Abstract
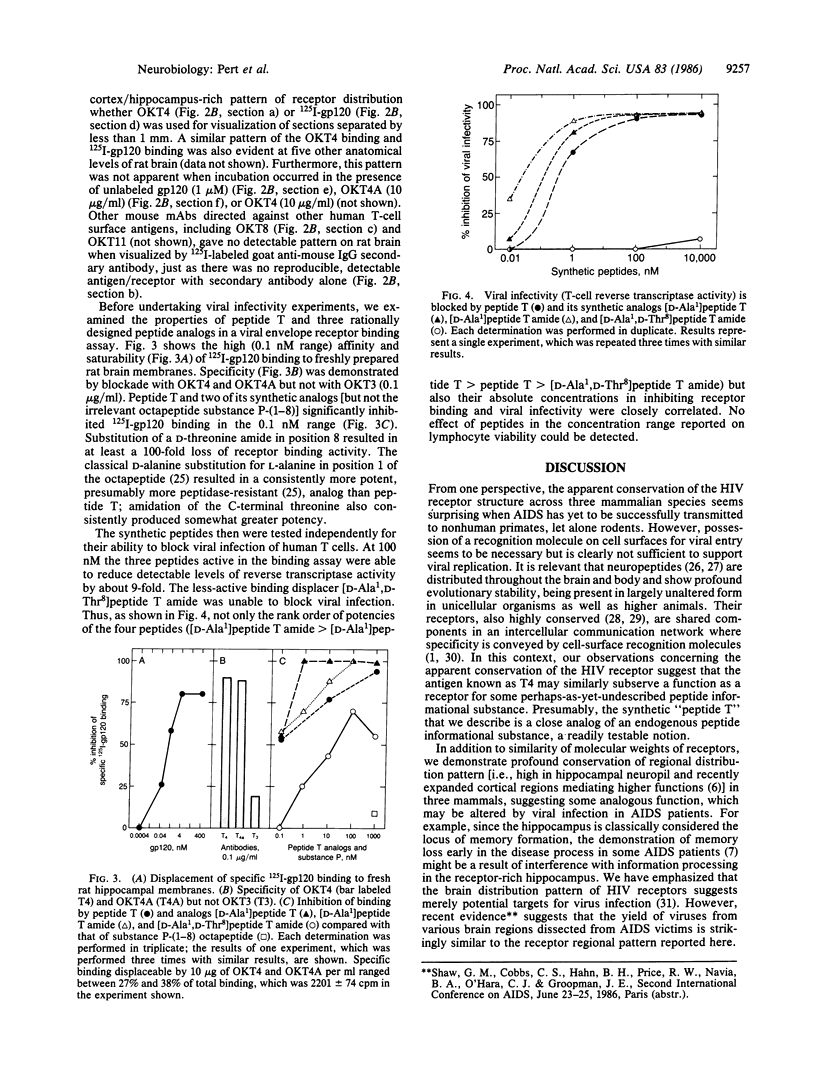
The differentiation antigen T4, present on the helper/inducer subset of T lymphocytes, is thought to serve as the receptor for the human immunodeficiency virus (HIV). We find that a 60-kDa protein, immunoprecipitable by monoclonal antibody (mAb) OKT4, is present on membranes from human brain as well as human T cells. Furthermore, the radioiodinated HIV envelope glycoprotein [125I-labeled gp120 (125I-gp120)] can be specifically covalently affixed to a molecule present on rat, monkey, and human brain membranes to yield a complex that is indistinguishable from that formed on human T cells. T4 antigen has been studied on unfixed squirrel monkey, rat, and human brain sections by autoradiography using the mAb OKT4. A highly conserved neuroanatomical pattern has been demonstrated, suggesting an analogous organization in these three mammalian brains. Furthermore, the localization of 125I-gp120 receptor binding appears similar to that of T4 and is highly reminiscent of patterns for many previously characterized neuropeptide receptors. A computer-assisted analysis of gp120 suggested that a previously unremarkable octapeptide sequence within the gp120 protein, which we have synthesized and termed "peptide T," may play an important role in HIV attachment. Thus, peptide T and three rationally designed peptide analogs, each with a systematic amino acid substitution, potently inhibit specific 125I-gp120 binding to brain membranes. Additionally, when tested in a viral infectivity assay, these peptides show the same rank order and similar absolute potency to block HIV infection of human T cells. Thus, peptide T may provide a useful pharmacological or immunological basis for the control and treatment of AIDS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Marsh Y. V., Schreiber A. B., Newman S. R., Todaro G. J., Nestor J. J., Jr Epidermal growth factor receptor occupancy inhibits vaccinia virus infection. Nature. 1985 Dec 19;318(6047):663–665. doi: 10.1038/318663a0. [DOI] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goochee C., Rasband W., Sokoloff L. Computerized densitometry and color coding of [14C] deoxyglucose autoradiographs. Ann Neurol. 1980 Apr;7(4):359–370. doi: 10.1002/ana.410070414. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Levels of quantitative analysis of receptor autoradiography: technical and theoretical issues. NIDA Res Monogr. 1985;62:13–29. [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982 Aug;2(8):1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. M., Farrar W. L., Pert C. B. Localization of the T4 antigen/AIDS virus receptor in monkey and rat brain: prominence in cortical regions. Psychopharmacol Bull. 1986;22(3):689–694. [PubMed] [Google Scholar]
