Abstract
DAS181 is a novel drug in development for the treatment of influenza as well as human parainfluenza viruses (hPIV). Previous studies demonstrated that DAS181 inhibited laboratory strains of hPIV, but no tests were conducted with primary clinical isolates of hPIV. To fill this gap, we studied six primary isolates including hPIV-2 and hPIV-3. First tests showed that the amplification of all viruses in vitro was reproducibly inhibited with DAS181 drug concentrations ranging between 0.1 and 1 nM. An hPIV-3 primary clinical isolate was then tested in a cotton rat model for sensitivity to 0.3-1 mg/kg drug treatments. Results showed that virus amplification in the lower respiratory tract was significantly and reproducibly inhibited by drug. Together, experiments demonstrated that DAS181 inhibited primary clinical isolates of hPIV in vitro and in vivo at doses similar to those previously described for inhibition of laboratory hPIV and influenza virus isolates.
Human parainfluenza viruses (hPIVs) belong to the paramyxovirus family and include subtypes 1, 2, 3, and 4. These subtypes cause diseases of different severity ranging from common cold symptoms to serious laryngotracheobronchitis (croup) or bronchiolitis. Human PIV-1 infections cause at least 50% of croup cases in the United States with an estimated 18,000 to 35,000 children younger than 5 years hospitalized each year (Henrickson, 2003). Hospitalizations with hPIV-3 infections are even more frequent and tend to associate with lower respiratory tract disease including bronchiolitis and pneumonia (Weinberg et al., 2009). HPIV-2 and hPIV-4 associate with fewer respiratory tract infections, but like the others, can cause serious disease in immunocompromised hosts (Boeckh, 2008;Falsey, 2012;Hall, 2001;Karron & Collins, 2007;Renaud & Englund, 2012;Weigt et al., 2011).
Currently, no standard prophylaxis or therapy exists for prevention or treatment of hPIV. Several vaccines have been tested, but none have yet reached licensure (Hurwitz et al., 1997;Jones et al., 2009;Jones et al., 2012;Karron et al., 2012;Karron & Collins, 2007;Skiadopoulos et al., 1999;Slobod et al., 2004). Ribavarin is used in some cases as treatment against hPIV in immunocompromised patients, but the results have been variable (Fuehner et al., 2011;Riner et al., 2009;Ustun et al., 2012), and candidate drugs such as BCX 2798 and BCX 2855 have not yet advanced to clinical testing (Alymova et al., 2004;Watanabe et al., 2009). The development of better treatment options for the hPIVs is therefore encouraged.
The host cell receptors for hPIVs are cell surface sialic acids (Ito, 2000). These are α-keto acids with 9-carbon backbones, usually found at the outermost positions of oligosaccharide chains attached to glycoproteins and glycolipids. The predominant type of sialic acid is N-acetylneuraminic acid (Neu5Ac), which is the biosynthetic precursor for most other types. Two major linkages between Neu5Ac and the penultimate galactose residues of the carbohydrate side chains are found in nature, Neu5Ac α(2,3)-Gal (α2,3-linked sialic acids) and Neu5Ac α(2,6)-Gal (α2,6-linked sialic acids). The hemagglutinin-neuraminidase (HN) molecules of hPIVs 1-4 recognize α(2,3)-linked sialic acids, while hPIV-3 is also known to recognize α(2,6)-linkages (Schauer, 1982;Suzuki et al., 2001). In some cases a second receptor site is exposed on the HN of hPIV-1 or hPIV-3 that may broaden receptor recognition (Alymova et al., 2012;Holmgren et al., 1980;Markwell et al., 1986;Markwell & Paulson, 1980;Mishin et al., 2010). In the cotton rat, often used as an animal model for hPIV infections, α(2,3)-linked sialic acid is present in the trachea and both α(2,3)- and α(2,6)-linked sialic acids are present in the lung (Blanco et al., 2013).
