Abstract
Behavioral studies have identified select experiences that can prime infants to attend to color information as the basis for individuating objects prior to the time they do so spontaneously. For example, viewing pretest events in which the color of an object predicts the function in which it will engage leads 9-month-olds (who typically do not attend to color differences) to demonstrate increased sensitivity to color information in a subsequent individuation task (Wilcox & Chapa, 2004). In contrast, viewing pretest events in which the color of an object predicts distinct object motions, but the motions are not functionally relevant, does not produce color priming. The purpose of the present research was to identify the cortical underpinnings of these behavioral effects. Infants aged 8 and 9 months viewed function or motion pretest events and then their capacity to individuate-by-color was assessed in an object individuation task. Behavioral and neuroimaging data were collected. Two main findings emerged. First, as predicted, the infants who viewed the function but not the motion pretest events showed prolonged looking to the test event, a behavioral indicator of object individuation. In addition, they evidenced increased activation in anterior temporal cortex, thought to be a cortical signature of object individuation. A second and unexpected finding was that viewing either type of pretest events led to increased activation in posterior temporal cortex, as compared to infants who did not see pretest events, revealing that prior exposure to the motion pretest events does influence infants’ processing of the test event, even though it is not evident in the behavioral results. The cognitive processes involved, and the cortical structures that mediate these processes, are discussed.
Keywords: object processing, color priming, temporal cortex, infants, near-infrared spectroscopy
I. Introduction
There are a growing number of reports in the adult literature of the effect of recent learning experiences on patterns of activation in the cortex (Eliassen, Souza, & Sanes, 2003; Op de Beeck, Baker, DiCarlo, & Kanwisher, 2006; van der Linden, van Tirennout & Indefrey, 2009; van der Linden, Murre & van Turennout, 2008; Weisberg, van Turennout, & Martin, 2007). For example, forming object categories through exemplar training can alter functional activation in superior temporal sulcus (STS) to objects belonging to the learned categories (van der Linden et al., 2009) and experience-dependent activation patterns are observed in fronto-parietal areas during visual-motor associative learning (Eliassen et al., 2003). Findings like these help cognitive neuroscientists better understand processes that underlie specific types of learning and properties of the brain that give rise to and support these processes. Reports of learning-related activation patterns in the immature brain remain elusive, in large part because of a lack of neuroimaging techniques available to study functional activation of the infant brain during cognitive tasks. However, with recent advances in fNIRS technology we now have a viable technique for investigating the extent to which recent experiences alter brain and behavior in the infant.
Effects of Color Priming on Object Individuation: Behavioral Results
We do know that the way that infants perceive, apprehend, and act on objects in the physical world can be influenced by recent experience. This has been demonstrated in a number of experimental contexts. For example, Needham and her colleagues (Libertus & Needham, 2010; Needham, Barrett, & Peterman, 2002) reported manipulatory experiences that facilitate object exploration and perception in 2- to 3-month-olds and Wang,Baillargeon, & Paterson (2005) identified experiences that facilitate 8-month-olds’ use of height information when interpreting uncovering events. Most relevant to the present research are experiences that can alter the type of information infants' use to individuate objects. There is evidence that within the context of object individuation tasks infants are more sensitive to some features than others. For example, infants use shape information to individuate objects by at least 4.5 months, yet they fail to use color information as the basis for individuating until about 11.5 months (Wilcox, 1999; Wilcox, Woods, Chapa, & McCurry, 2007). To be clear, infants can perceive the color differences, but they fail to draw on these differences to individuate objects. Subsequent studies have revealed, however, that infants younger than 11.5 months can be primed to individuate-by-color if they are first given experiences that highlight the value of attending to color differences (Wilcox & Chapa, 2004; Wilcox, Woods, & Chapa, 2008; Wilcox et al., 2007; Woods & Wilcox, 2012).
In one color priming experiment (Wilcox & Chapa, 2004), 9.5-month-olds were assigned to one of two conditions: function or motion. Infants tested in the function condition were first shown two pairs of pretest, or priming, trials in which the color of the objects (Figure 1 and Figure 2) predicted the function in which the object would engage (i.e., green containers pounded a nail and red containers scooped and poured salt). Next, infants’ ability to individuate two different-colored objects, a green ball and a red ball, was assessed in an individuation task (Figure 3). Infants assigned to the motion condition were tested using the same pretest-test protocol except that in the pretest events the actions in which the containers engaged were distinct (i.e., green containers made pounding motions and red containers made scooping and pouring motions) but not functionally relevant (i.e., not causally related to a function outcome). This was accomplished by moving the box with the nail/salt to the center of the platform, so that that the containers did not come in contact with the nail/salt as they underwent pound/pour motions. The results indicated that only the infants in the function condition individuated the green and red ball in the test events. That is, the experience of viewing the function but not the motion pretest events led to increased sensitivity to color information in the subsequent individuation task, a finding that has been replicated in studies using other types of function and motion events (Wilcox et al., 2008). Perhaps most striking about these results is the demonstration that observing one type of object event (different-colored containers performing different functions) can influence infant's interpretation of a very different type of object event (different-colored balls moving in and out of view).
Figure 1.
The pound and pour pretest events of the function condition used in Wilcox and Chapa (2004) and in Experiment 1. The pretest events of the motion condition were identical to those of the function condition except that the box with the nail or the box with the salt was moved 22 cm to the left, to the center of the stage. Hence, the cups never came in contact with the nail (pound event) or salt (pour event) when they underwent their motions.
Figure 2.
The cups used in the first and second pair of pretest events in Wilcox and Chapa (2004) and in Experiment 1. In each pair, the cup on the left was green and was used to pound and the cup on the right was red and was used to pour.
Figure 3.
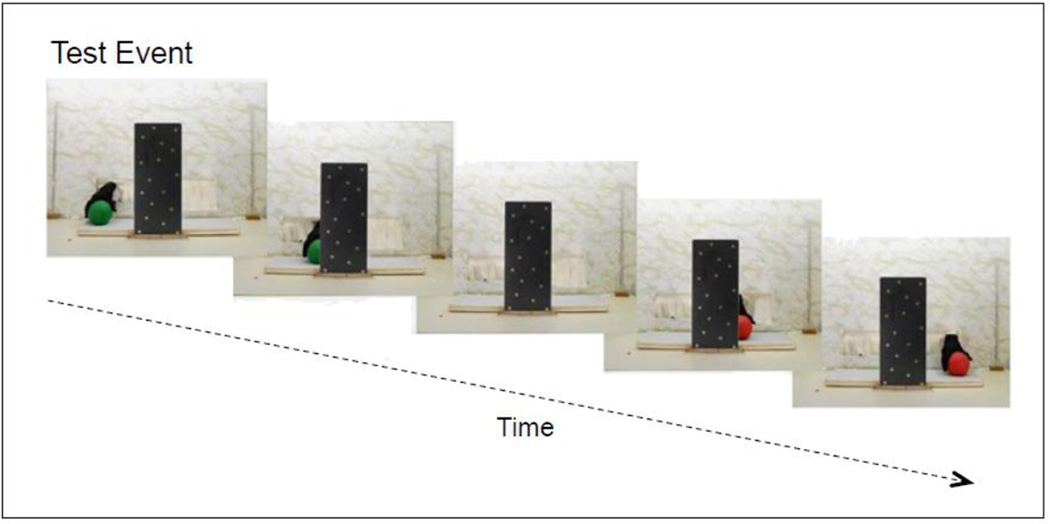
The narrow-screen green ball-red ball test event used in Wilcox and Chapa (2004) and in Experiments 1 and 2. The ball seen to the left of the screen was green and to the right of the screen was red. The screen was too narrow to hide both balls simultaneously. Prolonged looking to the narrow-screen green ball-red ball test event is taken as evidence that infant's individuated the objects (i.e., used the color differences to signal the presence of two objects and recognized that both objects could not fit simultaneously behind the screen; see Wilcox & Woods, 2009 for a review of the evidence).
