Abstract
The human-health risk posed by gardening near the Iron King Mine and Humboldt Smelter Superfund Site in Arizona was characterized in this study. Residential soils were used in a greenhouse study to grow common household vegetables, and local residents, after training, collected soil, water, and vegetables samples from their household gardens. Concentrations of arsenic measured in water, soil, and vegetable samples were used in conjunction with reported US intake rates to calculate the daily dose, Incremental Excess Lifetime Cancer Risk (IELCR), and Hazard Quotient for arsenic. Relative arsenic intake dose decreased in order: water > garden soils > homegrown vegetables, and on average, each accounted for 77, 16, and 7% of a residential gardener’s daily arsenic intake dose. The IELCR ranges for vegetables, garden soils, and water were 10−8 to 10−4, 10−6 to 10−4, and 10−5 to 10−2, respectively. All vegetables (greenhouse and home garden) were grouped by scientific family, and the risk posed decreased as: Asteraceae >> Fabaceae > Amaranthaceae > Liliaceae > Brassicaceae > Solanaceae >> Cucurbitaceae. Correlations observed between concentrations of arsenic in vegetables and soils were used to estimate a maximum allowable level of arsenic in soil to limit the excess cancer risk to 10−6. The estimated values are 1.56 mg kg−1, 5.39 mg kg−1, 11.6 mg kg−1 and 12.4 mg kg−1 for the Asteraceae, Brassicaceae, Fabaceae, and Amaranthaceae families, respectively. It is recommended that home gardeners: sample their private wells annually, test their soils prior to gardening, and, if necessary, modify their gardening behavior to reduce incidental soil ingestion. This study highlights the importance of site-specific risk assessment, and the need for species-specific planting guidelines for communities.
Keywords: Arsenic, Mining Waste, Exposure Assessment, Risk Characterization, Home Gardening, Vegetables
1. Introduction
Arsenic is an ubiquitous contaminant in water, sediment, and soil in many countries, and originates from both natural and anthropogenic sources. The mining and metal processing industry is a major anthropogenic contributor of arsenic to the environment, with the single largest input coming from copper smelting (40%) followed by other mining activities at 16% (Prohaska and Stingeder, 2005). For example, for every ton of copper produced, it has been estimated that 1.5 kg of arsenic is released into the environment, and mine tailings alone contribute 7,000-11,000 tons of arsenic per year (Nriagu and Pacyna, 1988). Additionally, arsenic has been reported at a majority (1,149 of 1,684) of the current and former U.S. Environmental Protection Agency’s (USEPA) National Priorities List (Superfund) sites in the U.S., and this number is expected to increase in the future as more sites are evaluated (Agency for Toxic Substances and Disease Registry (ATSDR, 2007). Thus, potential exposure to arsenic is a major human health concern for those living near mines and other hazardous waste sites.
Acute exposure to arsenic can lead to nausea, diarrhea, encephalopathy, and neuropathy. Chronic low-level arsenic exposure, which is currently the focus of much research, has been linked to diabetes, hypopigmentation/hyperkeratosis, and a probable role in promoting cancer of the bladder, lung, skin, and prostate (e.g., Soghoian and Sinert, 2009, Tchounwou el al, 2004). Due to the frequency at which it is found, combined with its toxicity and potential for human exposure, arsenic is ranked as the number one contaminant of concern by the ATSDR (ATSDR, 2011).
Inorganic arsenic is a Class 1, non-threshold carcinogen for which risk assessments are based on a linear dose response (Meharg and Raab, 2010). The World Health Organization’s (WHO) current provisional guideline for inorganic arsenic in drinking water, designed to be protective of human health, is 10 μg L−1. This value is also the drinking water standard in both the U.S. and the European Union (EU). Another source of arsenic in the environment is soil. There are currently no federal regulatory standards for arsenic in residential soils in the U.S., although some States have set clean up standards. For example, the State of Washington has a regulatory level for unrestricted land uses of 20 mg kg−1 (Title 173-340-900), and Arizona has established a soil remediation standard of 10 mg kg−1 for both residential and non-residential sites (Title 18-7-205). Such remediation standards can be problematic because in some areas they are lower than the natural background levels in soil. There are also no arsenic standards for home garden soils, except for the land application of biosolids for home vegetable gardens, which is set at 41 mg kg−1 (USEPA, Title 40, Section 503.13).
Food presents an additional source of arsenic exposure, yet there are currently few regulations on arsenic concentrations in food in the U.S. or EU (Meharg and Raab, 2010; Peralta-Videa et al., 2009; Francesconi, 2007; Munoz et al, 2002). The Food and Agricultural Organization (FAO) /WHO Expert Committee on Food Additives stated that more accurate information on the arsenic content of foods is needed to improve assessments of dietary exposures (FAO/WHO, 2010). It has been shown that arsenic in soil is the major source for arsenic uptake by crops (e.g., Huang et al. 2006), and inorganic arsenic is considered to comprise the vast majority of the total arsenic in vegetables (e.g., Smith et al., 2006). This is particularly relevant for communities neighboring mining waste (Gonzalez, Gonzalez and Chavez, 2006), given that arsenic in mine tailings can be dispersed by wind and water erosion to residential areas and garden soils (Castro-Larragoitia et al, 1997, O’Neill, 1995, Munshower, 1994). Human exposure to inorganic arsenic is of special concern in the U.S. Southwest due to elevated levels that often occur naturally in soils and drinking-water sources. Thus, potential exposure via consumption of affected garden crops and incidental soil ingestion may add to an already elevated exposure from drinking water. Considering these factors, the issue of arsenic in fruits and vegetables grown in gardens in environments with elevated arsenic concentrations is of growing concern (Bhattacharya et al., 2010; Murray et al, 2009, Lee et al., 2005, Alam et al., 2003; Munoz et al., 2002; Cobb et al., 2000), especially in light of an anticipated increase in food gardening among Americans (National Gardening Association, 2009).
Minimal information currently exists regarding the risks of arsenic exposure from water, soil, and homegrown vegetables for communities neighboring hazardous waste sites. The objective of this study was to estimate the daily arsenic dose for vegetable gardeners in Dewey-Humboldt, Arizona, and to characterize the risk posed by consumption of garden vegetables, incidental soil ingestion, and water consumption. The town of Dewey-Humboldt, Arizona is adjacent to the Iron King Mine and Humboldt Smelter Superfund (IKMHSS) site, which is known to have elevated levels of arsenic. An exposure assessment was conducted to evaluate the amount of arsenic introduced to an individual via the ingestion of homegrown vegetables, soils (incidental), and water. The potential risk was then characterized to determine the incremental excess lifetime cancer risk and the likelihood of non-cancer adverse health effects (hazard quotient). In addition, maximum arsenic limits for garden soils were estimated, which will allow vegetable gardeners to evaluate their residential property for gardening via soil testing.
2. Materials and Methods
2.1 Site description
The IKMHSS site is located in Dewey-Humboldt, Yavapai County, Arizona, and was listed on the USEPA National Priorities List in 2008. The site comprises a combination of sources and releases from two separate locations: the Iron King Mine property and the Humboldt Smelter property. A portion of the Town of Dewey-Humboldt is situated between the mine and the smelter. The smelter operated from the late 1800s until 1969. The Iron King Mine operated periodically from the late 1800s until the early 1960s for production of gold, silver, copper, lead and zinc. All mining and smelting ceased by 1969.
Large amounts of uncontrolled mine-tailings waste exist on both the smelter and tailings properties. The average composite concentration of arsenic in the Iron King Mine tailings pile (0-0.61 meters below ground surface) is 3,710 mg kg−1 (EA Engineering, Science, and Technology, Inc. 2010). A previous study determined that the IKMHSS mine tailings have a low pH (2.5), high electrical conductivity (13.5 ms cm−1), a loam texture (34.7% sand, 44.8% silt, and 20.4% clay), and total organic carbon and total nitrogen of 1.22 g kg−1 and 0.0423 g kg−1, respectively (Solís-Domínguez et al, 2012). The unprotected mining wastes on the two properties are point sources of pollution and are prone to eolian dispersion and water erosion. This is reflected in observations of elevated arsenic and lead concentrations in surface soil at off-site areas adjacent to the neighboring Chaparral Gulch or downwind of the mine tailings and smelter properties. The concentrations of arsenic and lead in shallow surface soil samples in these areas are higher than the concentrations of arsenic and lead in the deeper surface soil. The elevated lead and arsenic levels near the surface are likely due to wind dispersion or surface water transport, rather than being attributable to background conditions (EA Engineering, Science, and Technology, Inc. 2010).