Sialidases are a family of exoglycosidases that catalyze the removal of terminal sialic acid residues from various glycoconjugates, and that can inhibit influenza viruses and hPIVs in vitro (Ah-Tye et al., 1999;Air & Laver, 1995;Bergelson et al., 1982;Els et al., 1989;Griffin et al., 1983;Moscona & Peluso, 1991;Moscona & Peluso, 1992;Suzuki et al., 2001;Zhang et al., 2005). DAS181 is a protein comprising a sialidase fused to a respiratory epithelium-anchoring domain at the C-terminus. The sialidase domain derives from Actinomyces viscosus and the anchoring domain derives from human amphiregulin protein and binds heparin, heparin-like molecules or other glycosaminoglycans (GAGs), thereby securing the molecule onto epithelial cell surfaces. DAS181 has been used as an inhalant and has shown promise as either a prophylactic or therapeutic at the early stage of a virus infection. It is currently in Phase 2 clinical development for the treatment of influenza (Moss et al., 2012).
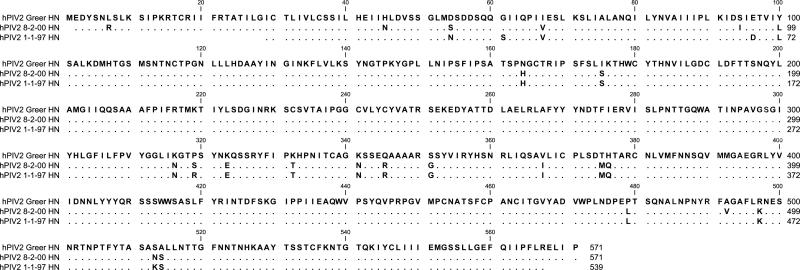
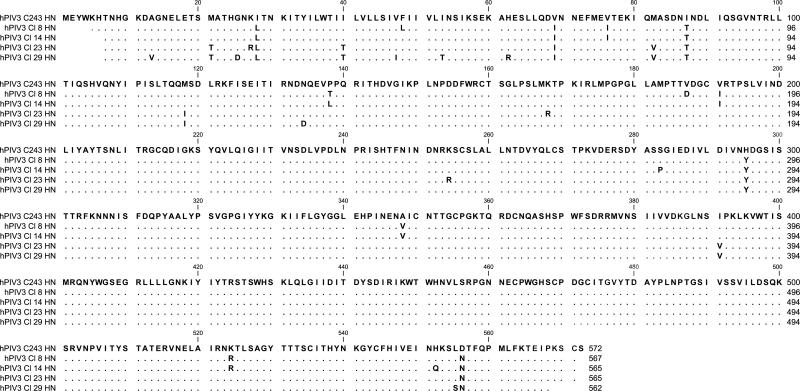
Characterization of the anti-hPIV activity of DAS181 has been previously accomplished with one laboratory isolate of each parainfluenza virus subtype (Moscona et al., 2010). Because these laboratory isolates are extensively passaged in vitro and may be selected for unique characteristics atypical of the clinical setting, we questioned whether a panel of primary virus isolates that had undergone limited passages would also be sensitive to drug. To this end, we collected six primary virus isolates (two hPIV-2 and four hPIV-3 isolates) from St. Jude Children's Research Hospital. Viruses were from pediatric samples collected from 1994-2009, which were saved for quality assurance purposes. Samples were completely deidentified prior to use, and the study was considered exempt from institutional review board (IRB) review. The hemagglutinin-neuraminidase (HN) sequences were determined for all six viruses and are shown in Figures 1A (hPIV-2) and B (hPIV-3). As demonstrated, all sequences were different from one another and from the template laboratory isolates, Greer (hPIV-2) and C243 (hPIV-3).
Figure 1. hPIV-2 and hPIV-3 protein sequences.