In summary, recent behavioral studies have revealed a developmental hierarchy in the type of featural information to which infants are most sensitive, with infants responding to shape differences early in the first year and color differences later in the first year. However, infants can be primed to attend to color differences, at an age younger than they do so spontaneously, by first viewing color-function events.
1.2 The Cortical Basis of Object Individuation
The introduction of fNIRS into the experimental setting has resulted in a growing number of studies that have investigated the cortical basis of object processing in infants (see Lloyd-Fox, Blasi, & Elwell, 2010 for a review). Many of these studies have focused on the cortical substrates that support infants' emerging capacity to track the identity of objects (Wilcox, Bortfeld, Armstrong, Woods, & Boas, 2009; Wilcox, Bortfeld, Woods, Wruck, & Boas, 2008) and, more specifically, infants' ability to individuate-by-feature (Wilcox, Haslup, & Boas, 2010; Wilcox, Stubbs, Hirshkowitz, & Boas, 2012). In the latter studies, infants ranging between 3 and 12 months of age were shown occlusion events similar to the one depicted in Figure 3 except that the screen was wider.1 In addition, the objects that emerged successively from behind the screen differed in shape (green ball-green box), color (green ball-red ball) or were identical in appearance (green ball-green ball). Neural activation was recorded in several cortical areas, including the occipital and temporal cortex. Two main findings emerged.
First, infants 3 to 7 months of age, who use shape but not color information to individuate objects (Wilcox, 1999), showed activation in the anterior temporal cortex when viewing the different-shape but not the different-color test event (Wilcox et al., 2010, 2012). It was not until 11 to 12 months, when infants first individuate-by-color (Wilcox, 1999; Wilcox et al., 2007), that infants showed activation in the anterior temporal cortex when viewing the different-color event. Finally, none of the age groups showed activation in anterior temporal cortex when viewing the control (green ball-green ball) test event, an event that infants interpret as involving a single object (Wilcox, 1999). In summary, different patterns of cortical activation were obtained for events in which infants used featural differences to signal the presence of distinct objects – events in which the individuation process was engaged – than for events in which this did not occur. This effect does not appear to be limited to individuation-by-feature. Wilcox et al. (2010) reported that neural activation is obtained in anterior temporal cortex when spatiotemporal information, such as a discontinuity in path or speed of motion, signals the presence of distinct objects.
What we have suggested, above, is that activation of the anterior temporal cortex observed in the Wilcox et al. studies is a cortical signature of object individuation: anterior temporal cortex mediates the individuation process and when this process is invoked neural activation is obtained. This interpretation is consistent with adult fMRI data implicating areas in the temporal cortex as important for mediating higher level object processes, such as object identification and categorization (Devlin et al., 2002; Humphreys, Price, & Riddoch, 1999; Malach et al., 1995). An alternative interpretation of the data is that the anterior temporal cortex is involved in the processing of small sets of objects and that this evokes cortical activation. Most adult fMRI data implicate parietal rather than temporal areas as important for numerical processing in the adult brain (Dehaene, 2007), which would argue against this interpretation. However, there is some evidence (Feigenson, Dehaene, & Spelke, 2004; Hyde & Spelke, 2008) that infants represent small sets as a group of distinct entities (object x and object y) rather than as a cardinal value (two objects), raising the possibility that infants' processing of small sets is mediated by a structure other than the posterior temporal cortex, such as the the anterior temporal cortex. Given that currently there is more support for the "object individuation" than the "small sets" hypothesis, we will adopt the former for the purpose of this paper. However, we acknowledge that these hypotheses require further testing.
The second main finding was that of age-related changes in patterns of cortical activation. In 3-to 7-month-olds, activation was obtained in posterior temporal areas in response to all of the test events (different-shape, different-color, control) and the magnitude of the response did not vary by event condition (Wilcox et al., 2010; Wilcox et al., 2012). Unexpectedly, a different pattern of results was obtained with older infants. In contrast to the 3- to 7-month-olds, 11- to 12-month-olds did not evidence a significant increase in posterior temporal activation in response to the test events (Wilcox et al., 2012). (Infants aged 8 to 10 months have not been tested so it is unclear how infants in that age group respond.) One explanation for this finding suggested by Wilcox et al. (2012) is that early in the first year multiple structures (or pathways) mediate processing of moving objects, but with time and experience some pathways are pared down. There is evidence from nonhuman primate studies (Bachevalier & Mishkin, 1994) that in early infancy recognition of familiar objects is mediated by two pathways that project from the inferior temporal cortex to the medial temporal cortex: TEO ⇒-H and TE ⇒ H. By the end of infancy only the latter pathway remains. However, if area TE is ablated before the TEO ⇒ H pathway is eliminated, then TEO ⇒ H remains functional and object recognition abilities are spared. Perhaps in the human infant there are multiple cortical structures involved in the tracking of individual objects, and with the paring down of object processing pathways posterior temporal cortex is eliminated from the circuit. The current research will assess the extent to which the posterior temporal cortex remains part of this circuit (i.e., is activated during an object individuation task) in 8- and 9-month-olds.
Finally, it is important to note that neural activation was obtained in occipital cortex in response to all of the events described above. This response tends to be quite robust, regardless of the age group tested or the nature of the occlusion event (Wilcox, Bortfeld, Woods, Wruck, & Boas, 2005; Wilcox et al., 2009; Wilcox et al., 2008; Wilcox et al., 2010; Wilcox et al., 2012). This result is not surprising: one would expect neural activation in occipital cortex to sensory processing of visual events.
1.3 The Present Research
The primary goal of the present research was to examine the extent to which recent experience influences behavioral and cortical responses in an object individuation task. Behavioral studies, reviewed above, have revealed that young infants' sensitivity to color information in an individuation task can be enhanced by first viewing color-function pretest events. In addition, fNIRS studies, also reviewed above, have revealed what appears to be a cortical signature of object individuation. If viewing color-function events primes infants younger than 11.5 months to attend to color differences in a subsequent object individuation task, and individuation-by-feature has a unique cortical signature, then younger infants who view function, but not motion, pretest events should show that cortical signature and demonstrate behavioral responses consistent with object individuation in subsequent test events.
2. Experiment 1
Infants aged 8 to 9 months were assigned to one of two conditions: function or motion. Infants in each condition were tested using a two-phase procedure: pretest (or priming) trials followed by test trials. In the pretest phase of the experiment, infants were shown either function or motion pretest events with green and red containers (Figure 1 and Figure 2). In the test phase of the experiment, all infants were shown a narrow-screen green ball-red ball test event (Figure 3). Prolonged looking to the narrow-screen green ball-red ball test event is taken as evidence that infant's individuated the objects). If those infants who saw the function (but not the motion) pretest events were primed to attend to color differences, they should show behavioral responses and hemodynamic responses consistent with sensitivity to color differences during the narrow-screen green ball-red ball test event.
2.1 Methods
2.1.1 Participants
infants' aged 8 and 9 months participated in Experiment 1 (N = 39; 17 males and 22 females; M age = 9 months, 1 day, range = 8 months, 1 day to 9 months, 28 days). ). Parents reported their infant’s race/ethnicity as Caucasian (n=25), Hispanic (n=5), Black (n=4), or of mixed race/other (n=5). Fourteen additional infants were tested but eliminated from the sample because of procedural problems (n = 5) or difficulty in obtaining an optical signal (n = 9). Infants were pseudo-randomly assigned to the function (n = 21) or motion (n = 18) condition.
In both experiments participants were recruited primarily from commercially produced lists. Parents were offered $5 or a lab T-shirt for participation.
