Abstract
Despite its critical role in supplying naïve T cells to the circulation, the thymus is particularly sensitive to immune injury, such as that caused by cytoreductive chemo- or radiation therapy, shock, infection and graft versus host disease (GVHD). Crucially, insufficient thymic recovery has been directly correlated with increased risk of opportunistic infections and poor clinical outcomes in recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT). Prolonged immune deficiency is particularly pronounced in older patients whose thymi are already significantly impaired due to age-related thymic involution. Previous preclinical studies have revealed several strategies that can enhance thymic function and immune reconstitution after transplant, including sex steroid ablation (SSA), growth factors (growth hormone, GH, keratinocyte growth factor, KGF, insulin-like growth factor 1, IGF-1, interleukin-7, IL-7) and ex vivo generated precursor T cells (preT). In addition, recent studies have shown that other approaches, such as interleukein-22 (IL-22) and nutritional changes, may represent additional candidates to enhance thymic regeneration. In this review we provide updates on these strategies and comment on their potential to be translated into clinical therapies.
Introduction
Allogeneic hematopoietic stem cell transplant (allo-HSCT) is a well-established therapy with curative potential for a variety of malignant and non-malignant diseases. Post-transplant immune deficiency can lead to opportunistic infections and malignant relapse that are major contributors to post-transplant morbidity and mortality. Delayed immune reconstitution, and in particular the slow recovery in the development of newly generated T cells, contributes towards poor patient outcome, not only in recipients of allo-HSCT, but also in other clinical settings of immune depletion, notably cancer chemotherapy, chronic infection, and radiation injury (1). Furthermore, an inverse relationship between a transplant recipient’s age and their capacity to generate T lymphocytes (especially CD4+ T cells) has been found, further complicating the use of allo-HSCT (2, 3). In fact, compared to children, de novo development of naive T cells in the thymus takes considerably longer in the adult and generates a more limited TCR repertoire leading to susceptibility to opportunistic infections (4). This aspect is of particular clinical importance given the number of allo-HSCT being performed in aged patients (≥50 years) has been increasing over the past two decades and projections predict further increases in this population (5). Although age-related decline of immune function is a multifactorial condition, one of the key players in this process is the progressive decline in thymic function (known as thymic involution) that begins as early as puberty. Therefore, developing strategies to regenerate thymic function and peripheral immune reconstitution represent an important clinical challenge with the capacity to improve outcome in immunocompromised patients. The central purpose of this review is to provide updates on promising regenerative strategies that have the potential to be translated into clinical therapies.
The importance of thymic regeneration
The thymus is the principal lymphoid organ responsible for generating and supplying naïve T cells, and a broad TCR repertoire, to the periphery. The process of T cell development is tightly regulated by the bidirectional crosstalk between thymic stromal cells and developing thymocytes (6). Thymic epithelial cells (TECs), endothelial cells, fibroblasts, and dendritic cells within the thymic stroma play a critical role to guide the differentiation of bone marrow (BM)-derived T cell progenitors through distinct developmental steps that ultimately lead to the formation of mature CD4+ or CD8+ T cells expressing an MHC-restricted, antigen-specific TCR.
Although the thymus is critically important for the supply of new T cells, it is particularly sensitive to endogenous and exogenous insults such as infection, shock, sex steroids, cytoreductive chemo- or radiation therapy and graft-versus-host disease (GVHD). While the thymus has a remarkable capacity for endogenous regeneration, this capacity declines considerably with age. However, while this process of age-related thymic involution does not represent a significant clinical problem in a healthy individual, reduced thymic function is detrimental when active recovery of thymopoiesis is required to sustain immune competence after clinically induced immune depletion. Insufficient recovery in thymopoiesis has been directly linked to opportunistic infections and an adverse clinical outcome in recipients of allo-HSCT (7).