Sanger sequencing was conducted to identify HN sequences for each primary virus isolate. Predicted amino acid sequences are aligned to the templates of Greer for hPIV-2 (1A) and C243 for hPIV-3 (1B). A blank position indicates lack of sequence information. A dot indicates a match with the template, and a single-letter code indicates an amino acid substitution.
Methods. Viral RNA was purified using a Qiagen QIAmp Viral RNA Mini Kit (Cat# 52904), and RT-PCR was conducted with virus sequence-specific primers (synthesized by Integrated DNA Technologies) and a RNA One-step PCR Kit from TaKaRa Bio, Inc. (Cat# RR024). Sanger sequencing was then performed at the Hartwell Center of St. Jude Children's Research Hospital using additional virus sequence-specific primers. For analyses, individual sequences were imported into DNA Lasergene 7 SeqMan software and aligned to create a consensus contig. Contigs were translated and aligned using CLC Main Workbench 5 software.
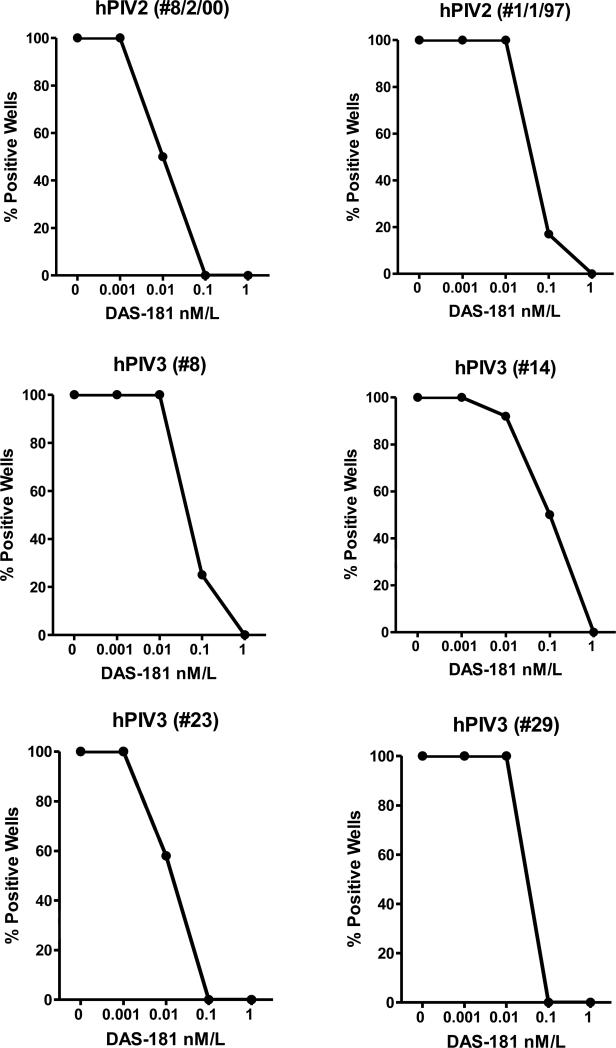
To determine if DAS181 inhibited hPIV isolates in vitro, virus was added to confluent LLC-MK2 cell cultures and incubated for 2 hours, after which plates were washed and replaced with media containing varying concentrations of DAS181. Plates were incubated for 4-5 additional days and then scored by hemagglutination (HA) assays. As demonstrated in Figure 2, all clinical isolates were completely inhibited following incubation with 0.1-1 nM DAS181. Studies with hPIV-1 were also conducted, but required 5 μg/ml acetylated trypsin for extracellular cleavage of the hPIV-1 fusion protein (Karron & Collins, 2007). Full virus inhibition was observed with DAS181, but higher drug concentrations were required for inhibition of hPIV-1 as compared to hPIV-2 and hPIV-3. The explanation for this result was unclear, although it is likely that the input of trypsin at high concentration digested and partially inactivated drug (data not shown).
Figure 2. DAS181 inhibited amplification of hPIV primary isolates in vitro.
