Introduction - ‘classic’ fight or flight physiology
Ca2+ is an essential ion and second messenger that plays a central role in determining membrane excitability and contractility in heart, while also participating in diverse and fundamental activities such as gene transcription, cell metabolism and survival, vesicle secretion, learning and memory. Voltage-gated Ca2+ channels (CaVx) are the primary entry point for extracellular Ca2+ to cross cell membranes and gain access to the intracellular space. In ventricular myocytes CaV1.2 is the most important pathway for Ca2+ entry and provides a trigger for release of intracellular Ca2+ from the sarcoplasmic reticulum that activates myofilament crossbridge formation to grade the strength of each heart beat. Thus, improved understanding of Ca2+ homeostatic mechanisms, generally, and CaV1.2 regulation, specifically, are important goals for cardiovascular science.
The increase in CaV1.2 current (ICa) following adrenergic receptor agonist stimulation is a fundamental aspect of ‘fight or flight’ physiology, but the molecular mechanisms underlying this pathway are incompletely understood. While Ca2+ was identified as a necessary ingredient for myocardial excitability by experiments of Sydney Ringer, over 100 years ago,1 knowledge of the signaling pathways that enhance cardiac contraction, in part by increasing ICa has accrued more recently. Highlights in this discovery time line include the concept of receptors for extracellular chemical signals by Alquist,2 the identity of cyclic adenosine monophosphate (cAMP) as a second messenger by Sutherland3 and enzymatic catalysis of amino acid phosphorylation by the cAMP kinase (PKA) to modify protein function by Krebs.4 Pioneering work by many investigators, including Numa who first cloned the pore forming α subunit of CaV1.25 and Catterall who identified key biochemical features of several voltage-gated ion channels, including CaV1.26 contributed to understanding of how agonist receptor stimulation leads to enhanced ICa. Ironically, ICa increases by PKA have served as a prototypical example of how the cAMP/PKA pathway affects myocardium but definitive understanding of where, if and how the CaV1.2 protein complex is phosphorylated in response to β adrenergic receptor stimulation to increase ICa remains stubbornly elusive.
Overview of CaV1.2 phosphorylation sites: what did we once think we knew?
I remember thinking that our field understood, at a molecular level, how CaV1.2 activity increases were driven by β adrenergic receptor agonist stimulation when the Hosey group identified the α subunit C terminus serine 1928 as a target for PKA-dependent phosphorylation and an indispensible site for increasing ICa.7 These studies used heterologous expression systems to build evidence for serine 1928 as an agonist effector. The apparent importance of this site seemed to grow after protein kinase C was also reported to catalyze phosphorylation of serine 1928.8 However, later Brian O’Rourke’s group reported that CaV1.2 mutants expressed in cultured adult ventricular myocytes lacking serine 1928 maintained responsiveness to adrenergic stimulation.9 Over the years there have been various reports identifying key regions and/or individual amino acids as important or essential for ‘fight or flight’ mediated increases in ICa. The majority of these reports have relied, at least in part, on heterologous expression systems because of the difficulty, time and expense required to make individual mutations in CaV1.2 and its auxiliary subunit proteins. Yang et al. in this issue of Circulation Research10 developed a transgenic model of CaV1.2 that harbored a mutation conferring relative insensitivity to dihydropyridine CaV1.2 antagonists driven by a tetracycline ‘on’ system to operationalize myocardial expression of mutant CaV1.2 channels in mice. The endogenous CaV1.2 could thus be mostly silenced by use of conventional CaV1.2 antagonist drugs, leaving the majority of residual ICa via the transgenically expressed, dihydropyridine-resistant CaV1.2. By this approach, Yang et al. produced new evidence that a recently identified site for proteolytic cleavage of the C terminus (alanine 1800) and a candidate PKA-targeted amino acid (serine 1700) were not by themselves required for isoproterenol-dependent increases in ICa.
Why is it so difficult to identify the culprit site for PKA-mediated increases in ICa?
