Abstract
Purpose
To assess risk of cartilage loss in the tibiofemoral joint in relation to baseline damage severity, and to analyze the association of nearby pathologic findings on the risk of subsequent cartilage loss.
Methods
The Multicenter Osteoarthritis (MOST) Study is a longitudinal study of individuals with or at high risk for knee osteoarthritis. Magnetic resonance imaging (MRI) examinations were assessed according to the Whole Organ Magnetic Resonance Imaging Score (WORMS). Included were all knees with available baseline and 30 months MRIs. Ordinal logistic regression was used to estimate risk of cartilage loss in each subregion in relation to the number of associated articular features including bone marrow lesions, meniscal damage and extrusion and also in regard to baseline damage severity, respectively.
Results
13524 subregions of 1365 knees were included. 3777 (27.9%) subregions exhibited prevalent cartilage damage at baseline and 1119 (8.3%) subregions showed cartilage loss at 30-month follow-up. Risk of cartilage loss was increased for subregions with associated features (ORs 2.53, 95% confidence interval [CI] 2.03-3.15 for one, 4.32 95% CI 3.42-5.47 for two and 5.30 95% CI 3.95-7.12 for three associated features; p for trend <0.0001). Subregions with prevalent cartilage damage showed increased risk for further cartilage loss compared to subregions with intact cartilage at baseline with small superficial defects exhibiting highest risk.
Conclusion
Risk of cartilage loss is increased for subregions with associated pathology and further increased when more than one type of associated feature is present. In addition, prevalent cartilage damage increases risk for subsequent cartilage loss.
Keywords: magnetic resonance imaging, osteoarthritis, risk factors, cartilage loss, meniscal damage, mensical extrusion, bone marrow lesions
Introduction
Osteoarthritis (OA) has long been regarded as a disease of “wear and tear” of cartilage. However, due to the application of magnetic resonance imaging (MRI) to large clinical cohorts a change in the paradigm has occurred in that it is now accepted that OA is not simply perceived as a disease of cartilage but is a whole-organ disorder involving multiple joint tissues leading eventually to joint failure.[1-5]
Longitudinal studies of knees with OA have suggested that MRI-detected tibiofemoral cartilage loss is associated with older age, female gender, higher body mass index (BMI), African-American ethnicity, varus malalignment, synovitis, large bone marrow lesions (BMLs), anterior cruciate ligament tears, meniscal tears and meniscal extrusion.[6-13] In addition, one of the strongest predictors of subsequent cartilage loss is prevalent cartilage damage.[6, 14-17] Excessively loaded regions of the knee are at higher risk for cartilage loss and OA compared to less loaded regions of the knee.[1, 18] BMLs are strongly related to loading as associations with malalignment have shown and BMLs could be considered as proxies for malalignment in a compartment.[1, 10] Also, cartilage damage itself may increase load to the underlying subchondral bone as manifested by baseline cartilage defects predicting site-specific BML progression.[19] Further, meniscal damage and extrusion increase ipsi-compartmental focal stress regardless of alignment.[12]
Whether as a consequence of their effect on loading or not, ipsi-compartmental meniscal damage, meniscal extrusion and prevalent BMLs in the same subregion, which we will refer to as co-localized “associated pathologies”, have been associated with cartilage loss but there has been little examination of their combined effect.[11, 12, 16]
Thus, aim was to evaluate the impact of co-localized pathology, i.e., directly underlying BMLs in the same subregion, or meniscal damage and extrusion in the same tibiofemoral compartment, on the risk of subsequent cartilage loss in the identical subregion at 30-month follow-up stratified by baseline cartilage damage severity. Further, we assessed risk of subsequent cartilage loss in relation to the presence of one, two or three associated pathologies and whether the degree of prevalent baseline cartilage damage had an impact on subsequent cartilage loss.
Materials and Methods
Study Design and Subjects
Subjects were participants in the Multicenter Osteoarthritis Study (MOST), a prospective epidemiological study of 3,026 people aged 50 to 79 years with a goal of identifying risk factors for incident and progressive knee OA in a population either with or at high risk of developing OA. Factors considered to contribute to a high risk of knee OA included being overweight or obese, having either knee pain, aching, or stiffness on most of the preceding 30 days, a prior knee injury that made it difficult to walk for at least one week, or previous knee surgery. They were recruited from two U.S. communities, Birmingham, Alabama and Iowa City, Iowa through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. The Health Insurance Portability and Accountability Act-compliant study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama at Birmingham, University of California at San Francisco and Boston University School of Medicine. We obtained written informed consent from all patients.
Subjects were not eligible to participate in MOST if they screened positive for rheumatoid arthritis [20] had ankylosing spondylitis, psoriatic arthritis, Reiter's syndrome, renal insufficiency that required hemo- or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), had or planned to have bilateral knee replacement surgery, were unable to walk without assistance, or were planning to move out of the area in the next three years.[21]
In the present study we included all participants with available baseline and 30-month follow-up MRIs.
