Abstract
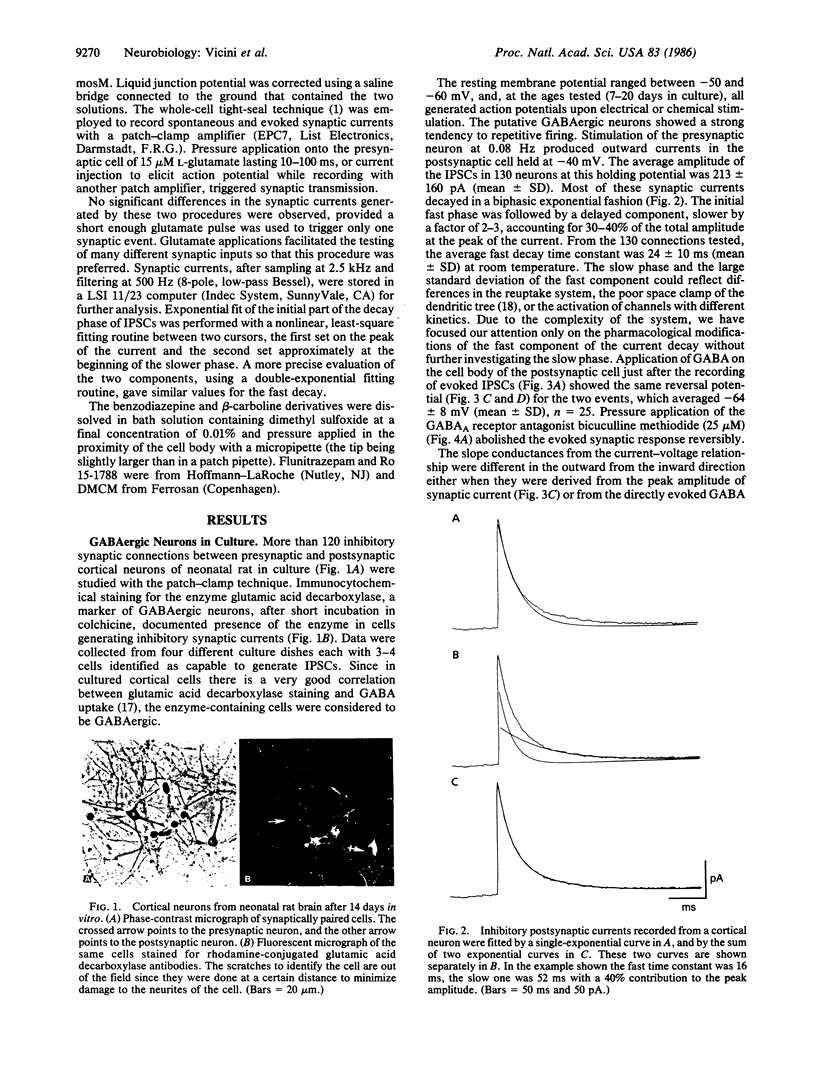
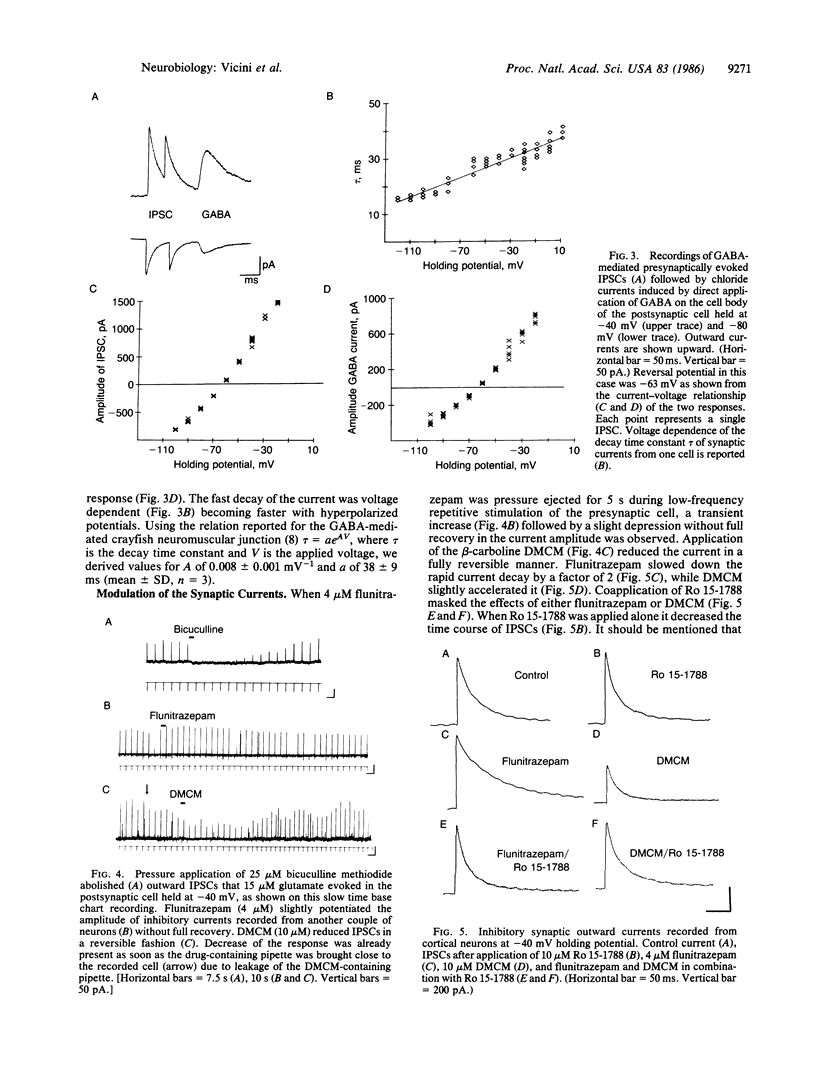
Inhibitory gamma-aminobutyric acid-mediated synaptic currents were studied in dissociated primary cultures of neonatal rat cortex with the whole-cell patch-clamp technique. Immunocytochemical staining of the cultures showed the presence of a large number of glutamic acid decarboxylase-containing neurons, and electrical stimulation of randomly selected neurons produced in many cases chloride-mediated and bicuculline-sensitive inhibitory synaptic currents in postsynaptic cells. The amplitude and decay time of the inhibitory synaptic currents were increased by flunitrazepam and decreased by the beta-carboline derivative methyl 6,7-dimethoxy-4-ethyl-beta-carboline-3-carboxylate, two high-affinity ligands for the allosteric regulatory sites of gamma-aminobutyric acid receptors. The imidazobenzodiazepine Ro 15-1788, another high-affinity ligand of the gamma-aminobutyric acid receptor regulatory sites that has negligible intrinsic activity, blocked the action of flunitrazepam and beta-carboline. However, Ro 15-1788 also increased the decay rate of the inhibitory synaptic currents. This might suggest that an endogenous ligand for the benzodiazepine-beta-carboline binding site is operative in gamma-aminobutyric acid-mediated synaptic transmission.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan C. Y., Farb D. H. Modulation of neurotransmitter action: control of the gamma-aminobutyric acid response through the benzodiazepine receptor. J Neurosci. 1985 Sep;5(9):2365–2373. doi: 10.1523/JNEUROSCI.05-09-02365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Gage P. W., Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol. 1984 Nov;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G. Inhibitory synaptic currents in voltage-clamped locust muscle fibres desensitized to their excitatory transmitter. Proc R Soc Lond B Biol Sci. 1984 May 22;221(1224):375–383. doi: 10.1098/rspb.1984.0039. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. Miniature and evoked inhibitory junctional currents and gamma-aminobutyric acid-activated current noise in locust muscle fibres. J Physiol. 1986 May;374:179–200. doi: 10.1113/jphysiol.1986.sp016074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M. A. Physiological identification of GABA as the inhibitory transmitter for mammalian cortical neurons in cell culture. Brain Res. 1980 May 19;190(1):111–121. doi: 10.1016/0006-8993(80)91163-4. [DOI] [PubMed] [Google Scholar]
