhibits degradation and metabolic processing of LDLs, resulting in elevation of cholesterol levels [9]. Prescription of statins in patients with hypercholesterolemia associated with undetected hypothyroidism may cause severe rhabdomyolysis via additive interaction as shown in this case. Other risk factors for statin induced muscle injury include old age, female gender, impaired renal function, impaired hepatic function, alcohol, excessive physical activity, and concomitant use of medications that are metabolized by cytochrome P450 3A4 [7]. Statin induced muscle injury usually do not require any other specific treatment except discontinuation of the medication. In Korea, there has been a report of mild form of lovastatin induced rhabdomyolysis [10]; however, as we have observed in this patient, if a relatively high dose of statin is administered in patients with untreated hypothyroidism, it can cause severe rhabdomyolysis complicated by acute renal failure requiring renal replacement therapy. Therefore, physicians must pay special attention to the possible presence of undetected hypothyroidism when administering statins in patients with hypercholesterolemia.
In summary, the authors experienced a case of severe rhabdomyolysis due to statin therapy in a patient with hypercholesterolemia and subclinical hypothyroidism. Rhabdomyolysis was accompanied by oliguric acute renal failure that required continuous renal replacement therapy. We reports this case together with a literature review in order to emphasize hypothyroidism as a possible risk factor for statin-induced muscle injury and to recommend testing for hypothyroidism before administration of statins.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 2.Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoA reductase inhibitors. Drug Saf. 2000;23:197–213. doi: 10.2165/00002018-200023030-00003. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 4.Oken DE, Arce ML, Wilson DR. Glycerol-induced hemoglobinuric acute renal failure in the rat. I. Micropuncture study of the development of oliguria. J Clin Invest. 1966;45:724–735. doi: 10.1172/JCI105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manoukian AA, Bhagavan NV, Hayashi T, Nestor TA, Rios C, Scottolini AG. Rhabdomyolysis secondary to lovastatin therapy. Clin Chem. 1990;36:2145–2147. [PubMed] [Google Scholar]
- 6.Harper CR, Jacobson TA. The broad spectrum of statin myopathy: from myalgia to rhabdomyolysis. Curr Opin Lipidol. 2007;18:401–408. doi: 10.1097/MOL.0b013e32825a6773. [DOI] [PubMed] [Google Scholar]
- 7.Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116:408–416. doi: 10.1016/j.amjmed.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Kisakol G, Tunc R, Kaya A. Rhabdomyolysis in a patient with hypothyroidism. Endocr J. 2003;50:221–223. doi: 10.1507/endocrj.50.221. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Arishima T, Kudo T, Nishihara E, Ohye H, Kubota S, Fukata S, Amino N, Kuma K, Sasaki I, Hiraiwa T, Hanafusa T, Takamatsu J, Miyauchi A. Effect of levo-thyroxine replacement on non-high-density lipoprotein cholesterol in hypothyroid patients. J Clin Endocrinol Metab. 2007;92:608–611. doi: 10.1210/jc.2006-1605. [DOI] [PubMed] [Google Scholar]
- 10.Park JM, Kim CW, Lee JH, Lim SK, Lee HC, Huh KB. A case of lovastatin induced rhabdomyolysis. Korean J Med. 1996;50:846–850. [Google Scholar]