Radiographs
At baseline, all subjects underwent weight-bearing posteroanterior (PA) fixed flexion knee radiographs using a plexiglass positioning frame (SynaFlexer™).[22] A musculoskeletal radiologist and two rheumatologists all with over 10 years experience reading study radiographs and blinded to clinical data, graded the x-rays according to the Kellgren-Lawrence (KL) scale.[23] Radiographs were presented sequentially with readers blinded to all clinical data and to MR images. Radiographic tibiofemoral OA was considered present if KL grade ≥2. The interrater reliability for pairs of readers among the three readers ranged from a w-kappa of 0.77 to 0.80 (for all p<.0001).
In addition, long-limb films were acquired and mechanical alignment was measured as the angle formed by the intersection of the femoral and tibial mechanical axes. Neutral alignment was defined as 179-181 degrees, varus malalignment as ≤178 degrees and valgus malalignment as ≥182 degrees.
MRI Acquisition
MRIs were obtained in both knees at baseline and 30-month follow-up with a 1.0 T dedicated extremity unit (OrthOne™, GE Healthcare, Wilmington, MA) with a circumferential extremity coil using fat-suppressed (FS) fast spin-echo proton density-weighted (PDw) sequences in two planes, sagittal (TR = 4800 ms, TE = 35 ms, 3 mm slice thickness, 32 slices, 288 × 192 matrix, 2 excitations (NEX), 140 × 140 mm field of view (FOV), echo train length (ETL) = 8) and axial (TR = 4680 ms, TE = 13 ms, 3 mm slice thickness, 20 slices, 288 × 192 matrix, 2 NEX, 140 × 140 mm FOV, ETL = 8), and a short tau inversion-recovery (STIR) sequence in the coronal plane (TR = 6650 ms, TE = 15 ms, TI = 100 ms, 3 mm slice thickness, 28 slices, 256 × 192 matrix, 2 NEX, 140 mm2 FOV, ETL = 8).
MRI Interpretation
Two musculoskeletal radiologists, with 7 and 9 years experience in standardized semiquantitative MRI assessment of knee OA, blinded to radiographic OA grade, and clinical data, graded cartilage status, BMLs, meniscal morphology and meniscal extrusion according to the WORMS system.[4,24] Baseline and follow-up MRIs were presented paired and sequentially to the readers, with the chronological order known to the readers. BMLs and cartilage status were scored in each of the 5 subregions in the medial and lateral tibiofemoral compartments, for a total of 10 subregions per knee (online supplementary Figure S1). Cartilage morphology and signal were scored semiquantitatively from 0 to 6 in each. In a modification of WORMS developed for longitudinal readings, the use of coding within-grade changes (i.e., definite change that does not cover a full grade increase or decrease in cartilage damage and consequently also including possible progression of grade 6 lesions) for cartilage assessment was introduced.[25]
BMLs were defined as poorly-delineated areas of hyperintensity directly adjacent to the subchondral plate on the STIR and PDw FS images.[26,27] BML size was scored from 0-3 based on the extent of regional involvement.
Meniscal status was graded from 0 to 4 in the anterior horn, the body, and the posterior horn of the medial and lateral meniscus. Extrusion of the medial and lateral meniscal body was assessed using coronal STIR images. The edge of the tibial plateaus (excluding osteophytes) was used as the reference to measure extrusion. Medial and lateral meniscal extrusion was graded from 0 to 2 (0 = no extrusion; 1 = extrusion ≤ 50% of the body; 2 = extrusion > 50% of the body.[11, 12]
The weighted kappa coefficients of inter-observer reliability (30 knees randomly selected read by both readers) were 0.66 for the readings of BMLs (comparing 0-3 scores in each subregion), 0.80 for meniscal morphology (comparing 0-4 scores in each subregion), 0.60 for meniscal extrusion (comparing 0-2 scores medially and laterally) and 0.78 for cartilage morphology (comparing 0-6 scores in each subregion).
Statistical analysis
Prevalent cartilage damage was defined as any grade ≥2 (=a focal superficial defect) detected at baseline. Cartilage loss was defined as at least within-grade progression in the same subregion. The unit of analysis was the subregion of the tibiofemoral compartments. We examined the association of cartilage loss in a given subregion with baseline BMLs and meniscal damage and extrusion directly underlying the same articular subregion in the same tibiofemoral compartment using logistic regression with generalized estimating equations to account for correlations among multiple subregions within a knee (using one knee per person). Subregions with associated articular pathology were categorized as having one, two or three associated risk factors (i.e., meniscal damage and/or meniscal extrusion and/or BMLs). Subregions without associated pathology but with the same degree of prevalent cartilage damage were the reference. All analyses were conducted unstratified for all cartilage morphology scores combined as well as stratified according to baseline subregional cartilage morphology score.