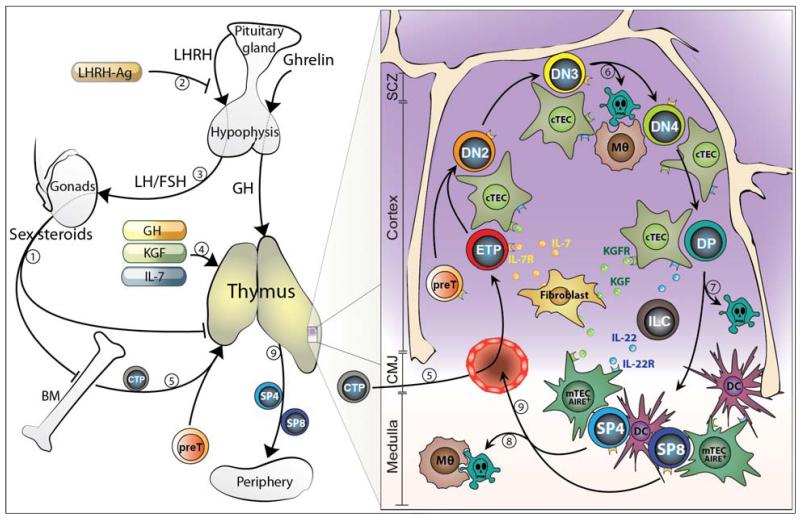
Previous preclinical studies have proposed several strategies that can protect and restore thymic function as well as boost the immune reconstitution after allo-HSCT. Sex steroid ablation (SSA), growth hormone (GH), growth factors (keratinocyte growth factor, KGF, and insulin-like growth factor 1, IGF-1), interleukin-7 (IL-7) and precursor T cells (preT), have all shown positive effects in enhancing thymic regeneration after injury (Figure 1).
Figure 1. Overview of steady-state thymopoiesis in addition to therapeutic strategies currently in clinical trials to enhance thymic recovery after HSCT.
The increase in sex steroids during life is generally thought to progressively impair BM and thymic lymphopoiesis (1). SSA using a LHRH-Ag (2) (that blocks the upstream signals stimulating sex steroid production (3)) reverses the age-related decline in BM and thymic function and promotes their rejuvenation. Similarly, therapeutic administration with GH, KGF or IL-7 promotes the regeneration of T cell lymphopoiesis (4). The process of thymopoiesis begins when circulating BM-derived T-lineage progenitors (CTP) seeds the thymus in the cortico-medullary junction (CMJ) (5) and undergo a series of well-defined maturation steps. This all occurs under the guidance and close interaction with the thymic stromal microenvironment; which is comprised of cTEC, mTEC, fibroblasts, macrophages (Mθ) and DC, and which provide survival, differentiation and homing factors to the developing thymocytes. Approximately 95% of thymocytes produced daily die during the process of beta-selection (6), positive selection (7) and negative selection (8) by apoptosis, which results in the generation of self-restricted and self-tolerant naïve CD4+ and CD8+ T cells (SP4 and SP8) (8). Several intrathymic factors have been shown to be important for endogenous T cell regeneration and may have some therapeutic benefit including IL-7, KGF and IL-22. SCZ (Sub-capsular zone), ILC (Innate lymphoid cells)
Keratinocyte growth factor
The murine and human fibroblast growth factor (FGF) family consists of at least 23 structurally related growth factors. FGF7 (also known as KGF) is a protein of 194 amino acids and with a hypothetical molecular weight of 22kDa (8). KGF is mainly expressed by non-hematopoietic stromal cells and induces the proliferation and repair of epithelial cells after injury (8). Within the thymus, KGF is expressed mainly by fibroblasts and mesenchymal cells, but is also expressed by thymocytes after the double negative (DN) stage. Although mice deficient for KGF have intact thymic architecture and thymopoiesis, suggesting redundancy in the steady-state function of KGF, thymic recovery after immune injury is significantly delayed in KGF−/− mice (9).