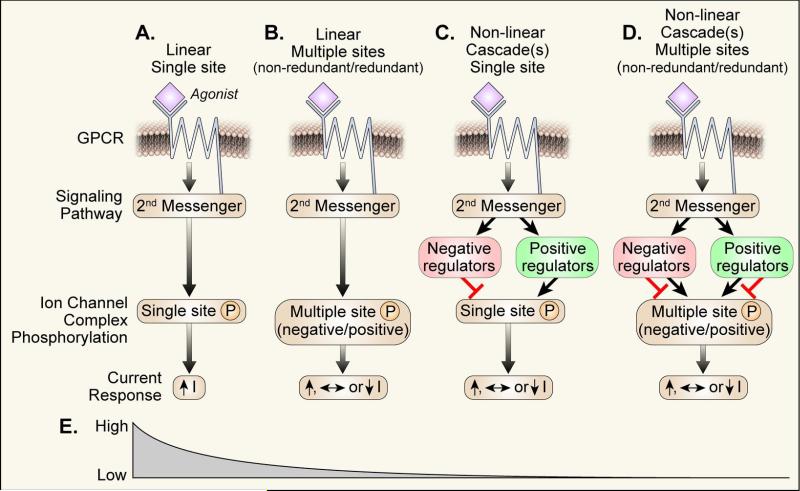
While it is now clear that identifying the key phosphorylation sites and other enabling post-translational modifications on CaV1.2 (and perhaps on any similarly formidable and complex protein) for increasing ICa in response to β adrenergic receptor agonist stimulation is not easy, the nature of the obstacles to successful discovery are less certain. My view is that the entire biological system may be far more complex than originally anticipated and that multiple challenges, yet unsolved, may lie in wait for our field before key post translational modifications governing fundamental CaV1.2 α subunit physiology will be definitively understood. Figure 1 schematizes various generic scenarios that may exist for a G protein coupled or other cell membrane receptor pathway to influence ion channel behavior. The leftmost scenario (a linear signaling pathway where a single ‘culprit’ amino acid target for PKA-mediated phosphorylation leads to ion current increases, Fig 1A) is the simplest and therefore the most likely to be resolved with our current experimental approaches. Unfortunately, the lack of a clear CaV1.2 α subunit candidate to emerge from experimental work in the post-patch clamp and molecular biology era suggests, at least to me, that this scenario is unlikely to be a valid or sufficiently nuanced reflection of adrenergic agonist stimulation to CaV1.2. Figure 1B illustrates another linear model (i.e. where agonist activation of a receptor increases ion channel phosphorylation without activating other ion channel-modifying kinases or phosphatases) but where phosphorylation at various sites can lead to increases, decreases or no net change in ionic current. While PKA consistently increases ICa, it is a formal possibility that this observed action could be a net result favoring stimulatory over inhibitory actions. It is possible that the history or order of phosphorylation events could be important for net ionic current responses to an upstream stimulus. For example, phosphorylation at site X in advance of site Y could be inhibitory while phosphorylation of site Y alone could enhance ionic current. The figure 1B scenario includes two potential subcategories: one where the various amino acids are completely redundant and another where the amino acid targets are unique or incompletely redundant for purposes of agonist stimulation. Although simpler than the more rightward scenarios (Fig 1C and D), the scenario in Fig 1B is already complex and could account for apparent dispensability of various bona fide PKA sites because the ‘key sites’ are redundant or partially redundant. Figure 1C shows a situation where a single receptor leads to activation of multiple signaling cascades, simplified here to show one stimulatory and one inhibitory pathway working through a single stimulatory phosphorylation site. The model in Figure 1C could potentially explain discrepancies between studies in native (primary) cells and cultured cells or in different animal models or in ‘identical’ animal models from different genetic backgrounds if the potential pathways were different, lacking or less coupled to the upstream G protein coupled receptor in one system compared to another. Work by a number of groups does support features of this scenario by suggesting that isoproterenol can recruit kinase pathways (e.g. CaMKII, PKC) in addition to PKA with the potential to enhance ICa. The recruitment of multiple kinase (and phosphatase) pathways targeting multiple redundant or non-redundant amino acids (Fig 1D) is further complicated by the potential for distinct phosphorylation targets to produce convergent biophysical phenotypes, such as mode 2 gating.11, 12 Ultimately, PKA dependent phosphorylation must modify CaV1.2 channel structure in a manner that enhances ICa. However, detailed structural understanding of these events faces formidable obstacles that will not be resolved when and if a CaV1.2 crystal structure is solved because such a structure will necessarily be static, while the gating consequences of agonist phosphorylation are dynamic.