2.2 Greenhouse and home garden studies
A greenhouse study was conducted in parallel with a home garden study to measure arsenic concentrations in edible vegetable tissues. These studies are described in detail in Ramirez-Andreotta et al. (2013). Briefly, three treatments were used for the greenhouse study: (T1) Residential soil with background levels of arsenic, mixed with 25% MiracleGro™ Gardening Soil Mix (MiracleGro™); (T2) Residential soil with elevated arsenic levels, mixed with 25% MiracleGro™; and (T3) Residential soil with elevated arsenic levels, mixed with 25% MiracleGro™ and 10% mine tailings waste from the Iron King Mine. Vegetables studied were bush bean (Fabaceae Phaseolus vulgaris), lettuce (Asteraceae Lactuca sativa), radish (Brassicaceae Raphanus sativus), and onion (Liliaceae Allium cepa).
In the home garden study, 25 participants collected garden soil, and irrigation water samples as previously described (Ramirez-Andreotta et al., 2013). Each participant chose their own vegetables to grow for the study, and each submitted up to four types of vegetables from their garden for analysis. Irrigation water samples were analyzed using Inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500ce, Agilent Technologies, Santa Clara, CA), with a quantifiable detection limit for As of 0.11 μg L–1. Independently, water samples were collected by University personnel from a selected subset of participant homes for QA/QC purposes, and consistent results were obtained from the two set of samples. Subsamples for each of the greenhouse soil treatments and home garden soils were subjected to acid digestion following USEPA Method 3051 (USEPA, 1986). The subsamples were treated with 2.5 mL HNO3 and microwave (CEM corporation, model number MDS 2100) digested for 1 h. The digested soil samples were analyzed using ICP-MS (Agilent 7500ce, Agilent Technologies, Santa Clara, CA), with a quantifiable detection limit for As of 0.11 μg L–1 or 0.0033 mg kg–1. These methods are described in more detail in Ramirez-Andreotta et al. (2013).
All vegetables from the greenhouse and community garden studies were harvested and washed in a 0.1 M HCl solution and rinsed with nanopure water to remove all soil particles before analysis to ensure that the arsenic measured represented that taken up into the plant tissue. Vegetable samples were dried, ground with a Wiley Mill for 1 min, passed through a 30 mesh (0.595 mm) screen, and analyzed for total arsenic content. 0.5000 g subsamples of each vegetable sample were digested using 2.5 mL HNO3, 2.5 mL H202 and 10 mL water. Baseline controls, subjected to identical processing, were employed consisting of: 1) distilled water and HNO3; and 2) standard reference tomato leaves were used as a check standard for validating accuracy and comparability within the environmental measurement community (Standard Reference Material 1573a, National Institute of Standards and Technology). The metal content of digested plant tissues was determined using ICP-MS. For this study, only the edible portions of the plant were analyzed. These methods are described in more detail in Ramirez-Andreotta et al. (2013).
2.3 Exposure assessment (ADD and LADD)
Arsenic concentrations in vegetable samples were compared to those reported in US FDA Total Diet Study Statistics on Element Results based on the 2006 – 2008 Market Baskets (US FDA Market Basket Study, 2010). The Market Basket Study involved purchasing samples of food throughout the U.S., preparing the food as it would be consumed, and analyzing the foods to measure the concentrations of selected elements and compounds. Market basket studies are generally conducted four times each year, once in each of four geographic regions of the country (West, North Central, South, and Northeast). The selected food samples are purchased by FDA personnel from supermarkets, grocery stores, and fast food restaurants in three cities in each region and are shipped to a central FDA laboratory (US FDA, 2010).
The Market Basket Study employs zeros for samples for which non-detects are reported. A more conservative approach is to employ a non-zero value that is related to the method detection limit, such as ½ the detection limit (USEPA, 1991a; USEPA, 2000). Thus, the average arsenic concentrations reported in the Market Basket Study were recalculated to incorporate this more conservative approach. For example, when an average of zero was reported, a modified average (MA1) was calculated using the limit of detection (LOD) as follows:
When an average was reported that included one or more zeros in the calculation, a second modified average (MA2) was calculated using the following equation:
The arsenic concentrations measured in the home garden soils (Cs), vegetables (Cv), and water samples (Cw) were used to calculate the average daily dose (ADD) and lifetime average daily dose (LADD). The ADD and LADD were calculated using the following equations (USEPA, 2011, EA Engineering, Science, and Technology, Inc. 2010, USEPA, 1991, Gerba, 2006):
where: IR = intake rate; BAF = bioavailability factor (EA Engineering, Science, and Technology, Inc. 2010); CF = conversion factor; EF = exposure frequency; ED = exposure duration; AT-NC = average time – non-cancer; AT-C = average time – cancer (AT-C); and BW = body weight. Values for these parameters (Table 1) were obtained from the 2011 USEPA Exposure Factors Handbook (USEPA, 2011) and the USEPA IKMHSS site Investigative Report (EA Engineering, Science, and Technology, Inc. 2010) and the RAGS for Superfund Volume I: Human Health Evaluation Manual. Supplemental Guidance: Standard Default Exposure Factors (USEPA, 1991b). To promote comparison of the ADD values from this study to values reported in the literature, the ADD values for this study were modified using the following equation:
where a mean body weight of 60 kg is assumed.
Table 1.
Exposure factor values used in the calculation of lifetime average daily dose, average daily dose, incremental excess lifetime cancer risk, hazard quotient and maximum arsenic soil concentrations.
| Factor/Parameter | Symbol | Units | Residential Value |
Data Source |
|---|---|---|---|---|
| Arsenic Concentration in Soil | Cs | mg kg−1 | Varied by soil treatment or household |
Ramirez-Andreotta et al, 2013 |
| Arsenic Concentration in Vegetable |
Cv | mg kg−1 (fresh weight) |
Varied by vegetable |
Ramirez-Andreotta et al., 2013 |
| Arsenic Concentration in Water |
Cw | mg L−1 | Varied by household |
Ramirez-Andreotta et al., 2013 |
| Ingestion Rate - Soil | IRsoil | mg day−1 | 1002 | USEPA, 2012c, EA Engineering, Science, and Technology, Inc. 2010, US EPA, 1991. |
| Ingestion Rate - Vegetable | IRveg | mg day−1 | Varies by vegetable |
US EPA, 2011 |
| Ingestion Rate – Potable Water |
IRw | L day−1 | 2 | EA Engineering, Science, and Technology, Inc. 2010, US EPA, 1991, |
| Bioavailability Factor | BAF | unitless | 0.8 | EA Engineering, Science, and Technology, Inc. 2010 |
| Exposure Frequency | EF | days year−1 | 350 | US EPA, 2011 |
| Exposure Duration | ED | years | 30 | US EPA, 2011 |
| Conversion Factor (used for soils and vegetables only) |
CF | kg mg−1 | 0.000001 | EA Engineering, Science, and Technology, Inc. 2010 |
| Body Weight | BW | kg | 601 | US EPA, 2011 |
| Average Time – Cancer | AT-C | days | 28470 | US EPA, 2011 |
| Average Time - Noncancer | AT-NC | days | 10950 | US EPA, 2011 |
| Arsenic Reference Dose | RfD | mg kg−1 day−1 |
0.0003 | US EPA, 2012b |
| Arsenic Cancer Slope Factor | CSF | 1.5 kg day mg−1 |
1.5 | US EPA, 2012b |
US EPA Exposures Handbook recommends using 60 kg with their predicted IR for the consumption of homegrown produce as they sampling efforts combined adults with children.
100 mg/day is the recommended default IR value (USEPA, 2012c, USEPA, 1991) and is what was used in the Iron King Mine Humboldt Smelter Superfund Site Dewey-Humboldt Remedial Investigative Report (EA Engineering, Science, and Technology, Inc. 2010).
The IR used for each vegetable was the average intake rate for home-produced food in non-metropolitan areas of the U.S., reported in the USEPA Exposure Factors Handbook (USEPA, 2011). This value incorporates the amount of vegetable lost during preparation and cooking, set by the EPA at 12.4%. Typically, the IR is reported for each vegetable. However, in the case of radish, celery, and Brussels sprouts, the specific vegetable IR was not reported. In these cases, the IR reported for total per capita consumption (Table 9-9, USEPA, 2011) was multiplied by the fraction of home produce consumed for the food classes “root vegetables” (radish) and “exposed vegetables” (celery and Brussels sprouts) as reported in Table 13-68 of the handbook. Squash was also listed in the category “exposed vegetables”, but there was no IR reported for total per capita consumption. For this case, the reported IR was divided by the number of vegetable types listed in that category.