2.1.2 Task and Procedure
The infants tested in the function condition were first presented with two pairs of pretest trials. Each pair of trials consisted of a pound trial followed by a pour trial (Figure 1). Each pretest trial was 30 s in duration, during which infants saw almost 4 complete cycles of the event, pound or pour, appropriate for that trial (i.e., each event cycle was 8 s in duration, but only 6 s of the last event cycle were seen). Each pair of pound-pour trials was seen with a different pair of green and red containers (Figure 2). The green container always pounded and the red container always poured, so that object color predicted the event in which the object would engage. Following the pretest phase of the experiment, infants were presented with a narrow-screen green ball-red ball (GB-RB) test event on four consecutive trials (Figure 3). Each test trial was 20 s in duration, during which infants saw two complete cycles of the occlusion event (i.e., each event cycle was 10 s in duration). The curtain was raised to begin, and lowered to end, each pretest and test trial. Because analysis of optical imaging data requires a baseline interval, each pretest and test trial was preceded by a 10 s baseline during which time the curtain remained lowered to occlude the stage of the apparatus. The infants in the motion condition were tested using the same protocol with one exception: the infants saw motion pound-pour pretest events rather than function pound-pour pretest events. In the motion pretest events the nail-box (pound trials) or salt-box (pour trials) was moved 22 cm leftward to the center of the stage (the width of each box was 19.5 cm). Hence, in the pound trials the green container moved up and down without coming in contact with the nail and in the pour trials the red container made scooping and pouring motions without acquiring and releasing salt.
Infants sat in a Bumbo® seat in a quiet, dark room and watched the events appropriate for their condition in the puppet-stage apparatus. Trained experimenters produced the pretest and test events live following a precise script. Two observers, who were naïve to the condition to which infants were assigned, monitored infants' looking behavior through peepholes in the frames to either side of the apparatus. Each observer held a game pad connected to a Dell computer and depressed a button when the infant attended to the event. The looking times recorded by the primary (and more experienced) observer were used in data analysis. Inter-observer agreement was calculated for pretest and test trials and averaged 94% (per trial and infant).
Total duration of looking (i.e., cumulative looking) to each pretest and test trial was obtained. Pretest trials in which infants looked < 15 s and test trials in which infants looked < 10 s were excluded from analysis. This ensured that group differences in hemodynamic responses could not be attributed to group differences in overall time spent viewing the pretest and test events.
Recall that we predicted that infants who individuated the green and red ball would find the narrow-screen GB-RB test event unexpected. However, it is difficult to obtain group differences in duration of looking to occlusion events during trials capped at 20 s trials. This necessitated finding another, more sensitive, looking time measure. To assess the extent to which infants' experienced a violation-of-expectation, we calculated the duration of infants' first look (i.e., time to infants' first look away) to the narrow-screen GB-RB test event on each test trial. Typically, infants take longer to disengage, or look away, from an event they find novel or unexpected. Duration of first look is considered a reliable measure of attentional engagement and active information processing (Cohen & Cashon, 2003; Olsen & Sherman, 1983; Striano, Vaish, & Benigno, 2006)
2.1.3 Instrumentation
The imaging equipment contained four fiber optic cables that delivered near-infrared light to the scalp of the participant (emitters), eight fiber optic cables that detected the diffusely reflected light at the scalp (detectors), and an electronic control box that served as the source of the near-infrared light and the receiver of the reflected light. The control box produced light at wavelengths of 690 nm, which is more sensitive to deoxygenated blood, and 830 nm, which is more sensitive to oxygenated blood, with two laser-emitting diodes (TechEn Inc). Laser power emitted from the end of the diode was 4 mW. Light was square wave modulated at audio frequencies of approximately 4 to 12 kHz. Each laser had a unique frequency so that synchronous detection could uniquely identify each laser source from the photodetector signal. Ambient illumination from the testing room did not interfere with the laser signals because environmental light sources modulate at a different frequency. Fiber optic cables were 2.5 mm in diameter and 5 m in length. Each emitter delivered both wavelengths of light and each detector responded to both wavelengths. The signals received by the electronic control box were processed and relayed to a DELL desktop computer. A custom computer program recorded and analyzed the signal.
Prior to the experimental session, infants were fitted with a custom-made headgear that secured the fiber optics to the scalp. Configuration of the sources and detectors within the headgear, placement of the sources and detectors on the infant's head, and location of the nine corresponding channels were identical to those of Wilcox and her colleagues (Wilcox et al., 2010, 2012) and are displayed in Figure 4. Source-detector separation was 2 cm. The headgear was not elastic so the distance between sources and detectors and between the four source-detector groups (O1, P3, T5, T3) remained fixed. The headgear was placed on the infant's head using O1 as the primary anchor and T3 and P3 as secondary anchors. The mean head circumference of infants in the function (M = 44.36 cm, SD = 1.40 cm) and motion (M = 43.86 cm, SD = 2.63 cm) conditions did not differ significantly, t < 1, df = 37.
Figure 4.
Configuration and placement of optodes. (a) Location of emitters (large red circles) and detectors (black squares) on the infant's head in relation to the 10–20 International EEG system (small black circles) used in the present experiments. This configuration was identical to that used by Wilcox et al. (2010, 2012). Note that an emitter was placed directly over O1, T5, and T3 and one emitter lay near P3. Also represented are the nine corresponding channels from which data were collected. Each detector read from a single emitter except for the detector between T3 and T5, which read from both emitters. The light was frequency modulated to prevent “cross-talk”. (b) Configuration of the emitters (red circles) and detectors (black squares), and the nine channels, in the headgear. Emitter-detector distances were all 2 cm. (c) Infants sat in a supportive seat to restrain excess movement. An elasticized headband was slid onto the infant’s head and secured by a chinstrap.
2.1.4 Processing of fNIRS Test Data
The fNIRS test data were processed, for each detector and event condition separately, using a procedure identical to that of Wilcox and her colleagues (Wilcox et al., 2005; Wilcox et al., 2010). Briefly, the raw signals were acquired at the rate of 200 samples per second, digitally low-pass-filtered at 10 Hz, a principal components analysis was used to design a filter for systemic physiology and motion artifacts, and the data were converted to relative concentrations of oxygenated (HbO) and deoxygenated (HbR) blood using the modified Beer-Lambert law.
For the pretest trials, changes in HbO and HbR were examined using the following time epochs: the 2 s prior to the onset of the pretest event, the 30 s pretest event, and the 10 s following the pretest event. The mean optical signal from −2 to 0 s (baseline) was subtracted from the signals and other segments of the time epoch were interpreted relative to this zeroed baseline. For the test trials, changes in HbO and HbR were calculated using the same procedure except that the test event was 20 (rather than 30) seconds. Optical signals were averaged across trials and then infants for each event condition. Trials objectively categorized as containing motion artifacts (a change in the filtered intensity greater than 5% in 1/20 s during the 2 s baseline and the pretest/test event) were eliminated from the mean. On the basis of this criterion, and the looking time criteria (see above), sixteen of 124 possible pretest trials (31 infants × 4 trials) were eliminated from analysis of the pretest data. These were distributed about equally between the two conditions (function condition, 5 of 64 possible trials and motion condition, 7 of 60 possible trials, z = .725 p > .05). In addition, seventeen of 136 possible test trials (34 infants × 4 trials) were eliminated from analysis of the test data. These were distributed about equally between the two conditions (function condition, 11 of 68 possible trials and motion condition, 6 of 68 possible trials, z = .304 p > .05).
2.2 Results
To be included in the sample, infants must have contributed behavioral and optical imaging data during the pretest and/or test phase of the experimental session. (Reasons for elimination are reported in 2.1.1. Participants). Of the n = 21 infants in the function condition, n = 16 contributed pretest data (behavioral and neuroimaging) and n = 17 contributed test data (behavioral and neuroimaging); n = 13 contributed both pretest and test data. Of the 18 infants in the motion condition, n = 15 contributed pretest data (behavioral and neuroimaging) and n = 17 contributed test data (behavioral and neuroimaging); n = 14 contributed both pretest and test data. Because the majority of infants contributed data for both pretest and test trials, and the same pattern of results was obtained when we included infants who completed both pretest and test trials (n = 27) and when we included infants who completed pretest and/or test trials (n = 39) the larger sample was retained.
2.2.1 Looking Time Data
Pretest trials
Total duration of looking to the pretest events was averaged across trials and infants for each of the two conditions, function and motion, and analyzed by means of an independent samples t-test. The infants in the function (n = 16, M = 26.23, SD = 2.31) and the motion (n = 15, M = 26.02, SD = 2.05) condition looked about equally to the pretest events, t < 1, df = 29.