Serially diluted DAS181 was tested for inhibition of hPIV-2 and hPIV-3 amplification in vitro.
Methods. To test sensitivity of viruses to DAS181, LLC-MK2 cells were plated in 96-well tissue culture plates and grown to confluency in MEM, 0.225% NaHCO3, 5% Fetal Bovine Serum, L-glutamine, and gentamicin at 37°C, 5% CO2. Viruses were diluted in DMEM, 0.1% BSA, L-glutamine, gentamicin to a concentration of 250 TCID50 per 100 μl. LLC-MK2 plates were washed with DPBS w/ Mg++ and Ca++ and 100 μl/well of the diluted virus were added and incubated for 2 hours at 37°C. After incubation, plates were aspirated, washed with DPBS, followed by addition of 100 μl DAS181 serially diluted from 1 to 0.001 nM in DMEM, 0.1% Bovine Serum Albumin. Twelve well replicates were performed for each virus and each drug dilution. Cultures were incubated for 4-5 days at 37°C after which wells were scored for hemagglutination (HA) activity. HA assays were conducted at 4 degrees C using turkey red blood cells (RBCs) for hPIV-3 isolates and guinea pig RBCs for hPIV-2 isolates. Percent inhibition was scored based on reduction in the number of HA-positive wells in the presence of drug. Control HA assays were set up with DAS181 and serially diluted hPIV-2 or hPIV-3 stocks, demonstrating that HA assays were not inhibited by the addition of 0-1 nm drug at 4 degrees C.
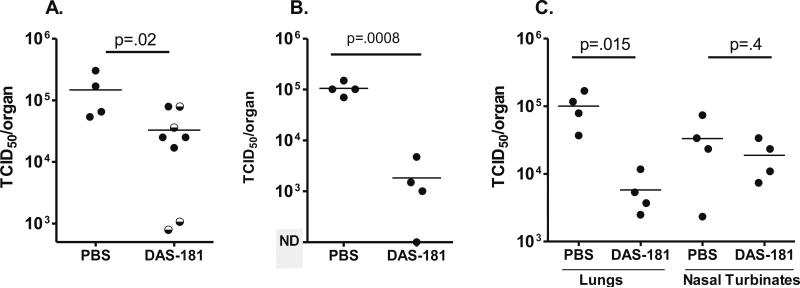
Evidence that clinical hPIV isolates could be inhibited in vitro prompted in vivo studies. After a test of viral isolates hPIV-2 8/2/00, hPIV-2 1/1/97 and hPIV-3#14 for amplification in cotton rats, the isolate that yielded the highest titer on days 3-4 after infection (hPIV-3#8) was selected for further study. In a preliminary experiment, animals were infected with ~1.5 × 10e6 TCID50 hPIV-3#8 followed four hours later with drug (0.3-1 mg/kg by the i.n. route, a concentration that was selected based on previous studies (Moscona et al., 2010)). Repeat drug doses were on days 1 and 2 and viral measurements were conducted on day 3. As shown in Figure 3A, there was a significant decrease in virus amplification. The next experiments assessed the anti-viral activity when 1 mg/kg drug was administered one hour before virus infection rather than four hours after infection. As shown in Figure 3B, DAS181-treated animals again showed significant reduction of virus, in this case with inhibition of virus approaching 2 logs. To determine whether the anti-viral effects of DAS181 were more evident in the upper or lower respiratory tract tissues (URT or LRT), experiments were next conducted to assess nasal turbinates and lungs in parallel. Results revealed that the reduction of hPIV-3 by DAS181 treatments was not statistically significant in the nasal turbinates, but was reproducibly inhibitory in the lung (Figure 3C).
Figure 3. DAS181 inhibits LRT hPIV-3 amplification in vivo in a cotton rat model.