There are uncertainties associated with the calculations above. It is assumed that an individual living in the area will be eating the vegetable for 350 days out of the year for 30 years over an average time of 30 (ADD) or 78 years (LADD). This is unlikely due to the vegetable growing seasons and fluctuations in garden productivity. Therefore, the calculated LADDs and ADDs are conservative, and the actual values would most likely be smaller. As noted above, all vegetables were washed in a 0.1 M HCl solution and rinsed with nanopure water to remove all soil particles before analysis to ensure that the arsenic measured represented that incorporated into the plant tissue. It is unrealistic to assume an average home gardener would wash all their vegetables with a 0.1 M HCl solution, and it can be inferred that some soil particles might remain on the vegetable. Potential exposure via ingestion of soil associated with the vegetables is accounted for through the assessment of incidental soil ingestion presented above.
2.4 Risk characterization (IELCR and HQ)
The carcinogenic risk was characterized by using the incremental excess lifetime cancer risk (IELCR), which is the LADD multiplied by the cancer slope factor (CSF). The CSF is the upper bound, approximating a 95% confidence limit, on the increased cancer risk from a lifetime exposure to an agent. The IELCR characterizes the excess cancer risk (beyond one’s existing chance of developing cancer) for an individual who consumes that vegetable, water, or incidentally ingests the garden soil following the assumptions used in the calculation of the LADD. IELCR is used to determine the theoretical maximum number of excess cancer cases that are expected to develop due to that exposure and concentration (USEPA, 2012b). The IELCR was calculated for vegetables, garden soils, and water using a CSF of 1.5 kg day mg−1 (USEPA, 2012b). The non-carcinogenic risk was characterized by using the hazard quotient (HQ), which is the ratio of the ADD to the reference dose (RfD). The RfD is an estimate of a daily oral exposure to the human population (including sensitive subgroups) that is likely to produce no appreciable risk of deleterious effects during a lifetime (USEPA, 2012b). The RfD for arsenic is 0.0003 mg kg−1 d−1 (USEPA, 2012b). A potential risk is considered to exist when the HQ is greater than 1.
2.5 Determination of Risk-Based Maximum Soil Arsenic Concentrations
Risk-based screening values are derived from equations combining exposure assumptions with toxicity data (ITRC, 2005). They provide a standard methodology to calculate risk-based soil screening levels for contaminants in soils that may be used to identify areas needing further investigation (USEPA, 1996). These calculated soil estimates represent the maximum allowable soil arsenic concentration for gardens such that consuming vegetables grown in the garden will not produce an IELCR exceedance at the selected target risk level. Soil screening levels for arsenic for planting a garden were developed as follows. First, the risk-based screening level (RBSL) equation (USEPA, 1996) was used to calculate the maximum arsenic concentration (Cv-max) allowable for the selected vegetables for specific cancer target risks (TR) ranging from 10−6 to 10−5:
In the RBSL calculation, the IR is the average intake rate for all the vegetables within one plant family. Second, correlations relating concentration of arsenic in vegetables to the concentrations in soils in which they were grown were used to estimate the soil arsenic concentrations corresponding to the calculated maximum allowable vegetable arsenic concentrations (Cv-max). This analysis was conducted for the Asteraceae, Brassicaceae, Fabaceae and Amaranthaceae families, which are the families for which robust correlations were reported (Ramirez-Andreotta et al, 2013).
3.0 Results
3.1 Water
Arsenic concentrations in water samples collected from the 25 homes participating in this study ranged from 1.40 to 2,030 μg L−1 (Ramirez-Andreotta et al., 2013). Using these values, the range of the LADD values was 1.38 × 10−5 to 9.98 × 10−3 mg kg−1 d−1, and the mean LADD was 1.18 × 10−4 mg kg−1 d−1. Seventeen out of the 25 community water samples had an IELCR greater than 1 ×10−4, and the remaining samples had an IELCR greater than 1 × 10−5 (Table 2). For reference, the IELCR for arsenic in drinking water at the current USEPA regulatory standard is 1.48 × 10−4. The ADD arsenic range was 3.59 × 10−5 to 2.60 × 10−2 mg kg−1 d−1, and the HQ range was 0.120 to 86.5. Fifteen samples exceeded a HQ value of 1. It should be noted that the participants were asked to collect water samples from their primary source of water for irrigation. In some, but not all cases, this water is also used for potable water (drinking water). Thus, in the discussion below, the water sources are considered as representing potable water for all participants.
Table 2.
Incremental Excess Lifetime Cancer Risk (IELCR) ranges for water, garden soils and plant families.
| Source | Mean (Standard Deviation) | Range |
|---|---|---|
| Water | 8.5 ×10−4 (3.0 × 10−3) | 2.1 × 10−5 - 1.5 × 10−2 |
| Soils | ||
| Greenhouse | 1.9 × 10−4 (1.9 × 10−4) | 2.0 × 10−5 - 4.0 × 10−4 |
| Garden | 2.6 ×10−5 (2.7 ×10−5) | 1.7 × 10−6 - 1.2 × 10−4 |
| Plant Families | ||
| Asteraceae | ||
| Greenhouse | 2.3 × 10−4 (2.3 × 10−4) | 2.6 × 10−5 - 6.7 × 10−4 |
| Household | 2.8 × 10−6 (2.9 × 10−6) | 6.0 × 10−7 - 4.8 × 10−6 |
| Fabaceae | ||
| Greenhouse | 2.5 × 10−5 (2.5 × 10−5) | 8.2 × 10−6 - 8.4 × 10−5 |
| Household | 8.1 × 10−6 (9.9 × 10−6) | 1.3 × 10−6 - 2.0 × 10−5 |
| Brassicaceae | ||
| Greenhouse (radish only) | 2.6 × 10−7 (2.5 × 10−7) | 3.3 × 10−8 - 8.3 × 10−7 |
| Household | 3.8 × 10−6 (4.5 × 10−6) | 2.7 × 10−9 - 1.1 × 10−5 |
| Amaranthaceae | 1.7 × 10−5 (1.7 × 10−5) | 7.3 × 10−7 - 4.6 × 10−5 |
| Liliaceae | ||
| Greenhouse (onion only) | 7.8 × 10−7 (7.3 × 10−7) | 1.1 × 10−6 - 6.6 × 10−6 |
| Household | 4.4 × 10−6 (6.8 × 10−6) | 3.1 × 10−7 - 2.2 × 10−5 |
| Solanaceae | 1.1 × 10−6 (9.6 × 10−7) | 9.3 × 10−7 - 4.1 × 10−6 |
| Cucurbitaceae | 2.5 × 10−7 (6.5 × 10−7) | 8.1 × 10−8 - 2.6 × 10−6 |
3.2 Soils
Arsenic concentrations for the three greenhouse treatments and 25 home garden soils ranged from 27.2 – 533 mg kg−1 and 2.35 – 374 mg kg−1, respectively (Ramirez-Andreotta et al., 2013). These were used to calculate the range of the LADD of arsenic from the greenhouse soils and home garden soils: 1.33 × 10−5 to 2.64 × 10−4 mg kg−1 d−1 and 1.16 × 10−6 to 1.84 × 10−4 mg kg−1 d−1, respectively. The IELCR for arsenic at the Arizona Soil Remediation Level is 7.38 × 10−6. All greenhouse soils treatments, and seventeen garden soils exceeded an IELCR of 1 ×10−5 (Table 2). The range in ADD for arsenic in the greenhouse soils and home garden soils are 3.45 × 10−5 to 6.85 × 10−4 mg kg−1 d−1 and 3.01 × 10−6 to 4.77 × 10−4 mg kg−1 d−1, respectively. The HQ range for the greenhouse soils and home garden soils are 0.115 to 2.28 and 0.0100 to 1.59, respectively. Soils for treatments 2 and 3 from the greenhouse, and one out of the 25 home garden samples exceeded the HQ value of 1.
3.3 Vegetables
Arsenic concentrations measured for the lettuce, onion, radish, and bean greenhouse samples ranged from 0.00843 to 5.28 mg kg−1 fresh weight (Table 3). The LADD and ADD values for all greenhouse vegetables had similarly wide ranges (Table 4). Overall, the IELCR posed by these crops for all greenhouse treatments decreased in order of Asteraceae (lettuce) >> Fabaceae (bean) > Liliaceae (onion) > Brassicaceae (radish) (Table 2). IELCR values for the USFDA Market Basket study for lettuce, onion, and bean were lower than the greenhouse values. All the lettuce replicates grown in T3 and one lettuce replicate in T2 exceeded the HQ value of 1.
Table 3.