Test trials
Total duration of looking to the test events was also averaged across trial and infants, for each condition separately, and analyzed by means of an independent t-test. As expected, infants who had received function pretest trials (n = 17, M = 20.25, SD = 5.27) did not differ significantly in the total amount of time they spent looking at the narrow-screen GB-RB test event than infants who had received motion pretest trials (n = 17, M = 21.63, SD = 2.02), t = 1, df = 32. To assess the extent to which infants' found the narrow-screen GB-RB test event unexpected, the duration of first look data were treated in the same manner as the total duration of looking data. As predicted, those infants who had seen the function pretest events (n = 17, M = 16.85, SD = 5.05) took significantly longer to first disengage from the narrow-screen GB-RB test event than those infants who had seen the motion pretest events (n = 17, M = 13.06, SD = 5.35), t = 2.12, df = 32, p = .042. The effect size was large as indicated by Cohen's d = .724 (Cohen, 1977). This outcome suggests that the infants who saw the function pretest events, but not the infants who saw the motion pretest events, found the narrow-screen GB-RB event unexpected. That is, only the infants who were primed by the function events used the color difference to individuate the green and red ball and recognized that the screen was too narrow to occlude both balls simultaneously. These results are consistent with previous behavioral reports that 8- and 9-month-olds can be primed, by viewing function (but not motion) events, to use color differences as the basis for individuating objects (Brower & Wilcox, 2012; Wilcox & Chapa, 2004; Wilcox et al., 2008).
2.2.2 Optical Imaging Data
On the basis of previous research (Wilcox et al., 2010, 2012), we expected to obtain activation in occipital and temporal, but not parietal, cortex in response to the pretest and test events. In addition, we hypothesized that the channels most likely to be activated in these areas were channels 9 (occipital cortex), 4 and 5 (posterior temporal cortex), and 2 (anterior temporal cortex). Preliminary analyses of the data confirmed this hypothesis and channels 1, 3, 6, 7 and 8 were excluded from further consideration. Note also that the data obtained at channels 6 and 7 (parietal cortex) were noisier than that obtained at the other channels, rendering the data less reliable. Finally, we focused our analyses on HbO responses, which are more robust that HbR responses (Strangman et al., 2003). However, HbR data are reported in the Appendix.
Pretest trials
Hemodynamic response curves for each condition and channel are presented in Figure 5. Relative changes in HbO were averaged over 10 to 30 s for each condition separately. This interval was chosen because one full cycle of the pretest event (function/motion) was complete by 8 s and, allowing 2 s for the hemodynamic response to become initiated, changes in HbO should be detectable by 10 s. Pilot data confirmed that hemodynamic responses are detectable by 10 s and persist through the 30 s trial. Two sets of analyses were conducted on HbO responses. First, mean responses obtained at channels 2, 4, 5, and 9 were compared to 0 (Table 1). One-tailed tests were used because our predictions were one-directional (i.e., an increase in HbO was expected); negative HbO responses are uncommon in this experimental context. Second, an independent samples t-test was conducted for each channel (2, 4, 5, and 9) to assess differences between groups. There is evidence that different patterns of activation are obtained in the temporal cortex when adults' view tools undergoing motion that is functionally, as compared to non-functionally, related (Beauchamp, Lee, Haxby, & Martin, 2002, 2003). If the infant brain is functionally organized in a similar way, group differences in HbO responses should be observed in the temporal cortex.
Figure 5.
Hemodynamic response curves (smoothed for presentation purposes with a 1 Hz low-pass filter) for Experiment 1. Relative changes in HbO and HbR (red and blue lines respectively) during pretest and test trials at each of the nine channels are displayed for the function and motion conditions separately. Time is on the x-axis and hemodynamic changes in µM cm on the y-axis. The channels associated with each of the four 10–20 coordinates are labeled accordingly. For the pretest trials, 1 to 30 s was the test event and 31 to 40 s was the silent pause (baseline). The hemodynamic response was averaged over 10 to 30 s, indicated by narrow grey shading. For the test trials, 1 to 20 s was the test event and 21 to 30 s was the silent pause (baseline). The hemodynamic response was averaged over 6 to 20 s, indicated by narrow grey shading. Asterisks indicate M (SD) HbO responses that differed significantly from baseline (* p < .05, ** p < .01, and *** p < .001, one-tailed).
Table 1.
Mean (SD) HbO responses obtained during the pretest and test trials of Experiment 1. One sample t-tests compared mean HbO responses, averaged over 10 to 30 s for pretest trials and 6 to 20 s for test trials, to zero at channels 2, 4, 5, and 9. Asterisks indicate M (SD) HbO responses that differed significantly from baseline (* p < .05, ** p < .01, and *** p < .001, one-tailed). Between groups comparisons using independent samples t-tests (two-tailed), were also performed for channels 2, 4, 5, and 9.
| EXPERIMENT 1: PRETEST TRIALS HBO |
ONE SAMPLE T-TESTS M (SD) | INDEPENDENT SAMPLES T- TEST |
||
|---|---|---|---|---|
| Neural Region | Channel | Function N = 16 | Motion N=15 |
Between Subjects Effects N= 31 |
| T3 | 1 | .0021 [.003) | −.0007 [.006) | |
| 2 | .0072 (.009)** | .0010 [.003) | t(29)= 2.249, p = .032 | |
| 3 | .0022 [.009) | .0041 [.008) | ||
| T5 | 4 | .0004 [.013) | .0053 (.009)* | t [2 9) < 1.5 |
| 5 | .0015 [.009) | .0024 [.006) | t[29) < 1.0 | |
| P3 | 6 | .0047 [.013) | −.0013 [.013) | |
| 7 | .0038 [.013) | −.0023 [.012) | ||
| 01 | 8 | .0048 [.008) | .0008 [.005) | |
| 9 | .0060 (.009)* | .0010 [.013) | t [2 9) < 1.5 | |
| EXPERIMENT 1: TEST TRIALS HBO | ONE SAMPLE T-TESTS M (SD) | INDEPENDENT SAMPLES T- TEST |
||
| Neural Region | Channel | Function Green ball-Red ball N = 17 |
Motion Green ball-Red ball N=17 |
Between Subjects Effects N= 34 |
| T3 | 1 | −.0005 [.002) | −.0002 [.003) | |
| 2 | .0032 (.005)* | −.0016 [.008) | t(32)= 2.096, p = .044 | |
| 3 | .0013 [.007) | .0027 [.007) | ||
| T5 | 4 | .0033 (.004)** | .0043 (.005)** | t[32) < 1.0 |
| 5 | .0032 (.003)*** | .0025 (.002)*** | t[32) < 1.0 | |
| P3 | 6 | −.0022 [.011) | −.0007 [.006) | valign="middle"> |
| 7 | .0017 [.006) | −.0013 [.009) | ||
| 01 | 8 | .0018 [.005) | .0022 [.005) | |
| 9 | .0051 (.006)** | .0052 (.010)* | t[32) < 1.0 | |
In the function condition, a significant increase in HbO relative to baseline was obtained at channel 9 (occipital cortex) and channel 2 (anterior temporal cortex). In the motion condition, a significant increase in HbO relative to baseline as obtained in channel 4 (posterior temporal cortex), only. The magnitude of the response obtained at channel 2 differed significantly by condition and the effect size associated with this comparison was large, d = .823. Significant group differences were not obtained at any of the other channels tested.
Test Trials
Hemodynamic response curves for each condition and channel are presented in Figure 5. The test data were treated in a manner similar to that of the pretest data except that relative changes in HbO were averaged over 6 to 20 s for each condition and channel. This interval was chosen for test trials because the first emergence of the object to the right of the screen began at 4 s and, allowing 2 s for the hemodynamic response to become initiated, changes in HbO should be detectable by 6 s and persist until the end of the trial at 20 s (see Wilcox et al., 2012 for supporting evidence). The mean responses obtained at channels 2, 4, 5, and 9 were compared to 0 (Table 1). In addition, an independent samples t-test was conducted for each of these channels.