(a) In the cotton rat model, DAS181 was tested for inhibition of hPIV-3#8 amplification. Briefly, cotton rats (Harlan, Inc., Indianapolis Indiana) were infected with ~1.5 × 106 TCID50 HPIV-3 in 100 μl i.n.. Four hours later, test animals were given a dose of DPBS or DAS181 at 0.3 or 1.0 mg/kg body weight in a volume of 100 μl by the i.n. route. Two more doses of drug were given 1 and 2 days after infection. On day 3 following infection, lungs were harvested and homogenized in 3-5 ml DPBS. For titering, samples were serially diluted from 10−1 through 10−6 and 200 μl/well were inoculated onto LLC-MK2 monolayers in replicate wells of 96-well plates. Plates were incubated at 33°C in 5% CO2 for 4 days. 50 μl supernatant from each well was removed, mixed with 50 μl turkey RBC, and incubated at 4°C for 45 minutes to 1 hour. Wells were scored as positive or negative for hemagglutination (HA). Virus titers (TCID50) were calculated based on HA data for each test animal using the Reed-Meunch formula. Each symbol represents the result from an individual animal. For DAS-181-treated animals, semi-solid and solid dots represent animals that received 0.3 mg/kg or 1 mg/kg drug, respectively. (b) Experiments were conducted as in (a), but the first dose of drug was administered one hour prior to infection rather than four hours after infection. (c) Experiments were conducted as in (b), but with measurements of viral titers in nasal turbinates as well as lungs. To harvest nasal turbinates soft tissues were removed from bony structures, suspended in 1 ml DPBS, and screened through a 70 micron filter.
Of note, we have not asked if drug can be initiated one or two days after viral infection in cotton rats, although clinical studies are addressing this question. We note that the protection afforded by drug administered prior to infection appeared to be best. This is consistent with previous literature, suggesting that prophylaxes associate with the best protective effects against paramyxoviruses (Groothuis et al., 2011).
Taken together, data add to our understanding of DAS181-mediated inhibition of the hPIVs. All hPIV-2 and hPIV-3 isolates were susceptible to full inhibition by DAS181 in vitro with drug doses of 0.1-1 nm. Of interest, this drug range was comparable to that described previously for inhibition of influenza viruses and laboratory strains of hPIV (Ah-Tye et al., 1999;Moscona et al., 2010). Our cotton rat model further illustrated that hPIV-3 could be inhibited by DAS181 in vivo, more so in the lung than in the nasal turbinates. Differences in locations might have been a simple consequence of drug distribution, as a relatively large volume of drug applied to sedated animals readily enters the lung (Burke et al., 2011). In addition, the differing distributions of epithelial cells and sialic acid moieties throughout the respiratory tract (Rackley & Stripp, 2012) may have influenced drug efficacy. The reduction of hPIV-3 amplification in lung tissue by DAS181 is a highly desirable attribute, given that the morbidity and mortality caused by hPIV-3 is predominantly due to LRT infections. URT infections with hPIV-3 are generally mild, and the short-term deposition of viral antigen has the potential to promote durable B and T cell residence in nasal associated lymphoid tissues (Rudraraju et al., 2011) to protect individuals from disease upon future virus exposure. Of note, DAS181 may be formulated in variable particle sizes to direct drug deposition either to URT or LRT tissues as desired.
DAS181 has recently been used to treat four severely ill immunocompromised patients who became infected with hPIV. Cases included stem cell and lung transplant patients, all of whom experienced an improved clinical outcome after treatment and two of whom experienced a reduced viral load (Chen et al., 2011;Drozd et al., 2013;Guzman-Suarez et al., 2012). Given that there is no licensed hPIV vaccine and that hPIV-infected patients are usually treated only with supportive care, DAS181 may provide an important new drug option for a currently unmet medical need in both adult and pediatric healthcare arenas.
Highlights.