Range of arsenic concentrations in vegetables compared to the US FDA Market Basket Study.
| Vegetable Family | Arsenic FWa (mg kg−1) |
|---|---|
| Asteraceae (Lettuce) | |
| Greenhouseb | 0.203 – 5.28 |
| Home garden (N=2) | 0.00550 - 0.0376 |
| US FDA Market Basket Study | 0.005c |
|
| |
| Fabaceae (bean) | |
| Greenhouse | 0.0110 – 0.240 |
| Home garden (N=3) | 0.00384 - 0.0560 |
| US FDA Market Basket Study | 0.005d |
|
| |
| Liliaceae | |
| Greenhouse (onion only)b | 0.00843- 0.04933 |
| Home garden (N = 10) | 0.00232 - 0.167 |
| US FDA Market Basket Study | 0.005d |
|
| |
| Brassicaceae | |
| Greenhouse (radish only)b | 0.102 – 2.56 |
| Home garden (N=7) | 0.00278 - 0.0674 |
| US FDA Market Basket Study | 0.003e (radish), 0.005d (broccoli), 0.004c (red cabbage) |
|
| |
| Amaranthaceae | |
| Home garden (N=7) | 0.00573 - 0.204 |
| US FDA Market Basket Study | 0.005c (beets), 0.005d (spinach) |
|
| |
| Solanaceae | |
| Home garden (N=15) | 0.00132 - 0.0100 |
| US FDA Market Basket Study | 0.006d (tomato), 0.005c (pepper, green) |
|
| |
| Cucurbitaceae | |
| Home garden (N=15) | 0.00211 - 0.0136 |
| US FDA Market Basket Study | 0.005c (squash), 0.013d (cucumber) |
Fresh weight
Range for all three treatments, N=12
Modified average 1
Modified average 2
Reported mean from the 2007 US FDA’s Total Diet Study, the 2010 Market Basket Study did not sample radish.
Table 4.
Lifetime average daily dose and average daily dose of arsenic for greenhouse samples.
| Vegetable Family | LADD (mg kg−1 day−1) |
ADD (mg kg−1 day−1) |
|---|---|---|
| Asteraceae - Lettuce | ||
| T1a | 1.86 × 10−5 – 4.06 × 10−5 | 7.68 × 10−5 – 1.06 × 10−4 |
| T2b | 4.24 × 10−5 – 1.42 × 10−4 | 1.10 × 10−4 – 3.68 × 10−4 |
| T3c | 1.88 × 10−4 – 4.49 × 10−4 | 4.89 × 10−4 – 1.10 × 10−3 |
|
| ||
| Fabaceae - Bean | ||
| T1 | 2.56 × 10−6 – 6.81 × 10−6 | 6.67 × 10−6– 1.77 × 10−5 |
| T2 | 1.81 × 10−5 – 8.91 × 10−6 | 1.42 × 10−5 – 4.71 × 10−5 |
| T3 | 2.23 × 10−5 – 5.57 × 10−5 | 5.80 × 10−5 – 1.45 × 10−4 |
|
| ||
| Brassicaceae - Radish | ||
| T1 | 2.21 × 10−8 – 3.93 × 10−8 | 5.74 × 10−8 – 1.02 × 10−7 |
| T2 | 8.54 × 10−8 – 2.01 × 10−7 | 2.22 × 10−7 – 5.23 × 10−7 |
| T3 | 1.60 × 10−7 – 5.53 × 10−7 | 4.17 × 10−7 – 1.44 × 10−6 |
|
| ||
| Liliaceae - Onion | ||
| T1 | 7.56 × 10−7 – 9.94 × 10−7 | 1.97 × 10−6 – 2.59 × 10−6 |
| T2 | 2.27 × 10−6 – 1.13 × 10−5 | 4.86 × 10−6 – 2.94 × 10−5 |
| T3 | 3.21 × 10−6 – 4.42 × 10−6 | 8.35 × 10−6 – 1.15 × 10−5 |
Values are range (N=4).
T1 = Greenhouse treatment 1
T2 = Greenhouse treatment 2
T3 = Greenhouse treatment 3
The vegetables collected from the home gardens were grouped by scientific family and the range of arsenic concentrations observed in the vegetable families are provided in Table 3, and their LADD and ADD values are presented in Table 5. Overall, the IELCR posed by the home garden crops for all households decreased in order of Amaranthaceae >> Fabaceae > Liliaceae > Brassicaceae > Asteraceae > Solanaceae >> Cucurbitaceae (Table 2). All Fabaceae, Amaranthaceae (except one Swiss chard sample), and Brassicaceae (except one radish and Brussels sprout sample) had an IELCR value greater than 1 × 10−6. In addition, one out of the two lettuce samples, three out of ten tomato samples, two out of the eight onion samples, and a cucumber sample exceeded 1 × 10−6. No homegrown vegetable samples exceeded the HQ value of 1.
Table 5.
Lifetime average daily dose and average daily dose of arsenic values for all home garden vegetables
| Vegetable Family | LADD (mg kg−1 day−1) |
ADD (mg kg−1 day−1) |
|---|---|---|
| Asteraceae | ||
| Lettuce | 3.20 × 10−6 | 8.32 × 10−6 |
| Lettuce | 6.68 × 10−7 | 1.22 × 10−6 |
|
| ||
| Fabaceae | ||
| Bean | 1.30 × 10−5 | 3.38 × 10−5 |
| Bean | 8.92 × 10−7 | 2.32 × 10−6 |
| Bean | 2.32 × 10−6 | 6.03 × 10−6 |
|
| ||
| Brassicaceae | ||
| Broccoli | 8.57 × 10−7 | 2.23 × 10−6 |
| Red Cabbage | 7.37 × 10−6 | 1.92 × 10−5 |
| Brussels Sprouts | 7.94 × 10−9 | 2.07 × 10−8 |
| Cabbage | 6.76 × 10−7 | 1.92 × 10−5 |
| Kale | 3.16 × 10−6 | 8.22 × 10−6 |
| Kale | 5.75 × 10−6 | 1.50 × 10−5 |
| Radish | 1.78 × 10−9 | 4.63 × 10−9 |
|
| ||
| Liliaceae | ||
| Onion | 1.41 × 10−6 | 3.66 × 10−6 |
| Onion | 1.40 × 10−6 | 3.64 × 10−6 |
| Onion | 2.08 × 10−7 | 5.42 × 10−7 |
| Onion | 7.05 × 10−7 | 1.83 × 10−6 |
| Onion | 4.93 × 10−7 | 1.28 × 10−6 |
| Onion | 4.42 × 10−7 | 1.15 × 10−6 |
| Onion | 3.31 × 10−6 | 8.60 × 10−6 |
| Onion | 1.50 × 10−5 | 3.90 × 10−5 |
| Chives | 1.01 × 10−6 | 2.62 × 10−6 |
| Garlic | 5.67 × 10−6 | 1.47 × 10−5 |
|
| ||
| Curcurbitaceae | ||
| Zucchini | 3.98 × 10−8 | 1.03 × 10−7 |
| Yellow Squash | 2.88 × 10−8 | 7.48 × 10−8 |
| Yellow Squash | 1.60 × 10−7 | 4.17 × 10−7 |
| White Squash | 4.39 × 10−8 | 1.14 × 10−7 |
| Zucchini | 7.58 × 10−8 | 1.97 × 10−7 |
| Yellow Squash | 4.66 × 10−8 | 1.21 × 10−7 |
| Zucchini | 2.49 × 10−8 | 6.46 × 10−8 |
| Zucchini | 5.40 × 10−8 | 1.40 × 10−7 |
| Zucchini | 4.03 × 10−8 | 1.05 × 10−7 |
| Zucchini | 2.88 × 10−8 | 7.48 × 10−8 |
| Spaghetti Squash | 4.42 × 10−8 | 1.05 × 10−7 |
| Zucchini | 1.07 × 10−7 | 2.79 × 10−7 |
| Delicata Squash | 3.26 × 10−8 | 8.47 × 10−8 |
| Cucumber | 1.73 × 10−7 | 4.56 × 10−6 |
|
| ||
| Solanaceae | ||
| Tomato | 4.29 × 10−7 | 1.11 × 10−6 |
| Tomato | 4.91 × 10−7 | 1.28 × 10−6 |
| Tomato | 4.77 × 10−7 | 1.24 × 10−6 |
| Tomato | 7.23 × 10−7 | 1.88 × 10−6 |
| Tomato | 4.89 × 10−7 | 1.27 × 10−6 |
| Tomato | 1.61 × 10−6 | 4.19 × 10−6 |
| Tomato | 2.76 × 10−6 | 7.18 × 10−6 |
| Tomato | 4.81 × 10−7 | 1.25 × 10−6 |
| Tomato | 5.09 × 10−7 | 1.32 × 10−6 |
| Tomato | 9.36 × 10−7 | 2.43 × 10−6 |
| Bell Pepper | 2.13 × 10−7 | 5.53 × 10−7 |
| Jalapeno | 4.92 × 10−7 | 1.28 × 10−6 |
| Jalapeno | 6.21 × 10−7 | 1.61 × 10−6 |
| Bell Pepper | 5.27 × 10−7 | 1.37 × 10−6 |
| Green Chili | 4.86 × 10−7 | 1.26 × 10−6 |
|
| ||
| Amaranthaceae | ||
| Swiss Chard | 4.89 × 10−7 | 1.27 × 10−6 |
| Swiss Chard | 7.13 × 10−7 | 1.85 × 10−6 |
| Beet | 1.93 × 10−6 | 5.03 × 10−6 |
| Beets | 1.39 × 10−6 | 3.60 × 10−6 |
| Spinach | 3.62 × 10−6 | 9.41 × 10−6 |
| Swiss Chard | 1.28 × 10−5 | 3.32 × 10−5 |
| Beet | 3.06 × 10−5 | 7.96 × 10−5 |
3.4 Comparing Gardenroots values to US FDA Market Basket Study
The arsenic concentrations measured for the greenhouse vegetables for all treatments were greater than the arsenic concentrations reported in the US FDA Market Basket. Arsenic concentrations in all the home grown vegetables from the Asteraceae and Amaranthaceae families and the majority of samples from the Fabaceae (2/3), Liliaceae (8/10), and Brassicaceae (6/7) families were greater than the arsenic concentrations reported in the US FDA Market Basket Study. In contrast, the majority of the Solanaceae samples (10/15) and Cucurbitaceae (12/15) samples were below the US FDA values. Within the Solanaceae family, four out of the five homegrown peppers were above the reported US FDA value for peppers (green).