In addition to this endogenous role in thymic function, exogenous administration of results in a two-fold increase in thymic cellularity in young mice and a four-fold increase in aged mice (9). Importantly, KGF administrati enhance thymopoiesis and peripheral T cell reconstitution after syngeneic and allogeneic HSCT (10), and in models of allogeneic umbilical cord blood transplantation (11). KGF enhances thymopoiesis by promoting the proliferation of TECs and protecting them from damage caused by radiation, chemotherapy or GVHD (12). Interestingly, in a non-human primate model, KGF improves thymic architecture and T cell recovery after CD34+ peripheral blood progenitor transplant (13).
KGF is a FDA-approved drug for the prevention of mucositis in patients receiving high dose chemotherapy before HSCT. A recent retrospective study with 251 patients showed that KGF does not increase transplant toxicity and it is safe for allo-HSCT recipients (14). However, additional prospective studies are required to clarify the potential of KGF as a thymopoietic factor in the setting of HSCT.
Interleukin-7
Interleukin 7 (IL-7) is a lymphopoietic cytokine that has been studied for its role in endogenous lymphocyte development in the thymus and BM, as well as its role in the function of mature T cells. IL-7 is primarily produced by non-hematopoietic stromal cells in the thymus and BM, while the IL-7 receptor (IL-7R) is expressed by several cell populations of developing lymphocytes, such as double negative (DN), CD4+ and CD8+ thymocytes, common lymphoid progenitors, innate lymphoid cells, pro- and pre-B cells and some dendritic cells (15). The outcome of IL-7 signaling appears to be in inhibiting apoptosis and in promoting differentiation (15).
Due to its role in directly promoting lymphocyte development, the potential of IL-7 for exogenous modulation of the immune system has been extensively studied in several mouse models. Pharmacological treatment of mice with recombinant IL-7 increases T cell reconstitution after syngeneic and allogeneic HSCT through increasing the proliferation of lymphocytes and lymphoid precursors, in addition to the homeostatic proliferation of mature circulating T cells (16). IL-7−/− mice exhibit a profound reduction in thymic size with few T cell produced and a total lack of T cells as IL-7 is indispensable for the recombination of chain of the TCR (17). In humans, a defect in IL-7 signaling leads to a severe combined immunodeficiency (SCID)(18).
Clinical trials have been performed to test the safety and efficacy of IL-7 as an immune boosting treatment. Previous studies have reported that both a non-glycosylated (CYT 99 007, Cytheris) and a glycosylated (CYT107, Cytheris) form of human IL-7 promotes a sustained dose dependent expansion of peripheral CD3+, CD4+ and CD8+ T cells and increases TCR repertoire diversity (19, 20). Although neither study could definitively show benefits in thymopoiesis, unlike the non-glycosylated form, the fully glycosylated IL-7 did not show signs of anti-IL-7 auto-antibody production. Further studies are required to achieve a better understanding of the therapeutic effect of IL-7 treatment in HSCT recipients.
Sex steroid ablation
In addition to their role in sexual dimorphism, sex steroids also play a critical role in regulating the immune system. It is well documented that the progressive decline in thymic size and function during life is in part correlated with an increase in sex steroids (21). Consistent with this, administration of androgens or estrogens induces thymic involution in young mice and their ablation promotes its regeneration (21). Perhaps unsurprisingly, the effects of sex steroids are not restricted exclusively to the thymus, and their ablation also enhances B cell lymphopoiesis (22). Goldberg and colleagues demonstrated that using the LHRH-agonist Lupron as a model of reversible chemical SSA, recipients of allogeneic HSCT showed accelerated engraftment post-HSCT, enhanced T cell reconstitution and increased T cell function (23). Clinical trials using the LHRH-agonist goserelin, showed increased CD4+ T-cell regeneration, enhanced TCR repertoire, and improved T cell function in auto- and allo-HSCT recipients (24). Even though the beneficial effects on immune reconstitution are not fully understood, SSA continues to be an attractive clinical therapy to restore immunocompetence in immunocompromised patients.