DAS181, a sialidase fusion protein, inhibited amplification of six different human parainfluenza virus (hPIV) clinical isolates in vitro
Full inhibition of hPIV clinical isolates in vitro was achieved with drug concentrations ranging between 0.1 and 1 nM
DAS181 inhibited amplification of a clinical isolate of hPIV in vivo when drug was administered prior to infection
DAS181 inhibited amplification of a clinical hPIV isolate in vivo when drug was administered after infection in a preliminary study
The inhibition of the clinical hPIV isolate in vivo was superior in the lower respiratory tract relative to the upper respiratory tract
ACKNOWLEDGEMENTS
This work was supported in part by NIH, NIAID grants P01-AI-054955, R01-AI088729, and R01-AI78819, NCI grant P30-CA21765 and the American Lebanese Syrian Associated Charities (ALSAC). We thank Drs. Allen Portner and Irina Alymova of St. Jude for constructive advice. We thank the St. Jude Hartwell Center for conducting Sanger sequencing. We thank Drs. R.L. Routh and R.B. Moss of Ansun Biopharma, San Diego, CA for provision of drug, for advice concerning experimental design, and for assistance with data interpretation and manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ah-Tye C, Schwartz S, Huberman K, Carlin E, Moscona A. Virus-receptor interactions of human parainfluenza viruses types 1, 2 and 3. Microb. Pathog. 1999;27:329–336. doi: 10.1006/mpat.1999.0313. [DOI] [PubMed] [Google Scholar]
- Air GM, Laver WG. Red cells bound to influenza virus N9 neuraminidase are not released by the N9 neuraminidase activity. Virology. 1995;211:278–284. doi: 10.1006/viro.1995.1401. [DOI] [PubMed] [Google Scholar]
- Alymova IV, Portner A, Mishin VP, McCullers JA, Freiden P, Taylor GL. Receptor-binding specificity of the human parainfluenza virus type 1 hemagglutinin-neuraminidase glycoprotein. Glycobiology. 2012;22:174–180. doi: 10.1093/glycob/cwr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alymova IV, Taylor G, Takimoto T, Lin TH, Chand P, Babu YS, Li C, Xiong X, Portner A. Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo. Antimicrob. Agents Chemother. 2004;48:1495–1502. doi: 10.1128/AAC.48.5.1495-1502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson LD, Bukrinskaya AG, Prokazova NV, Shaposhnikova GI, Kocharov SL, Shevchenko VP, Kornilaeva GV, Fomina-Ageeva EV. Role of gangliosides in reception of influenza virus. Eur. J. Biochem. 1982;128:467–474. doi: 10.1111/j.1432-1033.1982.tb06988.x. [DOI] [PubMed] [Google Scholar]
- Blanco JC, Pletneva LM, Wan H, Araya Y, Angel M, Oue RO, Sutton TC, Perez DR. Receptor characterization and susceptibility of cotton rats to avian and 2009 pandemic influenza virus strains. J Virol. 2013;87:2036–2045. doi: 10.1128/JVI.00638-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br. J Haematol. 2008;143:455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CW, Mason JN, Surman SL, Jones BG, Dalloneau E, Hurwitz JL, Russell CJ. Illumination of parainfluenza virus infection and transmission in living animals reveals a tissue-specific dichotomy. PLoS. Pathog. 2011;7:e1002134. doi: 10.1371/journal.ppat.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YB, Driscoll JP, McAfee SL, Spitzer TR, Rosenberg ES, Sanders R, Moss RB, Fang F, Marty FM. Treatment of parainfluenza 3 infection with DAS181 in a patient after allogeneic stem cell transplantation. Clin. Infect. Dis. 2011;53:e77–e80. doi: 10.1093/cid/cir501. [DOI] [PubMed] [Google Scholar]
- Drozd DR, Limaye AP, Moss RB, Sanders RL, Hansen C, Edelman JD, Raghu G, Boeckh M, Rakita RM. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transpl. Infect. Dis. 2013;15:E28–E32. doi: 10.1111/tid.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Els MC, Laver WG, Air GM. Sialic acid is cleaved from glycoconjugates at the cell surface when influenza virus neuraminidases are expressed from recombinant vaccinia viruses. Virology. 1989;170:346–351. doi: 10.1016/0042-6822(89)90394-2. [DOI] [PubMed] [Google Scholar]