3.5 Aggregate exposure dose
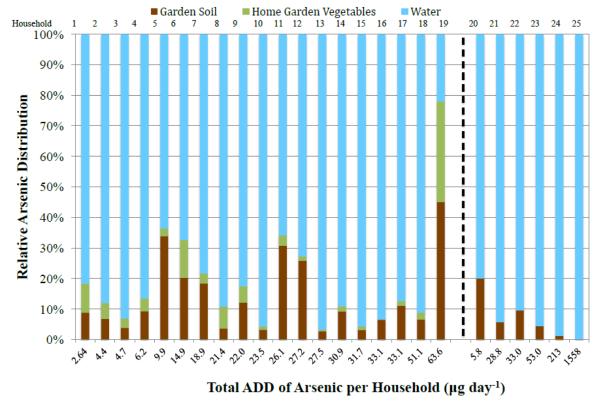
The combined ADD for water, soils, and vegetables for each household was determined, along with the relative contributions of each component to total arsenic exposure. Results showed that water contributed the largest amount of arsenic to a Dewey-Humboldt adult gardener, followed by incidental soil ingestion and homegrown vegetables (Figure 1). However, the results varied considerably. For example, household 19 had the largest soil arsenic concentration (373 mg kg−1) out of all the participants and one of the lower water arsenic concentrations (9.15 μg L−1). In this case soil and vegetable ingestion contributed nearly 80% of their ADD of arsenic. In contrast, households 15 and 18 had garden soil arsenic concentrations of 12.6 mg kg−1 and 43.2 mg kg−1, respectively, and soils and vegetable ingestion contributed less than 10% of their total ADD. The average of all ADDs for water, soil, and vegetables were 89.2 μg d−1, 3.39 μg d−1 and 1.66 μg d−1, respectively (Table 6). The average daily arsenic intake contribution from water, incidental soil ingestion and homegrown vegetables was 77, 16 and 7%, respectively.
Figure 1.
The total ADD (μg day−1) for potable water, soils and homegrown vegetables, and their relative arsenic exposure contribution per Dewey-Humboldt household. Household samples to the right of the dotted line are households that submitted only garden soils and potable water. Six of the 25 participants submitted only soil and water samples, and these data are presented along on the right side of the graph.
Table 6.
Estimated average daily intake of arsenic (μg day−1) from water, vegetables and soil for an adult from this study and others.
| Site | Water | Vegetables | Soil | Total | Reference |
|---|---|---|---|---|---|
| Greenhouse study, AZ, USA | – a | 6.97 (0.02 – 70.1) | 20.1 (2.07 – 41.1) | 27.1 (2.09 – 111.2) | This Study (ADD values) |
| Home Garden, AZ, USA | 89.2 (2.15 – 1557) | 1.66 (0.007 – 20.9) | 3.39 (0.232 – 26.8) | 94.3 (2.65−1558) | This Study (ADD values) |
| State of AZ, excluding mines | 3.4, 8.1b | – | 0.7, 1.4b | 38.5, 31.5 | O’Rourke et al, 1999 |
| Mining communities in AZ | 6.6, 15.2b | – | 0.8, 1.6b | 20.4, 39.4 | O’Rourke et al, 1999 |
| Coahuila, Mexicoc | 826, 505 | 262d | – | 1088, 767 | Del Razo et al, 2002 |
| Durango, Mexico c | 30.8, 17.5 | 14.0d | – | 44.8, 31.5 | Del Razo et al, 2002 |
| Village of Chiu Chui, Chile | 1331 | 69e | – | 1,400 | Diaz et al, 2004 |
| Village of Chiu Chui, Chilef | 92 | 39e | – | 131 | Diaz et al, 2004 |
| Songcheon area, South Korea | 186 | – | 0.354 | 186.4 | Lee et al, 2005 |
| Jalangi block, West Bengal, Indiag | 266, 399 | 9.17, 10.4 | – | 275, 409 | Roychowdhury et al. 2002, 2003 |
| Domkal block, West Bengal, Indiag | 200, 300 | 12.3, 10.6 | – | 212, 312 | Roychowdhury et al. 2002, 2003 |
| Santiago, Chile | – | 2.29 | – | 2.29 | Munoz et al, 2005 |
| Beijing, China | – | 5.11 | – | 5.11 | Bo et al, 2009 |
| Republic of Croatia | – | 0.114 | – | 0.114 | Sapunar-Postruznik, 1996 |
No Data
Reported Mean, 90th percentile
Reported by season: Summer, Winter
Pinto beans and potatoes only
Includes bread and maize
Second sampling after the administrative authority provided water that met Chilean regulations to Chui Chui village.
Arsenic total daily dietary intake for vegetables given for men, women.
3.6 Estimated maximum soil arsenic concentration
There was a direct correlation between the amount of arsenic that accumulated in the edible portion of the plant and the arsenic soil concentration for the vegetable families Asteraceae; Brassicaceae; Amaranthaceae and Fabaceae families (Ramirez-Andreotta et al, 2012). These regression equations can be used to estimate risk-based screening levels (i.e., maximum soil arsenic concentrations) for garden soil, such that consuming vegetables produced from the garden would not cause exceedance of IELCR at a specific target risk level. Table 7 provides the soil arsenic concentrations at target risks of 10−6, 10−5 and 10−4.
Table 7.
Estimated maximum arsenic levels in garden soils for Asteraceae, Brassicaceae, Fabaceae and Amaranthaceae at various target risk levels.
| 10−6 | 10−5 | 10−4 | ||
|---|---|---|---|---|
| Vegetable Family | Linear Regression Equation | (mg kg−1) | ||
| Asteraceae | y = 0.0343x | 1.56 | 15.6 | 156 |
| Brassicaceae | y = 0.008x | 5.39 | 53.9 | 539 |
| Fabaceae | y = 0.0018x | 11.6 | 116 | 1,160 |
| Amaranthaceae | y = 0.0036x | 12.4 | 124 | 1,242 |
4.0 Discussion
4.1 Arsenic daily intake from home garden vegetables
The results of this study show that the majority of the vegetables from five out of the seven plant families grown near the IKMHSS site had larger arsenic concentrations than the vegetables tested in the US FDA Market Basket study. In general, home garden tomato and squash samples were below the reported US FDA value. The mean aggregate daily intake rate of arsenic from home garden vegetables for a Dewey-Humboldt, Arizona community member was calculated to be 1.66 μg/day with a range of 0.007 to 20.9 μg/day. This mean value can be compared to values reported for other areas of the world (see Table 6).
Exposure to arsenic via vegetable consumption can be increased through cooking. Specifically, the results of several studies have revealed that boiling vegetables in water that is elevated in arsenic can increase arsenic concentrations in food (Del Razo et al, 2002). Thus, in arsenic-endemic areas, the high arsenic content in water used for cooking purposes is an additional source of inorganic arsenic exposure (Diaz et al, 2004, Roychowdhury et al. 2002). While the Gardenroots study reports arsenic values only for raw vegetables, it can be assumed based on the studies mentioned above that arsenic exposure may increase if the vegetable were cooked (boiled) with water containing arsenic. Individuals living in mining communities and arsenic-endemic areas need to be aware of the potential impact of arsenic in the water they are using to prepare food.