In the function condition,, a significant increase in HbO relative to baseline was obtained at channel 9 (occipital cortex), channels 5 and 4 (posterior temporal cortex), and channel 2 (anterior temporal cortex). In the motion condition, a significant increase in HbO was obtained at channels 4, 5, and 9. The magnitude of the response obtained at channel 2 in the infants who had viewed the function pretest events differed significantly from that of the infants who had viewed the motion pretest events, and the effect size associated with this comparison was large, d = .731. Significant group differences were not obtained at any of the other channels tested.
Finally, to explore the relation between behavioral and optical imaging data we conducted a correlational analysis between infants' mean first look away and their mean HbO response at channel 2. The outcome of this analysis revealed no significant correlation between these two factors, r2 = .103, n = 34, p > .05. (A similar outcome was obtained if we analyzed the data for each condition separately). Although one might expect, on the basis of the group differences observed in looking time and neuroimaging data, that these two factors would be highly correlated, this need not be the case. We predicted that viewing the pretest function event would prime infants to attend to color information in the test event, and that this would lead to (a) increased looking times and (b) increased activation, as measured by a relative change in HbO, in the anterior temporal cortex. However, we did not predict that these would be statistically dependent. Prolonged looking to the narrow-screen event is presumably a function of (a) successful object individuation and (b) perception of the event as unexpected or surprising. Hence, looking times are closely related to both cognitive (individuation) and attentional processes, the latter of which might be mediated by cortical structures not currently under investigation.
2.3 Discussion
In the pretest (or priming) phase of the experiment a different pattern of cortical activation was obtained for infants who viewed function as compared to motion pretest events. The infants in the function but not the motion condition evidenced significant activation in the anterior temporal cortex during the pretest event, and the difference between the two groups was statistically significant. There are a number of possible explanations for this result. Adult neuroimaging studies have reported distinct patterns of neural activation in temporal cortex in response to viewing tools undergoing functionally related motion as compared to motion that is not functionally-related (Beauchamp et al., 2002, 2003). In addition, after training with tools that perform specific functions adults show increased neural activation in middle temporal gyrus (Weisberg, van Turennout, & Martin, 2007). Hence, one possible explanation for the group difference observed in temporal activation has to do with the nature of the event; whether the event involved functionally relevant or irrelevant object motion. However, there are other differences between the pretest events that could have led to this pattern of results. Wilcox and her colleagues have argued that infants differentially engage in categorization processes when viewing function as compared to motion events (Wilcox & Chapa, 2004; Wilcox et al., 2008). More specifically, viewing different-colored objects engage in distinct functions, an object property to which infants are quite sensitive, leads infants to form event categories linking color to object function. This process increases infants' sensitivity to color in the subsequent test events. Viewing objects involved in distinct motions, for a number of reasons, does not lead infants to form such event categories. Neuroimaging studies conducted with adult participants have reported increased activation in temporal areas, such as superior temporal sulcus (STS), during object categorization processes (Chao et al., 1999; van der Linden, van Tirennout & Indefrey, 2009). Although speculative, these findings raise the possibility that the extent to which infants engage in event categorization can explain, at least in part, the pattern of results obtained. Because the current data do not allow us to distinguish between these two possibilities, further research will be needed to test between these, and potentially other, hypotheses.
In the test phase of Experiment 1 a different pattern of cortical activation was also obtained for infants who had viewed the function as compared to the motion pretest events The infants tested in the function condition, who were primed to attend to color differences in the test trials (i.e., they showed prolonged looking to the narrow-screen GB-RB event), evidenced activation in posterior and anterior areas of the temporal cortex. In comparison, the infants tested in the motion condition, who failed to use color differences to individuate the objects in the test trials, evidenced activation in posterior areas of the temporal cortex only. One might be concerned that hemodynamic responses obtained in the pretest trials were simply “carried over” to the test trials. This is unlikely, for two reasons. First, the pretest events involved different objects engaged in very different events. Given these differences, it is unlikely that viewing test events elicited the same hemodynamic responses as viewing pretest events, simply by virtue of temporal proximity. Second, the configuration of channels activated during the test phase differed from that activated during the pretest phase. For example, channels 4 and 5 were activated in both conditions during the test but not the pretest events, demonstrating that temporal activation was not simply “carried over” from pretest to test trials.
One expected finding was the lack of a significant hemodynamic response in the occipital cortex during the pretest events for the infants in the motion condition. However, the magnitude of the occipital responses observed in the motion infants did not differ significantly from those observed in the function infants, making it difficult to draw firm conclusions about this null finding. Given the fact that occipital activation is typically obtained in response to visual events, that occipital activation was obtained in the infants in the motion condition during the test phase, and the large standard deviation associated with channel 9 in the motion infants in the pretest phase, we suspect that this is a spurious outcome.
Whereas only the function infants evidenced activation in anterior temporal cortex, both the function and the motion infants evidenced activation in posterior temporal cortex. What is currently unclear is the extent to which posterior temporal activation can be explained by exposure to the pretest events prior to test. Recall that 3- to 7-month-olds, who have not been exposed to pretest events, evidence activation in posterior temporal cortex when viewing a green ball-red ball test event. In contrast, 11- to 12-month-olds, who also have not been exposed to pretest events, do not evidence activation in posterior temporal cortex when viewing a green ball-red ball test event. Because infants aged 8 to 9 months have not yet been tested, we do not know whether posterior temporal cortex is activated without prior exposure to pretest events. Experiment 2 tested this possibility.
3. Experiment 2
Experiment 2 assessed 8- and 9-month-olds' response to a narrow-screen GB-RB test event without first viewing pretest events. For control purposes, another group of infants were tested using a narrow-screen green ball-green ball (GB-GB) event. The GB-GB event was identical to the GB-RB event, except that a green (rather than a red) ball was seen to the right of the screen. Infants 3 to 12 months interpret this event as involving a single object that emerges successive to opposite sides of the screen (Wilcox, 1999; see Wilcox & Woods, 2009 for a review of the evidence).
3.1 Methods
3.1.1 Participants
Infants' aged 8 and 9 months participated in Experiment 2 (N = 34; 20 males and 14 females; M age = 9 months, 12 days, range = 8 months, 21 day to 9 months, 28 days). Parents reported their infant’s race/ethnicity as Caucasian (n=25), Hispanic (n=5), Black (n=2), or of mixed race/other (n=2).
Sixteen additional infants were tested but eliminated from the sample because of procedural problems (n = 6) or difficulty in obtaining an optical signal (n = 10). An equal number of infants were pseudo-randomly assigned to one of two narrow-screen conditions: green ball-red ball or green ball-green ball.
3.1.2 – 3.1.4 Procedure, Instrumentation, Data Processing
Infants were tested using the same procedure as Experiment 1 except that infants saw only the test events. Infants were presented with either the narrow-screen GB-RB event of Experiment 1 or a narrow-screen GB-GB event (i.e., a green ball was seen to both sides of the screen). Instrumentation and data processing were identical to that of Experiment 1.Interobserver agreement was calculated for test trials and averaged 94% (per trial and infant). The mean head circumference of infants in the GB-RB (M = 45.26 cm, SD = 1.32 cm) and GB-GB (M = 45.62 cm, SD = 1.53 cm) condition did not differ significantly, t < 1, df = 32.
On the basis of looking time and motion artifact criteria, nineteen (of 136 possible) test trials were eliminated from data analysis. Eliminated trials were distributed about equally between the two conditions (GB-RB condition, 11 of 68 possible trials and GB-GB condition, 8 of 68 possible trials, z = .742 p > .05).
3.2 Results
3.2.1 Looking Time Data
Mean duration of total (i.e., cumulative) looking to the test events was analyzed in the same manner as Experiment 1. The infants who viewed the narrow-screen GB-RB event (M = 20.94, SD = 2.99) did not differ significantly in the amount of time they spent looking during the test trials than the infants who viewed the narrow-screen GB-GB event (M = 20.52, SD = 3.31), t < 1, df = 32.