- Falsey AR. Current Management of Parainfluenza Pneumonitis in Immunocompromised patients: A Review. Infect Drug Resist. 2012;5:121–127. doi: 10.2147/IDR.S25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuehner T, Dierich M, Duesberg C, DeWall C, Welte T, Haverich A, Warnecke G, Simon AR, Gottlieb J. Single-centre Experience with Oral Ribavirin in Lung Transplant Recipients with Paramyxovirus Infections. Antivir. Ther. 2011;16:733–740. doi: 10.3851/IMP1811. [DOI] [PubMed] [Google Scholar]
- Graham AC, Hilmer KM, Zickovich JM, Obar JJ. Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling. J Immunol. 2013;190:4676–4684. doi: 10.4049/jimmunol.1202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JA, Basak S, Compans RW. Effects of hexose starvation and the role of sialic acid in influenza virus release. Virology. 1983;125:324–334. doi: 10.1016/0042-6822(83)90205-2. [DOI] [PubMed] [Google Scholar]
- Groothuis JR, Hoopes JM, Hemming VG. Prevention of serious respiratory syncytial virus-related illness. II: Immunoprophylaxis. Adv. Ther. 2011;28:110–125. doi: 10.1007/s12325-010-0101-y. [DOI] [PubMed] [Google Scholar]
- Guzman-Suarez BB, Buckley MW, Gilmore ET, et al. Clinical potential of DAS181 for treatment of parainfluenza-3 infections in transplant recipients. Transpl. Infect. Dis. 2012;14:427–433. doi: 10.1111/j.1399-3062.2012.00718.x. [DOI] [PubMed] [Google Scholar]
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N. Engl. J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- Henrickson KJ. Parainfluenza viruses. Clin. Microbiol. Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J, Svennerholm L, Elwing H, Fredman P, Strannegard O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc. Natl. Acad. Sci. U. S. A. 1980;77:1947–1950. doi: 10.1073/pnas.77.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz JL, Soike KF, Sangster MY, Portner A, Sealy RE, Dawson DH, Coleclough C. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine. 1997;15:533–540. doi: 10.1016/s0264-410x(97)00217-x. [DOI] [PubMed] [Google Scholar]
- Ito T. Interspecies transmission and receptor recognition of influenza A viruses. Microbiol. Immunol. 2000;44:423–430. doi: 10.1111/j.1348-0421.2000.tb02516.x. [DOI] [PubMed] [Google Scholar]
- Jones B, Zhan X, Mishin V, Slobod KS, Surman S, Russell CJ, Portner A, Hurwitz JL. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 2009;27:1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BG, Sealy RE, Rudraraju R, Traina-Dorge VL, Finneyfrock B, Cook A, Takimoto T, Portner A, Hurwitz JL. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine. 2012;30:959–968. doi: 10.1016/j.vaccine.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron RA, Collins PL. Parainfluenza Viruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. Lippincott Williams and Wilkins; 2007. pp. 1497–1526. [Google Scholar]
- Karron RA, Thumar B, Schappell E, Surman S, Murphy BR, Collins PL, Schmidt AC. Evaluation of two chimeric bovine-human parainfluenza virus type 3 vaccines in infants and young children. Vaccine. 2012;30:3975–3981. doi: 10.1016/j.vaccine.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell MA, Moss J, Hom BE, Fishman PH, Svennerholm L. Expression of gangliosides as receptors at the cell surface controls infection of NCTC 2071 cells by Sendai virus. Virology. 1986;155:356–364. doi: 10.1016/0042-6822(86)90199-6. [DOI] [PubMed] [Google Scholar]