4.2 Arsenic daily intake from all food sources
While this study focused on arsenic in home garden vegetables, other types of food contribute to an individual’s daily arsenic intake. Thus, the relative contribution of vegetables versus other foods was evaluated. The average dietary intake of arsenic from all food sources (excluding water) determined by market basket studies for an American adult is 53 μg/day, an Australian female is 53 μg/day, and an Australian male is 73 μg/day, with the main sources being fish, dairy, meat, and poultry (Prohaska and Stingeder, 2005, WHO, 2001, Yost et al., 1998, Australia New Zealand Food Authority, 1994). An average arsenic dietary intake of 77 μg/day was reported for a market basket study conducted in Santiago, Chile, with the main sources being seafood, followed by spices and meat (Munoz et al, 2005). An arsenic contribution from spices was also observed in West Bengal, India (Roychowdhury et al., 2002, 2003). Sixty to ninety-nine percent of the arsenic present in seafood comprises organo-arsenic species and thus is not considered a health hazard (Prohaska and Stingeder, 2005). Conversely, meat, poultry, dairy products, cereals, and vegetables contain higher proportions of the more toxic inorganic arsenic forms (Smith et al, 2006, Prohaska and Stingeder, 2005, Yost et al., 1998).
The range of arsenic intake rates for vegetables determined for this study (0.007 to 20.9 μg/day) can be compared to the reported average dietary intake of arsenic from all food sources for an American to evaluate the relative contribution of the homegrown vegetables to total food-based arsenic intake. The results of this comparison show that the relative contribution from homegrown vegetables ranges from negligible to a significant fraction (~39%) of the reported dietary intake. These data indicate that there are other significant sources of arsenic in the diet, and these sources should be considered in assessing total potential dietary intake for this community since they are not solely dependent on their garden. This was accomplished by adding the aggregate daily arsenic intake range values of the vegetables determined in this study to the average dietary exposure of 53 μg/day reported for Americans. These calculations resulted in estimated daily dietary intake rates of 53.0 μg/day (0.883 μg kg−1 per day) to 73.9 μg/day (1.23 μg kg−1 per day).
As stated above, meat, poultry, dairy products, cereals, and vegetables contain higher proportions of the more toxic inorganic arsenic forms. Given that the results of prior studies have shown that arsenic can accumulate in the edible portion of vegetables, and that there is a potential risk associated with the consumption of these home garden vegetables, regulatory standards and guidelines are being developed for arsenic in vegetables. For example, Poland has a fixed concentration for all fresh vegetables set at 0.2 mg kg−1, Japan has a set a limit of 1.0 mg kg−1 for spinach, tomatoes, and cucumbers, and in Spain the maximum content of arsenic in canned vegetables is 1.0 mg kg−1 (Peralta-Videa et al., 2009). Furthermore, the Codex Alimentarius Commission (Codex) developed by the FAO/WHO has arsenic standards at 0.1 mg kg−1 for edible fats and oils. Currently, the U.S. does not have regulatory standards for arsenic in vegetables or most other food products. The results of this study support the proposal that setting arsenic standards for food would be useful in combination with existing arsenic water standards for protecting public health (Munoz et al, 2002, Peralta-Videa et al., 2009, Meharg and Raab, 2010).
4.3 Arsenic daily intake from water and soil
The results of the study indicate that water is the major contributor to total arsenic exposure for the community (with the assumption that the water is employed for potable uses). This is consistent with the results of prior studies in Arizona and elsewhere. The National Human Exposure Assessment Survey conducted in Arizona reported that of the homes with the greatest potential arsenic intake doses, 47% of them were in mining communities (O’Rourke et al, 1999). The study also observed that in Ajo, Arizona (a mining community), 50% of the residents who had a total exposure to arsenic in the 90th percentile were consuming tap water with elevated arsenic levels (O’Rourke et al, 1999). The mean arsenic intake dose from water and soils obtained in the current study was greater than the intake doses reported by O’Rourke et al. (1999) for the specific population defined as Arizona mining communities (Table 6). A study was conducted near the Songcheon mine in South Korea to examine arsenic exposure. The estimated average daily intake rates for drinking water and incidental soil ingestion were 186 μg day−1and 0.35 μg day−1, respectively (Lee et al., 2005). The value reported for water is relatively similar to that reported herein, whereas the value for soil ingestion is approximately 10-times smaller.
The current study illustrates how the impacts of mining waste and geogenic sources of arsenic can combine to increase the risk of arsenic exposure for communities near mining sites that are located in arsenic-endemic areas. Arizona, and specifically Yavapai county, where the town of Dewey-Humboldt is located, have naturally high levels of arsenic in groundwater and soil due to: 1) granite bedrock, 2) the Colorado Plateau of northern Arizona and southern Utah, and 3) the arsenic-rich Supai Sandstone formation (Uhlman et al, 2009). These natural sources are the primary contributor to elevated levels of arsenic in groundwater. While the groundwater source is clear, discriminating among anthropogenic and geogenic sources of arsenic in soil is challenging given the potential contributions of arsenic releases to surface soils via air emissions, waste recycling, and soil amendments (Belluck et al, 2003). Regardless of the relative contributions of the specific sources, it is clear that the mine and natural geology of the area are both contributing to the elevated arsenic levels in the garden soils of the Dewey-Humboldt, AZ area. Furthermore, the current study did not measure exposure pathways such as indoor air, outdoor air, or house dust, all of which can add to total arsenic intake. For example, O’Rouke et al. (1999) reported that potential arsenic intake doses in the 90th percentile from dust in Arizona mining communities and the Verde Valley community (a mining community near Dewey-Humboldt, AZ) were 5.8 μg day−1 and 12.6 μg day−1 and, respectively. These values are larger than the value, 3.4 μg day−1, reported for the state of Arizona, excluding mining communities.
4.4 Relative contributions of food and water
The estimated intake of arsenic via ingestion of home garden vegetables was generally relatively low compared to water consumption (Figure 1). Furthermore, water consumption provides the majority of arsenic intake for most of the households compared to the total food intake (i.e., sum of mean U.S. dietary intake rate and homegrown vegetable intake rate). These results are consistent with those reported in prior studies, wherein water consumption provided the majority of arsenic intake (Diaz et al., 2004, Roychowdhury et al., 2003, Roychowdhury et al., 2002, Del Razo et al., 2002).
When totaling the arsenic daily intake from all foods (using the assumption above in section 4.2) and water, the range of arsenic ingestion for a Dewey-Humboldt, AZ community member is estimated to be 55.4 μg day−1 (0.921 μg kg−1 day−1) to 1,610 μg day−1 (26.8 μg kg−1 day−1), with a mean of 140 μg day−1 (2.33 μg kg−1 day−1). For comparison, in 2010 the FAO/WHO Expert Committee on Food Additives reviewed the latest scientific evidence, and determined the lower limit on the benchmark dose for a 0.5% (BMDL0.5) increased incidence of lung cancer from epidemiological data to be 3.0 μg kg−1 day−1 using a range of assumptions to estimate total exposure to inorganic arsenic from drinking water and food. Two households in this study had an intake that exceeded that value, with the primary contribution being water (these households did not submit vegetable samples). When also considering soil ingestion, the same two households remained the only ones in exceedance of the BMDL0.5.
4.5 Estimated maximum arsenic soil levels, and their application for this community
A tool to estimate soil screening levels or maximum soil arsenic concentrations would be of great benefit for vegetable gardeners who neighbor mine tailing waste, live in an arsenic-endemic area, or in the vicinity of a hazardous waste site. In this study, maximum soil arsenic concentrations were estimated for specific plant families by combining the observed soil-plant interactions with risk based screening calculations. The use of such correlations to develop soils screening levels represents, to our knowledge, a novel approach for managing dietary intake of arsenic from edible plants and incidental soil ingestion.
The calculations indicate limiting arsenic concentration in garden soils from this area to 1.56 mg kg−1 would maintain a target risk of 1 ×10−6 for incidental soil ingestion, as well as arsenic ingestion from Asteraceae, Brassicaceae, Amaranthaceae and Fabaceae families. These estimated soil arsenic concentrations values (Table 7) are recommended solely to residents of Dewey-Humboldt, Arizona since they may change for each soil type and situation considered. While this is a site-specific calculation, when the results of this study were compared with data reported in the literature, statistically significant correlations were observed for the Fabaceae and Brassicaceae families (Ramirez-Andreotta et al., 2013). These observations warrant further investigation to evaluate the potential for developing more widely applicable correlations.