Ghrelin, GH, IGF-1
GH is a peptide hormone that is produced primarily by the anterior pituitary gland and GH receptor expression is present in the thymus and BM on both hematopoietic and non-hematopoietic compartments (25). Exogenous administration of GH in mice increases thymic cellularity after HSCT, protects BM from radiation injury, delays thymic involution and positively regulates T cell migration (26). Studies have also shown that GH administration in HIV-infected patients increases thymic cellularity and peripheral immune response (27). Ghrelin and IGF-1 are believed to be involved in the GH pathway, where ghrelin promotes secretion of GH, and IGF-1 is one of the main mediators of the effects of GH (25). Clinical trials are ongoing to determine whether GH administration to patients undergoing allogeneic stem cell transplant will enhance immune reconstitution and protect from post-transplant complications.
Emerging strategies
T cell progenitors
In humans, the development of thymic-derived T cells can take from months to years and this process is even longer in aged recipients and patients affected by post-transplant complications such as GVHD. Therefore, strategies that can enhance the formation of thymic-derived T cells have the potential to reduce the period of post-transplant lymphopenia. Previous studies have demonstrated that a large number of precursor T (preT) cells can be generated using an ex vivo culture system with forced expression of Notch ligands Delta-like (DLL) 1 or 4, key mediators of T cell commitment and differentiation (28, 29). The adoptive transfer of preT cells, with T cell depleted BM or purified LSK into lethally irradiated recipients increases thymic cellularity, improves T cell chimerism and enhances peripheral T and NK reconstitution (30). Adoptive cell therapy with preT cells in HSCT recipients can also enhance regeneration of the thymic stroma, through the restoration of the bidirectional thymic cross-talk (30). In addition, preT cells may be used “off the shelf” across MHC barriers, since they were educated and selected in the recipients (31). Importantly, preT cells have also the potential to be genetically engineered with viral or tumor specificity (32).
Caloric restriction
Caloric restriction (CR) has been known for some time to have beneficial effects on animal lifespan and longevity. There is also increasing evidence that nutritional changes can have important implications in regulating immune responses (33). Several reports have demonstrated that CR enhances thymopoiesis and prevents mice from age-related thymic involution through the restoration of the TCR repertoire diversity and the increase in the proportion of naïve to memory T cells in the periphery (34). In contrast, high-fat diet-fed mice show premature thymic involution, increased thymocyte apoptosis, and accelerated age-related reduction in T-cell receptor excision circles (TRECs) and TCR diversity (35). These effects are not only restricted to the thymus, with CR also impacting BM lymphopoiesis (36).
Although CR has shown promising results as a new strategy to enhance immune function, many questions concerning its applicability still remain. Several groups have reported that CR can actually compromise the recovery and clearance of some infections, in part by impairing NK maturation and function. Therefore, the identification of specific therapeutic targets underlying the mechanism of CR could represent a better approach to enhance immune reconstitution in immunocompromised patients. Several putative targets have emerged including sirtuin (silent mating type information regulation 2 homologue) protein family, which increases after CR treatment and mediates some of the beneficial effects associated with low caloric intake (37). SIRT1 has been shown to increase in response to CR in many tissues and SIRT1−/− mice do not display some of the metabolic changed observed after CR treatment. Therefore, the development of specific SIRT1 activators could mimic CR effects.
Interleukin-22
Interleukine-22 (IL-22) is an IL-10 family cytokine that is primarily expressed by TH17 cells and innate lymphoid cells (38). IL-22 has been implicated in promoting epithelial integrity and antimicrobial immunity at mucosal surfaces (39). Recently we have revealed a framework of endogenous thymic regeneration that is centered on the production of IL-22 (40). In this model, the depletion of CD4+CD8+ double positive thymocytes triggers the production of IL23 by DCs, which in turn induces the production of IL-22 by thymic ILC. IL-22 can act directly on TECs, promoting their survival and proliferation, which ultimately leads to the rejuvenation of thymopoiesis. Importantly, exogenous administration of IL-22 promoted accelerated thymic recovery after sub-lethal irradiation in young mice. These findings suggest that IL-22 represents a novel therapeutic strategy for immune regeneration. Currently, clinical trials are on going to evaluate the safety and pharmacokinetic profile of IL-22 treatment in healthy volunteers.