In a separate analysis we estimated the risk of subsequent cartilage loss in relation to baseline cartilage damage severity using subregions without prevalent baseline cartilage damage (i.e., grades 0 and 1 combined) as the reference. This analysis was performed separately for subregions with and without associated pathology.
Adjustment was performed for potential confounders, i.e., baseline effusion-synovitis, Hoffa-synovitis, BMI, age, gender, radiographic osteoarthritis severity and malalignment. Cochran-Armitage test for trend was applied to analyze the associations between increasing number of associated pathologies and the odds for cartilage loss at follow-up. All statistical calculations were performed using SAS® software (Version 9.1 for Windows; SAS Institute; Cary, NC).
Results
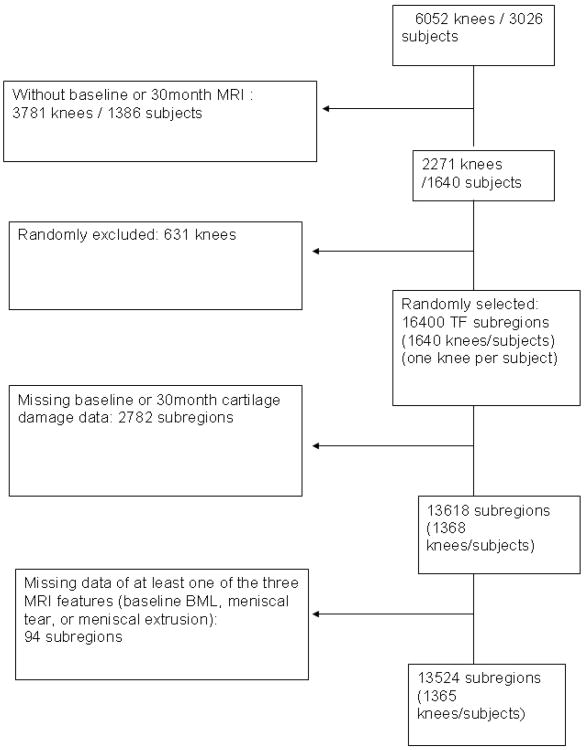
There were 1365 subjects (1365 knees) who met our inclusion criteria for this study. On average the subjects were elderly (mean age 62.1 ±7.9 years) and overweight (mean BMI 29.9 ± 4.8), and there were more women than men (61.2% female subjects). The majority (n=812, 59.5%) of knees did not have established TF OA (K/L 0: 585 [42.9%] knees; K/L 1: 227 [16.6%] knees) at baseline. 262 (19.2%) knees had a baseline K/L grade of 2, 239 (17.5%) were K/L 3 and 52 (3.8%) had a K/L grade of 4 at baseline. 366 (26.8%) participants reported frequent knee pain and 188 (13.7%) had symptomatic OA (defined as knee pain on most days in the last 30 days and radiographic OA K/L ≥ 2) at baseline. There were 643 limbs with varus malalignment (47.4%) and 264 (19.5%) with valgus malalignment. Altogether 13524 readable subregions were analyzed (Figure 1).
Figure 1.
Flowchart of subject inclusion.
Assessing all baseline grades combined, 375 subregions (4.4%) of subregions with no associated feature, 268 (10.9%) with one associated feature, 338 (18.2%) with two associated features and 138 (22.0%) with three associated features showed cartilage loss at follow-up. A detailed overview of baseline cartilage grades and subregions showing cartilage loss in relation to number of baseline associated pathologies is presented in Table 1.
Table 1. Frequencies of subregions exhibiting cartilage loss according to WORMS baseline cartilage score and number of associated pathologies.