- Hill J. M., Ruff M. R., Weber R. J., Pert C. B. Transferrin receptors in rat brain: neuropeptide-like pattern and relationship to iron distribution. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4553–4557. doi: 10.1073/pnas.82.13.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Le Roith D., Shiloach J., Roth J., Lesniak M. A. Evolutionary origins of vertebrate hormones: substances similar to mammalian insulins are native to unicellular eukaryotes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6184–6188. doi: 10.1073/pnas.77.10.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroith D., Liotta A. S., Roth J., Shiloach J., Lewis M. E., Pert C. B., Krieger D. T. Corticotropin and beta-endorphin-like materials are native to unicellular organisms. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2086–2090. doi: 10.1073/pnas.79.6.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M., Mathieson B., Sharrow S., McHugh L., Hämmerling U., Kanellopoulos-Langevin C., Brideau D., Jr, Thomas C. A., 3rd Preparative nonlytic separation of Lyt2+ and Lyt2- T lymphocytes, functional analyses of the separated cells and demonstration of synergy in graft-vs.-host reaction of Lyt2+ and Lyt2- cells. Eur J Immunol. 1981 Mar;11(3):228–235. doi: 10.1002/eji.1830110312. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Pert C. B., Kuhar M. J., Snyder S. H. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert C. B., Ruff M. R., Weber R. J., Herkenham M. Neuropeptides and their receptors: a psychosomatic network. J Immunol. 1985 Aug;135(2 Suppl):820s–826s. [PubMed] [Google Scholar]
- Pert C. B., Snyder S. H. Opiate receptor: demonstration in nervous tissue. Science. 1973 Mar 9;179(4077):1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Pert C. B. Small cell carcinoma of the lung: macrophage-specific antigens suggest hemopoietic stem cell origin. Science. 1984 Sep 7;225(4666):1034–1036. doi: 10.1126/science.6089338. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Schmitt F. O. Molecular regulators of brain function: a new view. Neuroscience. 1984 Dec;13(4):991–1001. doi: 10.1016/0306-4522(84)90283-5. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Simpson D. M., Nielsen S., Gold J. W., Metroka C. E., Posner J. B. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983 Oct;14(4):403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Venter J. C., Eddy B., Hall L. M., Fraser C. M. Monoclonal antibodies detect the conservation of muscarinic cholinergic receptor structure from Drosophila to human brain and detect possible structural homology with alpha 1-adrenergic receptors. Proc Natl Acad Sci U S A. 1984 Jan;81(1):272–276. doi: 10.1073/pnas.81.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]