Site-specific risk assessments need to be conducted in residential areas near Superfund sites, arsenic-endemic areas, and/or other locations where home and community gardens are located. Given the current risk assessment paradigm, the USEPA Residential Regional Screening Levels of 0.39 to 39 mg kg−1 correspond to a one-in-one-million to one-in-ten-thousand IELCR range for ingestion of soils (USEPA, 2012a, EA Engineering, Science, and Technology, Inc. 2010, Belluck et al., 2003), and many residential areas exceed these screening levels (Belluck et al., 2003). For example, the home garden mean arsenic soil concentration for this study was 44.1 mg kg−1 (Ramirez-Andreotta et al., 2013).
Previous studies have observed that soil type can affect arsenic bioavailability, and this can have implications for health risk assessment (e.g. Oolmen et al., 2002, Ruby, et al., 1999). For this study, it was assumed that 80% of the arsenic ingested was bioavailable (Table 1), and Oolmen et al., 2002, observed that arsenic bioavailability values ranged from 6-95% for three different soils. It is therefore recommended that further investigations be conducted to determine the bioavailability of arsenic from garden soils to improve risk assessments.
5.0 Conclusions
Based upon the results of this study, home gardeners living adjacent to the IKMHSS site have an estimated average daily arsenic intake ranging from 2.65 μg day−1 - 1,558 μg day−1. This study demonstrated that vegetables grown in soils neighboring mine waste, on average accumulated more arsenic than store bought vegetables. Although the ingestion of home garden vegetables may contribute more arsenic than that of store bought vegetables, the exposure was generally relatively low compared to exposure from water ingestion. Treatment of the water to reduce arsenic concentrations, or use of alternative water sources for drinking water would typically reduce the over arsenic intake rate, while increasing the relative contribution of homegrown vegetables to that reduced value.
This study illustrates how the impacts of mining waste and geogenic sources of arsenic can combine to increase the risk of arsenic exposure for communities near mining sites that are located in arsenic-endemic areas. It is recommended that community members living in such environs take steps to mitigate their arsenic exposure. USEPA is currently working on a Remedial Investigation Addendum and Feasibility Study to evaluate cleanup options for the site. In the meantime, two interim removal actions have occurred and a total of 15 residential yards have been remediated by replacing up to two feet of soil with clean backfill, a small tailings pile has been relocated, and a temporary dust fixative has been applied to the Humboldt Smelter ash piles (USEPA, 2012d). Given that the greatest exposure dose is typically from ingestion of potable water, addressing arsenic in drinking water is of primary concern. Ensuring that the concentration of arsenic is below the arsenic drinking water standard will have the largest impact on reducing total arsenic exposure. The USEPA, University of Arizona Cooperative Extension, and the National Ground Water Association recommend that private well owners test their water annually, since private wells are exempt and not regulated at the state and federal level (e.g., Uhlman, 2008). Methods for gardeners to reduce their incidental soil ingestion have been developed (available at http://garden-roots.org/). Lastly, risk-based maximum soil arsenic levels in soils for Asteraceae, Brassicaceae, Fabaceae and Amaranthaceae families were estimated that could help community members make more informed gardening decisions that may reduce their overall arsenic exposure from homegrown vegetables and soils.
Highlights.
Daily arsenic intake decreased in order of: water > soil > homegrown vegetables
The excess cancer risk range for consumption of homegrown vegetables was 10−8 to 10−4
The excess cancer risk range for incidental soil ingestion was 10−6 to 10−4
Risk-based maximum As levels in garden soils are estimated
Acknowledgements
This research was funded by the National Institute of Environmental Health Sciences Superfund Research Program (Grant P42 ES04940), the USEPA Office of Research and Development, the NASA Space Grant Program, The University of Arizona TRIF Water Sustainability Program, and the Alfred P. Sloan Foundation.
The authors would like to give a special thanks to the Gardenroots’ citizen science participants, the community of Dewey-Humboldt, Arizona, Mike Kopplin (The University of Arizona Superfund Research Program’s Hazard Identification Core) and Atasi Ray-Maitra (The University of Arizona Water Quality Laboratory).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR [Accessed 14 February 2013];Toxicological Profile for Arsenic. 2007 Available at: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3.
- ATSDR [Accessed 11 February 2013];Detailed Table for the 2011Priority List of Hazardous Substances that will be the Subject of Toxicological Profiles. 2011 Available at: http://www.atsdr.cdc.gov/SPL/index.html.
- Alam MGM, Snow ET, Tanaka A. Arsenic and heavy metal contamination of vegetables grown in Samta village. Bangladesh The Science of the Total Environment. 2003;308:83–96. doi: 10.1016/S0048-9697(02)00651-4. [DOI] [PubMed] [Google Scholar]
- Arizona Department of Human Health Services [Accessed 19 June 2012];Health Consultation: Iron King Mine and Humboldt Smelter, Dewey-Humboldt, Yavapai County, Arizona. 2009 Available: http://azmemory.lib.az.us/cdm/singleitem/collection/feddocs/id/1912/rec/20.
- Australia New Zealand Food Authority . A total diet survey of pesticides and contaminants. Australia New Zealand Food Authority; Canberra: The 1994 Australian Market Basket Survey. [Google Scholar]
- Belluck DA, Benjamin SL, Baveye P, Sampson J, Johnson B. Widespread arsenic contamination of soils in residential areas and public spaces: an Emerging regulatory or medical crisis? International Journal of Toxicology. 2003;22:109–128. doi: 10.1080/10915810305087. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Samal AC, Majumdar J. Arsenic Contamination in rice, wheat, pulses, and vegetables: a study in an arsenic affected area of West Bengal, India. Water, Air & Soil Poll. 2010;213:3–13. [Google Scholar]
- Castro-Larragoitia J, Kramar U, Puchelt H. 200 years of mining activities at La Paz, San Luis Potosi, Mexico - consequences for environment and geochemical exploration. J Geochem Explor. 1997;58:81–91. [Google Scholar]
- Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E. Accumulation of heavy metals by vegetables grown in mine wastes. Environ Tox & Chem. 2000;19(3):600–607. [Google Scholar]
- Csavina J, Field J, Taylor MP, Gao S, Landázuri A, Betterton EA, Sáez AE. A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Sci Total Environ. 2012;43:58–73. doi: 10.1016/j.scitotenv.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Razo LM, Garcia-Vargas GG, Garcia-Salcedo J, Sanmiguel MF, Rivera M, Hernandez MC, Cebrian ME. Arsenic levels in cooked food and assessment of adult dietary intake of arsenic in the Region Lagunera, Mexico. Food Chem. Toxicol. 2002;40:1423–1431. doi: 10.1016/s0278-6915(02)00074-1. [DOI] [PubMed] [Google Scholar]
- Diaz OP, Leyton I, Muñoz O, Nuñez N, Devesa V, Súñer MA, Vélez D, Montoro R. Contribution of water, bread and vegetables (raw and cooked) to dietary intake of inorganic arsenic in a rural village of northern Chile. Journal of Ag & Food Chem. 2004;52:1773–1779. doi: 10.1021/jf035168t. [DOI] [PubMed] [Google Scholar]
- EA Engineering, Science, and Technology, Inc [accessed 19 June 2012];Remedial Investigation Report Iron King Mine Humboldt Smelter Superfund Site Dewey-Humboldt, Yavapai County, Arizona. 2010 Available: http://yosemite.epa.gov/r9/sfund/r9sfdocw.nsf/3dc283e6c5d6056f88257426007417a2/9ff58681f889089c882576fd0075ea2f!OpenDocument.