Mean duration of infants' first look away was also analyzed in the same manner as Experiment 1. The infants who viewed the narrow-screen GB-RB (M = 12.68, SD = 4.93) and the narrow-screen GB-GB (M = 11.42, SD = 5.56) test event did not differ significantly in the time they took to first disengage from the test events, t < 1, df = 32. Across experiment analyses revealed that the mean first look away of the infants in Experiment 2 who saw the GB-RB test event differed significantly from that of the infants in Experiment 1 who saw the GB-RB test event after having seen function pretest events, t = 2.44, df = 32, p = .02, d = .724, but did not differ significantly from that of the infants in Experiment 1 who saw the GB-RB test event after having seen motion pretest events, t < 1, df = 32. These results suggest that the GB-RB infants of Experiment 2, like the motion GB-RB infants of Experiment 1, failed to use the color difference to individuate the two balls.
3.2.2 Optical Imaging Data
Hemodynamic responses were obtained for each channel for infants tested in the narrow-screen GB-RB and narrow-screen GB-GB conditions. The neuroimaging test data were processed and analyzed in the same manner as the neuroimaging test data of Experiment 1. Mean (SD) HBO responses are reported in Table 2. Mean (SD) HbR responses can be found in the Appendix.
Table 2.
Mean (SD) HbO responses obtained during the test trials of Experiment 2. One sample t-tests compared mean HbO responses, averaged over 10 to 30 s for pretest trials and 6 to 20 s for test trials, to zero at channels 2, 4, 5, and 9. Asterisks indicate M (SD) HbO responses that differed significantly from baseline (* p < .05, ** p < .01, and *** p < .001, one-tailed). Between groups comparisons using independent samples t-tests (two-tailed), were also performed for channels 2, 4, 5, and 9.
| EXPERIMENT 2: TEST TRIALS HBO |
ONE SAMPLE T-TESTS M (SD) | INDEPENDENT SAMPLES T-TEST |
||
|---|---|---|---|---|
| Neural Region | Channel | Green ball-Red ball N = 17 |
Green ball-Green ball N = 17 |
Between Subjects Effects N= 34 |
| T3 | 1 | .0005 [.003) | −.0011 [.004) | |
| 2 | −.0006 [.005) | .0001 [.005) | t[32) < 1 | |
| 3 | .0004 [.007) | .0026 [.005) | ||
| T5 | 4 | −.0014 [.005) | .0009 [.005) | t[32) < 1.5 |
| 5 | −.0012 [.006) | .0009 [.005) | t[32) < 1.5 | |
| P3 | 6 | −.0021 [.004) | .0023 [.008) | |
| 7 | .0002 [.010) | −.0006 [.005) | ||
| 01 | 8 | .0017 [.004) | .0031 [.005) | |
| 9 | .0050 (.007)** | .0051 (.007)** | t[32) < 1 | |
The infants who viewed the narrow-screen GB-RB test event and the infants who viewed the narrow-screen GB-GB test event both showed a significant increase in HbO relative to baseline at channel 9 (occipital cortex). The groups did not differ significantly in the magnitude of the response.
Across experiment analyses revealed that the infants in Experiment 1 who saw the motion pretest events demonstrated significantly greater activation at channel 5 (t = 2.57, df = 32, p = .015, d = .883) and channel 4 (t = 3.10, df = 32, p = .004, d = 1.056), but not at channel 2 (t < 1, df = 32), in response to the narrow-screen GB-RB test event than the infants in Experiment 2, who saw only the test event. Furthermore, the infants in Experiment 1 who saw function pretest events demonstrated significantly greater activation at channel 5 (t = 2.82, df = 32, p = .008, d = .970), channel 4 (t = 2.99, df = 32, p = .005, d = 1.024), and channel 2 (t = 2.26, df = 32, p = .031, d = .774) in response to the GB-RB test event than the infants in Experiment 2. These results indicate that viewing the motion event led to increased activation in posterior temporal areas whereas viewing the function event led to increased activation in posterior and anterior temporal areas.
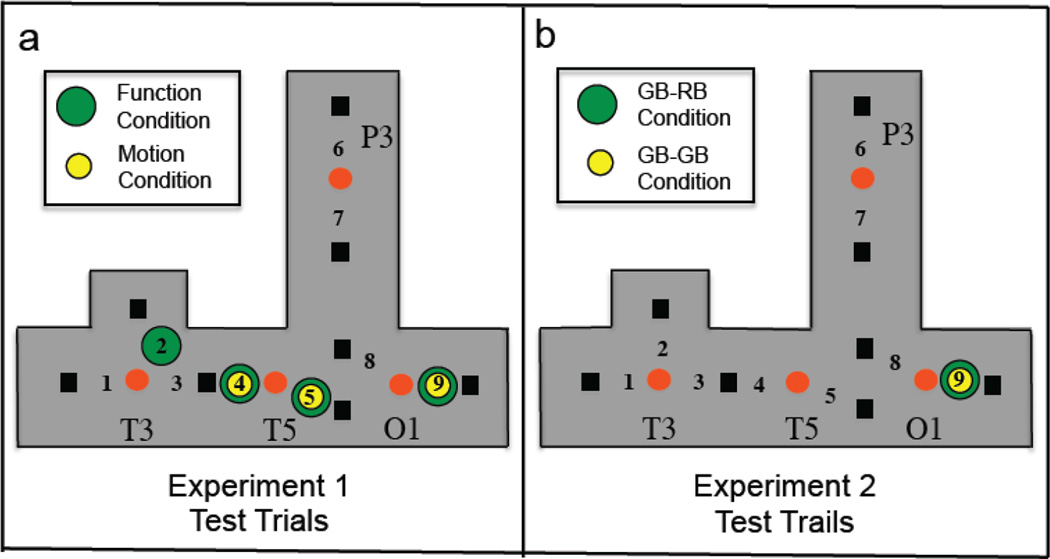
A summary of the test results obtained in Experiment 1 and 2 is depicted in Figure 6.
Figure 6.
Summary representation of the significant HbO responses obtained in the test phase of Experiments 1 and 2. The colored dots indicated that neural activation was obtained for that event condition at that channel. (a) Patterns of neural activation obtained in response to the narrow-screen green ball-red ball test event of Experiment 1 for the infants in the function and motion condition. (b) Patterns of neural activation obtained in response to the narrow-screen green ball-red ball (GB-RB) and narrow-screen green ball-green ball (GB-GB) test events of Experiment 2.
3.3 Discussion
The results of Experiment 2 are consistent with previous behavioral and neuroimaging studies reporting that infants younger than 11.5 months do not spontaneously use color differences to individuate objects. In addition, these results, when compared to those obtained in Experiment 1, reveal the influence of the function and motion pretest events on patterns of neural activation in the temporal cortex. Across experiment comparisons revealed that prior exposure to the motion pretest events led to increased activation in posterior temporal cortex during the narrow-screen GB-RB test event. In contrast, prior exposure to the function pretest events led to increased activation in posterior and anterior temporal object processing areas during the narrow-screen GB-RB test event. The effect sizes indicated that these findings were remarkably robust.
One might be concerned that exposure to the pretest events, in and of itself, influenced infants' response to the test events. For example, perhaps infants in Experiment 1 were more fatigued and/or less interested in the test events because they had already viewed pretest events, leading to across experiment differences in performance. There are two pieces of evidence that argue against this interpretation. First, mean duration of looking was similar across the two experiments, suggesting that overall attention to the test events did not vary as a function of whether or not infants had first viewed pretest events. Second, the effect of prior experience was event specific. Behavioral and cortical responses to the test events differed as a function of the type of pretest event previously viewed and not simply as a result of having viewed a pretest event.
4. Conclusion
Developmental scientists have demonstrated, in a number of experimental contexts, that the way that infants perceive, apprehend, and act on objects in the physical world can be altered by select experiences (Libertus & Needham, 2010; Needham et al, 2002; Wang & Baillargeon, 2005; Wilcox et al., 2007, 2008; Woods & Wilcox, 2012). These findings have raised a number of intriguing questions about the malleability of early knowledge and the mechanisms that underlie different types of learning. The current research explored changes in brain and behavior as a result of color priming in 8- and 9-month-olds.