- Markwell MA, Paulson JC. Sendai virus utilizes specific sialyloligosaccharides as host cell receptor determinants. Proc. Natl. Acad. Sci. U. S. A. 1980;77:5693–5697. doi: 10.1073/pnas.77.10.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishin VP, Watanabe M, Taylor G, DeVincenzo J, Bose M, Portner A, Alymova IV. N-linked glycan at residue 523 of human parainfluenza virus type 3 hemagglutinin-neuraminidase masks a second receptor-binding site. J. Virol. 2010;84:3094–3100. doi: 10.1128/JVI.02331-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A, Peluso RW. Fusion properties of cells persistently infected with human parainfluenza virus type 3: participation of hemagglutinin-neuraminidase in membrane fusion. J Virol. 1991;65:2773–2777. doi: 10.1128/jvi.65.6.2773-2777.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A, Peluso RW. Fusion properties of cells infected with human parainfluenza virus type 3: receptor requirements for viral spread and virus-mediated membrane fusion. J Virol. 1992;66:6280–6287. doi: 10.1128/jvi.66.11.6280-6287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A, Porotto M, Palmer S, et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J Infect Dis. 2010;202:234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RB, Hansen C, Sanders RL, Hawley S, Li T, Steigbigel RT. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect. Dis. 2012;206:1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin. Invest. 2012;122:2724–2730. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud C, Englund JA. Antiviral Therapy of Respiratory Viruses in Haematopoietic Stem Cell Transplant Recipients. Antivir. Ther. 2012;17:175–191. doi: 10.3851/IMP2060. [DOI] [PubMed] [Google Scholar]
- Riner A, Chan-Track KM, Murray J. Intravenous Ribavirin-Review of the FDA's Emergency Investigational New Drug Database (1997-2008) and Literature Review. Postgrad. Med. 2009;121:139–146. doi: 10.3810/pgm.2009.05.2014. [DOI] [PubMed] [Google Scholar]
- Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology. 2011;410:429–436. doi: 10.1016/j.virol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv. Carbohydr. Chem. Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Skiadopoulos MH, Tao T, Surman SR, Collins PL, Murphy BR. Generation of a parainfluenza virus type 1 vaccine candidate by replacing the HN and F glycoproteins of the live-attenuated PIV3 cp45 vaccine virus with their PIV1 counterparts. Vaccine. 1999;18:503–510. doi: 10.1016/s0264-410x(99)00227-3. [DOI] [PubMed] [Google Scholar]
- Slobod KS, Shenep JL, Lujan-Zilbermann J, Allison K, Brown B, Scroggs RA, Portner A, Coleclough C, Hurwitz JL. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22:3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Portner A, Scroggs RA, Uchikawa M, Koyama N, Matsuo K, Suzuki Y, Takimoto T. Receptor specificities of human respiroviruses. J Virol. 2001;75:4604–4613. doi: 10.1128/JVI.75.10.4604-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun C, Slaby J, Shanley RM, Vydra J, Smith AR, Wagner JE, Weisdorf DF, Young JA. Human Parainfluenza Virus Infection after Hematopoietic Stem Cell Transplantation: Risk Factors, Managemnet, Mortality, and Changes over Time. Biol. Blood marrow Transplant. 2012;18:1580–1588. doi: 10.1016/j.bbmt.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Mishin VP, Brown SA, et al. Effect of hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 on growth and pathogenicity of Sendai/human parainfluenza type 3 chimera virus in mice. Antimicrob. Agents Chemother. 2009;53:3942–3951. doi: 10.1128/AAC.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigt SS, Gregson AL, Deng JC, Lynch JP, 3, Belperio JA. Respiratory Viral Infections in hematopoietic Stem Cell and Solid Organ Transplant Recipients. Semin. Respir. Crit. Care Med. 2011;32:471–493. doi: 10.1055/s-0031-1283286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J. Pediatr. 2009;154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]