Combination strategies
Multiple strategies have been proposed to boost thymic recovery in periods of immune distress, however, although promising in preclinical studies, many of these have failed to generate compelling clinical evidence as to their effectiveness. Given that the thymus requires reciprocal cellular communication between the stromal and hematopoietic compartments for its development and ongoing maintenance, one possible approach to improve the clinical effectiveness of immune regeneration therapies would be to develop a combination strategy able to boost both compartments. For example, KGF has been shown to have synergistic effects when administered with preT (30), SSA (41) and p53 inhibition in preclinical studies (42). Moreover, a clinical trial is ongoing at MSKCC to address if combination therapy with KGF and SSA can promote faster immune recovery following stem cell transplant.
Conclusions
The prerequisite for recovery of robust thymopoiesis to establish effective adaptive immunity after periods of immune distress is a significant clinical problem. The therapeutic approaches outlined in this review have the potential to provide a rational basis for novel clinical strategies that enhance thymic function and immune recovery, not only in patients undergoing allo-HSCT but also in other situations of immune depletion, such as chronic infection, radiation exposure and aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Current opinion in hematology. 2012;19:324–335. doi: 10.1097/MOH.0b013e328353bc7d. [DOI] [PubMed] [Google Scholar]
- 2.Small TN, Avigan D, Dupont B, Smith K, Black P, Heller G, Polyak T, O’Reilly RJ. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biol Blood Marrow Transplant. 1997;3:65–75. [PubMed] [Google Scholar]
- 3.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–425. [PubMed] [Google Scholar]
- 4.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, Gress RE. Age-dependent incidence, time course, and consequences of thymic renewal in adults. The Journal of clinical investigation. 2005;115:930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2012 Available at: http://www.cibmtr.org.
- 6.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 7.Wils EJ, van der Holt B, Broers AE, Posthumus-van Sluijs SJ, Gratama JW, Braakman E, Cornelissen JJ. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica. 2011;96:1846–1854. doi: 10.3324/haematol.2011.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Advances in cancer research. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 9.Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, Suh D, Muriglan SJ, Boyd RL, van den Brink MR. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, Lacey DL, Blazar BR, Weinberg KI. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Chen G, Qiao S, Ma X, Tang X, Sun A, Wu D. Keratinocyte growth factor enhanced immune reconstitution in murine allogeneic umbilical cord blood cell transplant. Leukemia & lymphoma. 2011;52:1556–1566. doi: 10.3109/10428194.2011.573037. [DOI] [PubMed] [Google Scholar]
- 12.Rossi SW, Jeker LT, Ueno T, Kuse S, Keller MP, Zuklys S, Gudkov AV, Takahama Y, Krenger W, Blazar BR, Hollander GA. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109:3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seggewiss R, Lore K, Guenaga FJ, Pittaluga S, Mattapallil J, Chow CK, Koup RA, Camphausen K, Nason MC, Meier-Schellersheim M, Donahue RE, Blazar BR, Dunbar CE, Douek DC. Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood. 2007;110:441–449. doi: 10.1182/blood-2006-12-065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg JD, Zheng J, Castro-Malaspina H, Jakubowski AA, Heller G, van den Brink MR, Perales MA. Palifermin is efficacious in recipients of TBI-based but not chemotherapy-based allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. 2013;48:99–104. doi: 10.1038/bmt.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Alpdogan O, Schmaltz C, Muriglan SJ, Kappel BJ, Perales MA, Rotolo JA, Halm JA, Rich BE, van den Brink MR. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 17.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki JI, Ikuta K. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasher D, Dalal I. The genetic basis of severe combined immunodeficiency and its variants. The application of clinical genetics. 2012;5:67–80. doi: 10.2147/TACG.S18693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I, Stetler-Stevenson M, Engel J, Buffet R, Morre M, Amato RJ, Pecora A, Mackall CL, Gress RE. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perales MA, Goldberg JD, Yuan J, Koehne G, Lechner L, Papadopoulos EB, Young JW, Jakubowski AA, Zaidi B, Gallardo H, Liu C, Rasalan T, Wolchok JD, Croughs T, Morre M, Devlin SM, van den Brink MR. Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood. 2012;120:4882–4891. doi: 10.1182/blood-2012-06-437236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell Immunol. 2008;252:122–138. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Dudakov JA, Goldberg GL, Reiseger JJ, Chidgey AP, Boyd RL. Withdrawal of sex steroids reverses age- and chemotherapy-related defects in bone marrow lymphopoiesis. J Immunol. 2009;182:6247–6260. doi: 10.4049/jimmunol.0802446. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC, Samstein RM, Dudakov JA, Chidgey AP, Chen-Kiang S, Boyd RL, van den Brink MR. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol. 2009;182:5846–5854. doi: 10.4049/jimmunol.0801458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutherland JS, Spyroglou L, Muirhead JL, Heng TS, Prieto-Hinojosa A, Prince HM, Chidgey AP, Schwarer AP, Boyd RL. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:1138–1149. doi: 10.1158/1078-0432.CCR-07-1784. [DOI] [PubMed] [Google Scholar]
- 25.Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Current opinion in pharmacology. 2010;10:408–424. doi: 10.1016/j.coph.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Experimental hematology. 2003;31:953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 27.Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert EL, Ng MM, Clor JL, Epling L, Sinclair E, Baum PD, Li K, Killian ML, Bacchetti P, McCune JM. Growth hormone enhances thymic function in HIV-1-infected adults. The Journal of clinical investigation. 2008;118:1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Smedt M, Hoebeke I, Plum J. Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood cells, molecules & diseases. 2004;33:227–232. doi: 10.1016/j.bcmd.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 29.La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 30.Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM, Muriglan SJ, Suh D, Smith OM, Grubin J, Patel N, Chow A, Cabrera-Perez J, Radhakrishnan R, Diab A, Perales MA, Rizzuto G, Menet E, Pamer EG, Heller G, Zuniga-Pflucker JC, Alpdogan O, van den Brink MR. Adoptive transfer of Tcell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nature medicine. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 31.Holland AM, Zakrzewski JL, Goldberg GL, Ghosh A, van den Brink MR. Adoptive precursor cell therapy to enhance immune reconstitution after hematopoietic stem cell transplantation in mouse and man. Seminars in immunopathology. 2008;30:479–487. doi: 10.1007/s00281-008-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zakrzewski JL, Suh D, Markley JC, Smith OM, King C, Goldberg GL, Jenq R, Holland AM, Grubin J, Cabrera-Perez J, Brentjens RJ, Lu SX, Rizzuto G, Sant’Angelo DB, Riviere I, Sadelain M, Heller G, Zuniga-Pflucker JC, Lu C, van den Brink MR. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nature biotechnology. 2008;26:453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging and disease. 2012;3:91–129. [PMC free article] [PubMed] [Google Scholar]
- 34.Dixit VD. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Current opinion in immunology. 2010;22:521–528. doi: 10.1016/j.coi.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Astle CM, Harrison DE. Hematopoietic senescence is postponed and hematopoietic stem cell function is enhanced by dietary restriction. Experimental hematology. 2003;31:1097–1103. doi: 10.1016/s0301-472x(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 37.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 38.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunological reviews. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 39.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 40.Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, Boyd RL, van den Brink MR. Interleukin-22 drives endogenous thymic regeneration in mice. Science. 2012;336:91–95. doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, Taylor PA, Boyd RL, Hollander GA, Blazar BR. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008;111:5734–5744. doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly RM, Goren EM, Taylor PA, Mueller SN, Stefanski HE, Osborn MJ, Scott HS, Komarova EA, Gudkov AV, Hollander GA, Blazar BR. Short-term inhibition of p53 combined with keratinocyte growth factor improves thymic epithelial cell recovery and enhances T-cell reconstitution after murine bone marrow transplantation. Blood. 2010;115:1088–1097. doi: 10.1182/blood-2009-05-223198. [DOI] [PMC free article] [PubMed] [Google Scholar]