4.1 Color-Priming: Brain and Behavior
The primary finding obtained in the present research was that of priming-dependent patterns of functional activation during the object individuation task. Viewing color-function pretest infants led 8- and 9-month-olds, who do not spontaneously use color to individuate objects, to individuate-by-color in a subsequent object individuation task. This was evidenced in both behavioral and cortical responses. Infants who saw function pretest events showed prolonged looking to the narrow-screen GB-RB test event and increased activation in anterior temporal cortex. In contrast, infants who saw motion pretest events, and infants who were not presented with pretest events at all, did not show prolonged looking to the narrow-screen GB-RB test event or increased activation in anterior temporal cortex. The pattern of activation observed in the anterior temporal cortex of infants who had experienced color priming was similar to that of older infants who spontaneously use color to track the identity of objects (Wilcox et al., 2010; Wilcox et al., 2012).
What led infants who viewed the function (but not the motion) pretest events to show increased sensitivity to color in the test trials? What processes were involved? One hypothesis is that when viewing pound-pour events in which the color of an object predicts the function in which it will engage infants form event categories in which color is linked to object function (Wilcox & Chapa, 2004). It is this process that leads to increased sensitivity to color differences in a subsequent test event. The fact that color priming is obtained when infants see function but not motion pretest events indicates that infants do not form associations between any co-occurring object properties but, rather, this process is selective. There is a large body of research demonstrating that infants are sensitive to the functional properties of objects (see Wilcox et al., 2008 and Wilcox & Woods, 2009 for a review of the evidence). For example, infants detect functional relations between object parts and surfaces and expect objects to move and interact in ways that are consistent with these relations. In addition, infants readily use function-related information to guide learning about new objects. Hence, it makes sense that infants would be particularly sensitive to object function in priming situations. What evidence is there that infants were forming function-related event categories when viewing the pretest events? Color priming is obtained only when infant see at least two pairs of pretest trials with two different pairs of green and red containers. If infants see two pairs of pretest trials, but with the same pair of green and red containers, color priming is not obtained. A number of studies have now demonstrated that viewing multiple exemplars of the relation between color and function is critical to color priming (Wilcox & Chapa, 2004; Wilcox et al., 2008; see also Woods & Wilcox, 2012). Collectively, these data support the hypothesis that it is the extraction of the relation between color and function across multiple exemplars – the formation of an event category in which color is linked to object function – that leads to increased sensitivity to color information in a subsequent test event.
An alternative hypothesis is that color priming is an example of acquired distinctiveness (Bonardi, Graham, Hall, & Mitchell, 2005; Jitsumori, Ohkita, & Ushitani, 2011; Lawrence, 1949; Reese, 1972). Acquired distinctiveness, a form of discrimination learning, occurs when participants learn that one source of information reliably predicts an outcome and future learning is constrained and guided by the nature of this relation. Typically, in acquired distinctiveness experiments animals (e.g., humans, rodents, pigeons) are trained to discriminate between, and respond on the basis of, a stimulus dimension that is not inherently predictive. For example, children can be trained to associate the presentation of a nonsense figure (A and B) with a subsequent label (“ding” and “dong”, respectively). Once the relevant association is learned it becomes functionally dominant – the stimulus dimensions acquire distinctiveness – and this influences learning in subsequent trials (Reese, 1972). Acquired distinctiveness may explain, at least in part, color-function priming. In the pretest trials, the color of the container reliability predicted the function in which it would engage. Forming an association between color and function could have led infants to identify object color, a previously irrelevant object feature, as an important source of information. That is, the color-function pairing rendered the difference between green and red a relevant distinction. At the same time, acquired distinctiveness cannot fully explain color-function priming. Theoretically, discrimination learning should apply to a wide range of situations and stimulus properties. Yet we know that color priming occurs in the context of function but not motion events. It may be that acquired distinctiveness and event categorization both play a role in the priming process. For example, perhaps acquired distinctiveness is the process by which infants identify that different colors predict different outcomes and event categorization is the process by which infants link color to object function.
4.2 Activation in Posterior Temporal Cortex: An Unexpected Finding
Both the infants who viewed the function pretests events and those who viewed the motion pretest events evidenced a significant increase in HbO in the posterior temporal cortex during test trials. Across experiment comparisons indicated that these responses differed significantly from those obtained in infants who saw the narrow-screen GB-RB test event without prior exposure to function or motion pretest events (these infants did not evidence increased activation in posterior temporal cortex). Contrary to our predictions, viewing motion pretest events did influence infants' processing of the narrow-screen GB-RB event, a result that was not apparent in our behavioral data or predicted by previous behavioral studies.
One hypothesis consistent with these results is that posterior and anterior temporal cortex play separate, and perhaps hierarchically organized, roles in object processing. According to this hypothesis, viewing pretest events in which the color of an object reliability predicts an event outcome, regardless of whether the outcome is functionally significant, heightens infants' attention to color information in a subsequent test event. This is evidenced by increased activation in posterior temporal cortex, a cortical area implicated in mid-level object processing in the adult (Grill-Spector, 2003; Kanwisher, 2003). In comparison, viewing pretest events in which color reliably predicts an event outcome and the event outcome is functionally significant, not only heightens infants' sensitivity to color differences, as evidenced by increased activation in posterior temporal cortex, but also primes infants' to use the color difference as the basis for individuating objects. Color priming is evidenced by prolonged looking to the narrow-screen GB-RB event and increased activation in anterior temporal cortex, a cortical area implicated in higher-level object processing in the adult (Devlin et al., 2002; Humphreys et al., 1999; Malach et al., 1995). The charge of future research will be to test this hypothesis and delineate the functional role of each of these cortical areas and how their roles might change during the first year of life. As reported by Wilcox and her colleagues (Wilcox et al., 2010, 2012) infants 7 months and younger evidence neural activation in posterior temporal cortex during a narrow-screen GB-RB test event without first viewing pretest events. Yet, as seen in the present studies, 8- and 9-month-olds evidence activation in posterior temporal cortex in response to the narrow-screen GB-RB test event only after first viewing a pretest event. This outcome suggests functional reorganization within object processing areas of the temporal cortex during the first year.
4.3 Final Comments
During the last 40+ years developmental scientists have learned a great deal about infants' emerging perceptual and cognitive capacities and the kinds of experiences that support the acquisition of new knowledge. With the introduction of fNIRS into the experimental setting we now have the capacity to assess brain-behavior relations during development. As the current research demonstrates, the use of this technique can provide insight into the cortical structures that mediate and support the development of object processing capacities in the infant. We look forward to continued exploration of the infant mind using this technique.
Supplementary Material
Mean (SD) HbR responses obtained during the pretest and test trials of Experiment 1 and the test trials of Experiment 2.
Highlights.
Color-function pretest events prime 8- and 9-month-old infants to attend to color differences in an individuation task.
Color priming was evident in behavioral and neuroimaging data.
Anterior temporal cortex mediates object individuation in the infant.
Color-motion pretest events do not prime infants to attend to color in an individuation task.
Acknowledgements
We thank Tracy Smith Brower, Jennifer Armstrong Haslup, Mariam Massoud, Jennifer Moore Norvell, Jessica Stubbs, Kayla Boone Upshaw, Lesley Wheeler, and the staff of the Infant Cognition Lab at Texas A&M University for help with data collection and management, and the infants and parents who so graciously participated in the research. This work was support by grants BCS-0642996 and R01-HD057999 to TW and grant P41-RR14075 to DAB. DAB is an inventor on a technology licensed to TechEn, a company whose medical pursuits focus on noninvasive, optical brain monitoring. DAB's interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In a wide-screen event the screen is sufficiently wide to hide both objects (green ball and green box or green ball and red ball) simultaneously. The width of the screen does not influence whether infants individuate the objects, but it does influence whether infants perceive the event as unexpected. For example, if infants use color differences to indivdiuate objects, they show prolonged looking to a green ball-red ball test event only when the screen is two narrow to occlude both objects simultaneously (see Wilcox & Woods, 2009 for a review of the evidence). They do not show prolonged looking to a green ball-red ball event seen with a wide screen.