- FAO/WHO Expert Committee on Food Additives, Rome . Rome, Food and Agriculture Organization of the United Nations. World Health Organization; Geneva: Feb 16–25, 2010. JECFA/72/SC; http://www.who.int/foodsafety/chem/summary72_rev.pdf. [Google Scholar]
- Francesconi KA. Toxic metal species and food regulations— making a healthy choice. The Analyst. 2007;132:17–20. doi: 10.1039/b610544k. 2007. [DOI] [PubMed] [Google Scholar]
- Gerba CP. Risk Assessment. In: Pepper IL, Gerba CP, Brusseau ML, editors. Environmental and Pollution Science. Elsevier Inc; Amsterdam: 2006. [Google Scholar]
- Huang RQ, Gao SF, Wang WL, Staunton S, Wang G. Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China. Sci Total Environ. 2006;368:531–541. doi: 10.1016/j.scitotenv.2006.03.013. [DOI] [PubMed] [Google Scholar]
- ITRC (Interstate Technology & Regulatory Council) RISK-1. Interstate Technology & Regulatory Council, Risk Assessment Resources Team; Washington, D.C.: [accessed October 2012]. 2005. Examination of Risk-Based Screening Values and Approaches of Selected States. Available at: http://www.itrcweb.org. [Google Scholar]
- Lee J-S, Chon H-T, Kim K-W. Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Env Geochem & Health. 2005;27:185–191. doi: 10.1007/s10653-005-0131-6. [DOI] [PubMed] [Google Scholar]
- Meharg AA, Raab A. Getting to the bottom of arsenic standards and guidelines. Environ Sci and Tech. 2010;44:4395–4399. doi: 10.1021/es9034304. 2010. [DOI] [PubMed] [Google Scholar]
- Muñoz O, Diaz OP, Leyton I, Nuñez N, Devesa V, Súñer MA, Vélez D, Montoro R. Vegetables collected in the cultivated andean area of northern Chile: total and inorganic arsenic contents in raw vegetables. Journal of Ag & Food Chem. 2002;50(3):642–647. doi: 10.1021/jf011027k. [DOI] [PubMed] [Google Scholar]
- Muñoz O, Bastias JM, Araya M, Morales A, Orellana C, Rebolledo R, Vélez D. Estimation of the dietary intake of cadmium, lead, mercury, and arsenic by the population of Santiago (Chile) using a total diet study. Food & Chem Tox. 2005;43:1647–1655. doi: 10.1016/j.fct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Munshower FF. Practical handbook of disturbed land revegetation. Lewis Publishing; Boca Ratton, FL: 1994. [Google Scholar]
- National Gardening Association [Accessed 6 July 2010];The impact of home and community gardening in America. 2009 Available: http://www.gardenresearch.com/home?q=show&id=3126.
- Nriagu JO, Pacyna JM. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;33:3134–139. doi: 10.1038/333134a0. 1988. [DOI] [PubMed] [Google Scholar]
- Nriagu JO, editor. Arsenic in the Environment. Wiley and Sons; New York: 1994. [Google Scholar]
- Oomen AG, Hack A, Minekus M, Zeijdner E, Cornelis C, Schoeters G, Verstraete W, Van de Wiele T, Wragg J, Rompelberg CJM, Sips AJAM, Van Wijnen JH. Comparison of Five In Vitro Digestion Models To Study the Bioaccessibility of Soil Contaminants. Environ Sci Tech. 2002;36(15):3326–3334. doi: 10.1021/es010204v. [DOI] [PubMed] [Google Scholar]
- O’Rourke MK, Rogan SP, Jin S, Robertson GL. Spatial distribution of arsenic exposure and mining communities from NHEXAS Arizona. Journal of Exposure Analysis & Env Epi. 1999;9:446–455. doi: 10.1038/sj.jea.7500050. [DOI] [PubMed] [Google Scholar]
- Ramirez-Andreotta MD, Brusseau ML, Artiola JF, Maier RM. A Greenhouse and Field-Based Study to Determine the Accumulation of Arsenic in Common Homegrown Vegetables Grown in Mining-Affected Soils. Science Total Environment. 2013;443:299–306. doi: 10.1016/j.scitotenv.2012.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury T, Tokunaga H, Ando M. Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from the arsenic-affected areas of West Bengal, India. Sci Total Environ. 2003;308:15–35. doi: 10.1016/S0048-9697(02)00612-5. [DOI] [PubMed] [Google Scholar]
- Roychowdhury T, Uchino U, Tokunaga H, Ando M. Survey of arsenic in food composites from an arsenic-affected area of West Bengal India. Food & Chem Tox. 2002;40:1611–1621. doi: 10.1016/s0278-6915(02)00104-7. [DOI] [PubMed] [Google Scholar]
- Ruby MV, Schoo R, Brattin W, Goldade M, Post G, Harnois M, Mosby DE, Casteel SW, Berti W, Carpenter M, Edwards D, Cragin D, Chappell W. Advances in Evaluating the Oral Bioavailability of Inorganics in Soil for Use in Human Health Risk Assessment. Environ Sci Tech. 1999;33(21):3697–3705. [Google Scholar]
- Sapunar-Postruznik J, Bazulic D, Kubala H. Estimation of dietary intake of arsenic in the general population of the Republic of Croatia. Sci Total Environ. 1996;191:119–123. doi: 10.1016/0048-9697(96)05253-9. [DOI] [PubMed] [Google Scholar]
- Solís-Domínguez FA, White SA, Borrillo Hutter T, Amistadi MK, Root AA, Chorover J, Maier RM. Response of key soil parameters during compost-assisted phytostabilization in extremely acidic tailings: effect of plant species. Environ Sci Tech. 2012;46:1019–1027. doi: 10.1021/es202846n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NM, Lee R, Heitkemper DT, Cafferky KD, Haque A, Henderson AK. Inorganic arsenic in cooked rice and vegetables from Bangladeshi households. Sci Total Environ. 2006;370:294–301. doi: 10.1016/j.scitotenv.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Song B, Lei M, Chen T, Zheng Y, Xie Y, Li X, Gao D. Assessing the health risk of heavy metals in vegetables to the general population of Beijing, China. Journal Env Sci. 2009;21:1702–1709. doi: 10.1016/s1001-0742(08)62476-6. [DOI] [PubMed] [Google Scholar]
- Uhlman K. Well Owners’ Guide to Ground Water Resources in Yavapai County, AZ 1451. The University of AZ Cooperative Extension; [Accessed 14 September 2012]. 2008. Available at: http://arizona.openrepository.com/arizona/handle/10150/146412. [Google Scholar]
- USEPA . SW-846. Third Ed Office of Solid Waste and Emergency Responses; Washington DC: 1986. Methods of analysis of hazardous solid wastes. [Google Scholar]
- USEPA . Region III. Office of Superfund Hazardous Waste Management; Nov, 1991a. Chemical Concentration Data Near the Detection Limit. EPA/903/8-9/001. [Google Scholar]
- USEPA . Supplemental Guidance: Standard Default Exposure Factors. Office of Enforcement and Compliance Assurance; Washington, D.C.: Jun, 1991b. RAGS for Superfund Volume I: Human Health Evaluation Manual. [Google Scholar]
- USEPA [Accessed 13 August 2012];Office of Solid Waste and Emergency Response. Soil Screening Guidance: User’s Guide. 1996 Jul; Publication 9355.4-23. Available at: http://www.epa.gov/superfund/health/conmedia/soil/index.htm#user.
- USEPA . Assigning Values to Non-Detected/Non-Quantified Pesticide Residues in Human Health Food Exposure Assessments. Office of Pesticide Programs; Washington, DC: 2000. [Google Scholar]
- USEPA . Exposure Factors Handbook 2011 Edition (Final) US Environmental Protection Agency; Washington, DC: [Accessed 20 September 2012]. 2011. EPA/600/R-09/052F. Available at: http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252#Download. [Google Scholar]
- USEPA [Accessed 02 November 2012];Regional Screening Levels. Residential Soil Supporting Table. 2012a Available at: http://www.epa.gov/region9/superfund/prg/index.html.
- USEPA [accessed August 2012];Integrated Risk Information System (IRIS) 2012b Available at: http://www.epa.gov/IRIS/
- USEPA [accessed August 2012];Regional Screening Levels for Chemical Contaminants at Superfund Sites, RSL Calculator. 2012c Available at: http://epa-prgs.ornl.gov/cgi-bin/chemicals/csl_search.
- USEPA [Accessed on 14 February 2013];Iron King Mine and Humboldt Smelter Superfund Site Interim Removal Report Available Online Site Update. 2012d Sep; Available at: http://yosemite.epa.gov/r9/sfund/r9sfdocw.nsf/3dc283e6c5d6056f88257426007417a2/a2bf66d306e0e40188257a75005beb9e!OpenDocument.
- USFDA . US Food and Drug Administration; [accessed 18 November 2012]. Total Diet Study 2010. Available at: http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/TotalDietStudy/default.htm. [Google Scholar]
- Washington State Legislature [21 September 2012]; Available at: http://apps.leg.wa.gov/wac/default.aspx?cite=173-340-900.
- World Health Organization [Accessed 19 June 2012];Exposure to arsenic: a major public health concern. 2010 Available: www.who.int/ipcs/features/arsenic.pdf.
- World Health Organization, International Programme on Chemical Safety . Arsenic and arsenic compounds. 2nd ed. Vol. 224. Environmental Health Criteria; Geneva: [Accessed 07 September 2012]. 2001. Available at: http://whqlibdoc.who.int/ehc/WHO_EHC_224.pdf. [Google Scholar]
- Yost LJ, Schoof RA, Aucoin R. Intake of inorganic arsenic in the North American diet. Hum Ecol Risk Assess. 1998;4:137–152. [Google Scholar]