Figure 1.
Schematic diagram of various possible scenarios for receptor signaling to ion channels.
The culprit site concept may be too simplistic
The increasing complexity of scenarios depicted in figure 1A-D suggest that the likelihood of identifying a culprit phosphorylation site is relatively high for scenario A but steeply declines thereafter (Fig 1E). Phosphorylation of various amino acids likely lead to increases in ICa by lowering the energy barrier (increasing the probability) to assemble activated conformations. Assuming that upstream signaling is conserved in various cell systems, species and strains, the discovery of a culprit phosphorylation site will depend upon the existence of an unique phosphorylation-enabled conformation to achieve increased ICa after adrenergic receptor agonist stimulation. However, at this point we have no evidence to suggest a unique phosphorylation site is in control of this process. If we ‘discover’ such a site, our field should contemplate this new information with caution because a phosphorylation-resistant mutant protein may produce other unanticipated effects, including allosteric resistance to post-translational modifications at ‘distant’ sites.
Consider a story where space aliens visit planet earth and by observation conclude that it will be essential to understand the mechanism of action of automobiles to grasp fundamentals of human society. The alien scientists might first characterize the performance of cars, trucks and, perhaps, other motorized vehicles. Second, they would disarticulate the machinery to understand the mechanism of propulsion. One scientist might remove the non-redundant right front tire of a four wheeled vehicle and observing that the car was no longer functional conclude that the right front tire was the pathway/mechanism for motorized propulsion. Another scientist might produce conflicting results and could conclude that loss of the non-redundant left rear tire was also essential by its removal. Investigation of complex conveyances, such as a multi-wheeled semi trailer, could lead to a conclusion that any individual (non-redundant) tire was dispensable for the mechanism of propulsion because multiple wheels need to be removed before a short term change could be observed. Thus it may be with ion channels, such as CaV1.2, where we vainly seek simple, unique solutions to a complex, non-linear signaling system with multiple upstream inputs and redundant pathways favoring high activity conformations.
Acknowledgements
This work was funded in part by National Institutes of Health Grants R01-HL 079031, R01-HL096652, and R01-HL070250, R01-HL071140 and the Fondation Leducq “Transatlantic Alliance for CaMKII Signaling”, 08CVD01. Thanks to Long-Sheng Song for helpful discussions and to Ms. Sydney Harned for clerical support and to Mr. Shawn Roach for artwork.
Footnotes
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures - no disclosures
References
- 1.Miller DJ. Sydney ringer; physiological saline, calcium and the contraction of the heart. J Physiol. 2004;555:585–587. doi: 10.1113/jphysiol.2004.060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- 3.Hardman JG, Robison GA, Sutherland EW. Cyclic nucleotides. Annu Rev Physiol. 1971;33:311–336. doi: 10.1146/annurev.ph.33.030171.001523. [DOI] [PubMed] [Google Scholar]
- 4.Kemp BE, Bylund DB, Huang TS, Krebs EG. Substrate specificity of the cyclic amp-dependent protein kinase 1. Proc Natl Acad Sci U S A. 1975;72:3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 6.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: A 212-kda form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. Camp-dependent regulation of cardiac l-type Ca2+ channels requires membrane targeting of pka and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Liu G, Zakharov SI, Morrow JP, Rybin VO, Steinberg SF, Marx SO. Ser1928 is a common site for CaV1.2 phosphorylation by protein kinase c isoforms. J Biol Chem. 2005;280:207–214. doi: 10.1074/jbc.M410509200. [DOI] [PubMed] [Google Scholar]
- 9.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of l-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1c but not serine 1928 13. Circ.Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang LK,A, Samad T, Morrow J, Weinberg R, Marx SO. β-adrenergic regulation of the L-type Ca2+ channel does not require phosphorylation of a1c ser1700. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301926. xyz:xyz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koval OM, Guan X, Wu Y, Joiner ML, Gao Z, Chen B, Grumbach IM, Luczak ED, Colbran RJ, Song LS, Hund TJ, Mohler PJ, Anderson ME. CaV1.2 beta-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci U S A. 2010;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]