Contributor Information
Teresa Wilcox, Department of Psychology, Texas A&M University, College Station, TX 77843.
Amy Hirshkowitz, Department of Psychology, Texas A&M University, College Station, TX 77843.
Laura Hawkins, Department of Psychology, Texas A&M University, College Station, TX 77843.
David A. Boas, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA 02129
References
- Bachevalier J, Mishkin M. Effects of selective neonatal temporal lobe lesions on visual recognition in rhesus monkeys. Journal of Neuroscience. 1994;14:2128–2139. doi: 10.1523/JNEUROSCI.14-04-02128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp M, Lee K, Haxby J, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34:140–150. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp M, Lee K, Haxby J, Martin A. fMRI responses to video and point-light displays of moving humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15(7):991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Graham S, Hall G, Mitchell C. Acquired distinctiveness and equivalence in human discrimination learning: Evidence for an attentional process. Psychonomic Bulletin & Review. 2005;12(1):88–92. doi: 10.3758/bf03196351. [DOI] [PubMed] [Google Scholar]
- Brower T, Wilcox T. Priming infants to use color in an individuation task: Does social context matter? Infant Behavioral and Development. 2012;35:323–327. doi: 10.1016/j.infbeh.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- Cohen LB, Cashon CH. Handbook of psychology: Developmental psychology. Vol. 6. NJ: Wiley & Sons; 2003. Infant perception and cognition; pp. 65–89. [Google Scholar]
- Dehaene S. Symbols and quantities in parietal cortex: elements of a mathematical theory of number representation. In: Haggard P, Rossetti Y, editors. Attention and performance. XXII. Sensory-motor foundations of higher cognition. Cambridge, MA: Harvard University Press; 2007. [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, et al. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40:54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Eliassen J, Souza T, Sanes J. Experience dependent activation patterns in human brain during visual-motor associative learning. The Journal of Neuroscience. 2003;23(33):10540–10547. doi: 10.1523/JNEUROSCI.23-33-10540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson L, Dehaene S, Spelke ES. Core systems of number. Trends in Cognitive Science. 2004;8:307–314. doi: 10.1016/j.tics.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Current Opinion in Neurobiology. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Price CJ, Riddoch MJ. From objects to names: A cognitive neuroscience approach. Psychological Research. 1999;62:118–130. doi: 10.1007/s004260050046. [DOI] [PubMed] [Google Scholar]
- Hyde DC, & Spelke ES. All numbers are not equal: an electrophysiological investigation of small and large number representation. Journal of Cognitive Neuroscience. 2008;21:1039–1053. doi: 10.1162/jocn.2009.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitsumori M, Ohkita M, Ushitani T. The learning of basic-level categories by pigeons: The prototype effect, attention, and effects of categorization. Learn Behav. 2011;39:271–287. doi: 10.3758/s13420-011-0028-4. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. The Ventral Visual Object Pathway in Humans: Evidence from fMRI. In: Chalupa, Werner J, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2003. pp. 1179–1189. [Google Scholar]
- Lawrence D. Acquired distinctiveness of cues: I. Transfer between discriminations on the basis of familiarity with the stimulus. Journal of Comparative and Physiological Psychology. 1949;52(3):770–784. doi: 10.1037/h0058097. [DOI] [PubMed] [Google Scholar]
- Libertus K, Needham A. Teach to Reach: The Effects of Active Versus Passive Reaching Experiences on Action and Perception. Vision Research. 2010;50(24):2750–2757. doi: 10.1016/j.visres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Elwell CE. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neuroscience and Biobehavioral Reviews. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceedings of the National Academy of Sciences. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham A, Barrett T, Peterman K. A Pick-Me-Up for infants' Exploratory Skills: Early Stimulated Experiences Reaching for Objects Using ‘Sticky Mittens' Enhances Young Infrants' Object Exploration Skills. Infant Behavior and Development. 2002;25:279–295. [Google Scholar]
- Olsen GM, Sherman T. Attention, learning and memory in infants. In: Haith M, Campos J, editors. Manual of child psychology: Vol. 2, Infant and developmental psychobiology. New York: Wiley; 1983. pp. 1001–1080. [Google Scholar]
- Op de Beeck HP, Baker CI, DiCarlo JJ, Kanwisher NG. Discrimination training alters object recognition in human extriastriate cortex. Journal of Neuroscience. 2006;26:13025–13036. doi: 10.1523/JNEUROSCI.2481-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese HW. Acquired distinctiveness and equivalence of cues in young children. Journal of Experimental Child Psychology. 1972;13:171–182. [Google Scholar]
- Strangman G, Franceschini M, Boas D. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage. 2003;18:865–879. doi: 10.1016/s1053-8119(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Striano T, Vaish A, Benigno JP. Them meaning of infants' looks: Information seeking and comfort seeking? British Journal of Developmental Psychology. 2006;24:615–630. [Google Scholar]
- van der Linden M, Murre JMJ, van Turennout M. Formation of category representation in superior temporal sulcus. Journal of Cognitive Neuroscience. 2008;22(6):1270–1282. doi: 10.1162/jocn.2009.21270. [DOI] [PubMed] [Google Scholar]
- van der Linden M, van Turennout M, Indefrey P. Birds of a feather flock together: Experience-driven formation of visual object categories in human ventral temporal cortex. PlosOne. 2009;3:1–11. doi: 10.1371/journal.pone.0003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Baillargeon R, Paterson S. Detecting continuity violations in infancy: A new account and new evidence from covering and tube events. Cognition. 2005;95:129–173. doi: 10.1016/j.cognition.2002.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg J, van Turennout M, Martin A. A neural system for learning about object function. Cerebral Cortex. 2007;17:513–521. doi: 10.1093/cercor/bhj176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T. Object individuation: infants' use of shape, size, pattern, and color. Cognition. 1999;72:125–166. doi: 10.1016/s0010-0277(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Using near-infrared spectroscopy to assess neural activation during object processing in infants. Journal of Biomedical Optic. 2005;10:011010-1–011010-9. doi: 10.1117/1.1852551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas D. Hemodynamic response to featural changes in the occipital and inferior temporal cortex in infants: A preliminary methodological exploration. Developmental Science. 2008;11:361–370. doi: 10.1111/j.1467-7687.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Armstrong J, Woods R, Boas D. Hemodynamic response to featural and spatiotemporal information in the infant brain. Neuropsychologia. 2009;47:657–662. doi: 10.1016/j.neuropsychologia.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Chapa C. Priming infants to attend to color and pattern information in an individuation task. Cognition. 2004;90:265–302. doi: 10.1016/s0010-0277(03)00147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Haslup J, Boas DA. Dissociation of processing of featural and spatiotemporal information in the infant cortex. NeuroImage. 2010;53:1256–1263. doi: 10.1016/j.neuroimage.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Stubbs J, Hirshkowitz A, Boas DA. Object processing and functional organization of the infant cortex. NeuroImage. 2012;62:1833–1840. doi: 10.1016/j.neuroimage.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Woods R. Experience primes infants to individuate objects: Illuminating learning mechanisms. In: Needham A, Woodward A, editors. Learning and the infant mind. New York, NY: Oxford University Press; 2009. pp. 117–143. [Google Scholar]
- Wilcox T, Woods R, Chapa C. Color-function categories that prime infants to use color information in an object individuation task. Cognitive Psychology. 2008;57:220–261. doi: 10.1016/j.cogpsych.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Woods R, Chapa C, McCurry S. Multisensory exploration and object individuation in infants. Developmental Psychology. 2007;43:479–495. doi: 10.1037/0012-1649.43.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RJ, Wilcox T. Posture support improves object individuation in infants. Developmental Psychology. 2012 Oct 8; doi: 10.1037/a0030344. Advance online publication: doi:10.1037/a0030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean (SD) HbR responses obtained during the pretest and test trials of Experiment 1 and the test trials of Experiment 2.