- Dichter M. A. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978 Jun 30;149(2):279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- Dudel J. Voltage dependence of amplitude and time course of inhibitory synaptic current in crayfish muscle. Pflugers Arch. 1977 Oct 19;371(1-2):167–174. doi: 10.1007/BF00580786. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hunkeler W., Möhler H., Pieri L., Polc P., Bonetti E. P., Cumin R., Schaffner R., Haefely W. Selective antagonists of benzodiazepines. Nature. 1981 Apr 9;290(5806):514–516. doi: 10.1038/290514a0. [DOI] [PubMed] [Google Scholar]
- Jensen M. S., Lambert J. D. The interaction of the beta-carboline derivative DMCM with inhibitory amino acid responses on cultured mouse neurones. Neurosci Lett. 1983 Sep 30;40(2):175–179. doi: 10.1016/0304-3940(83)90298-7. [DOI] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- King G. L., Knox J. J., Dingledine R. Reduction of inhibition by a benzodiazepine antagonist, Ro15-1788, in the rat hippocampal slice. Neuroscience. 1985 Jun;15(2):371–378. doi: 10.1016/0306-4522(85)90219-2. [DOI] [PubMed] [Google Scholar]
- Krespan B., Springfield S. A., Haas H., Geller H. M. Electrophysiological studies on benzodiazepine antagonists. Brain Res. 1984 Mar 19;295(2):265–274. doi: 10.1016/0006-8993(84)90975-2. [DOI] [PubMed] [Google Scholar]
- Levi G., Aloisi F., Ciotti M. T., Gallo V. Autoradiographic localization and depolarization-induced release of acidic amino acids in differentiating cerebellar granule cell cultures. Brain Res. 1984 Jan 2;290(1):77–86. doi: 10.1016/0006-8993(84)90737-6. [DOI] [PubMed] [Google Scholar]
- Neale E. A., Oertel W. H., Bowers L. M., Weise V. K. Glutamate decarboxylase immunoreactivity and gamma-[3H] aminobutyric acid accumulation within the same neurons in dissociated cell cultures of cerebral cortex. J Neurosci. 1983 Feb;3(2):376–382. doi: 10.1523/JNEUROSCI.03-02-00376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. An analysis of the inhibitory post-synaptic current in the voltage-clamped crayfish muscle. J Physiol. 1979 Jan;286:265–282. doi: 10.1113/jphysiol.1979.sp012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Skerritt J. H., Macdonald R. L. Benzodiazepine Ro 15-1788: electrophysiological evidence for partial agonist activity. Neurosci Lett. 1983 Dec 30;43(2-3):321–326. doi: 10.1016/0304-3940(83)90208-2. [DOI] [PubMed] [Google Scholar]
- Snodgrass S. R., White W. F., Biales B., Dichter M. Biochemical correlates of GABA function in rat cortical neurons in culture. Brain Res. 1980 May 19;190(1):123–138. doi: 10.1016/0006-8993(80)91164-6. [DOI] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]