| Subregions exhibiting cartilage loss at 30 months follow-up | ||||||
|---|---|---|---|---|---|---|
| Cartilage status in subregion at baseline (WORMS grade) | Subregions with no associated pathology (%) | Subregions with one associated pathology (%) | Subregions with two associated pathologies (%) | Subregions with three associated pathologies (%) | All | |
| 0 and 1 n=9747 | 180 / 7286 (2.5) | 99 / 1633 (6.1) | 70 / 792 (8.8) | 3 /36 (8.3) | 352 / 9747 (3.6) | |
| 2 n=476 | 66 / 292 (22.6) | 35 / 121 (28.9) | 31 / 58 (53.5) | 1 / 5 (20.0) | 133 / 476 (27.9) | |
| 2.5 n=173 | 11 / 103 (10.7) | 12 / 42 (28.6) | 9 / 22 (40.9) | 2 / 6 (33.3) | 34 / 173 (2.0) | |
| 3 n=427 | 106 / 753 (14.1) | 93 / 472 (19.7) | 167 / 604 (27.7) | 61 / 154 (39.6) | 427 / 1983 (21.5) | |
| 4 n=49 | 1 / 9 (11.1) | 3 / 10 (30.0) | 3 / 22 (13.6) | 4 / 8 (50.0) | 11 / 49 (22.4) | |
| 5 and 6 n=1096 | 11 /148 (7.4) | 26 / 185 (14.1) | 58 / 363 (16.0) | 67 / 400 (16.8) | 162 / 1096 (14.8) | |
| All grades | 375 / 8591 (4.4) | 268 / 2463 (10.9) | 338 /1861 (18.2) | 138 / 609 (22.7) | 1119 / 13524 (8.3) | |
The presence of only one associated pathologic feature was associated with an increased risk of cartilage loss among regions that had a baseline cartilage morphology score of 0, 2.5, or 3. The presence of two pathologies increased the risk of cartilage loss for almost all subregions regardless of baseline score (apart from the rare grade 4). Finally, the presence of 3 pathologies increased the risk of cartilage loss among regions with baseline scores of 0 and 3. For all grades combined, the odds for subsequent cartilage loss were significantly increased for one (adjusted odds ratio [aOR] 2.53 95% confidence interval 2.03-3.15), two (aOR 4.32, 95% confidence interval 3.42-5.47) and three associated pathologies (aOR 5.30 95%confidence interval 3.95-7.12) (Table 2).
Table 2. Cartilage loss in the tibiofemoral joint stratified by baseline cartilage score in regard to number of associated pathologies.
| Cartilage status in subregion at baseline (tibiofemoral joint – 10 subregions) | |||||||
|---|---|---|---|---|---|---|---|
| Subregions and associated risk factor status | All Grades | Grades 0 and 1 | Grade 2 | Grade 2.5 | Grade 3 | Grade 4 | Grades 5 and 6 |
| Subregions included (%) | 13524 (100.0) | 9747 (72.1) | 476 (3.5) | 173 (1.3) | 1983 (14.7) | 49 (0.3) | 1096 (8.1) |
| Subregions with cartilage loss (%) | 1119 (8.3) | 352 (3.6) | 133 (27.9) | 34 (19.7) | 427 (21.5) | 11 (22.4) | 162 (14.8) |
|
| |||||||
| Subregions with cartilage loss and no associated pathologies(%) | 375 (4.4) | 180 (2.5) | 66 (22.6) | 11 (10.7) | 106 (14.1) | 1 (11.1) | 11 (7.4) |
| aOR1 (Reference) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Subregions with cartilage loss and one associated pathologies2 (%) | 268 (10.9) | 99 (6.1) | 35 (28.9) | 12 (28.6) | 93 (19.7) | 3 (30.0) | 26 (14.1) |
| aOR1 (95% CI) p | 2.53 (2.03-3.15) <.0001* | 2.46 (1.74-3.46) <0.001* | 1.45 (0.89-2.38) 0.13 | 3.60 (1.40-9.32) 0.008* | 1.43 (1.00-2.04) 0.047* | 3.54 (0.25-50.2) 0.34 | 1.95 (0.79-4.83) 0.15 |
| Subregions with cartilage loss and two associated pathologies3 (%) | 338 (18.2) | 70 (8.8) | 31 (53.4) | 9 (40.9) | 167 (27.7) | 3 (13.6) | 58 (16.0) |
| aOR1 (95% CI) p | 4.32 (3.42-5.47) <.0001* | 3.70 (2.51-5.44) <0.001* | 3.62 (1.98-6.63)<0.001* | 4.86 (1.64-14.5) 0.004* | 2.25 (1.58-3.21) <0.001* | 1.47 (0.14-15.8) 0.74 | 2.47 (1.00-6.07) 0.048* |
| Subregions with cartilage loss and three associated pathologies4 (%) | 138 (22.7) | 3 (8.3) | 1 (20.0) | 2 (33.3) | 61 (39.6) | 4 (50.0) | 67 (16.8) |
| aOR1 (95% CI) p | 5.30 (3.95-7.12) <.0001* | 3.59 (1.10-11.7) 0.03* | 1.03(0.09-11.9)0.98 | 4.73(0.74-30.2)0.09 | 3.75 (2.40-5.85) <0.001* | 16.29 (0.89-296) 0.06 | 2.57 (0.97-6.83) 0.06 |
|
| |||||||
| p for trend | <.0001* | <.0001* | 0.0004* | 0.0011* | 0.0152* | 0.1798 | 0.0798 |
aOR – adjusted odds ratio. Adjusted for confounders baseline effusion-synovitis, Hoffa-synovitis, body mass index, age, gender, radiographic osteoarthritis severity and malalignment
one associated feature: BML or meniscal damage or meniscal extrusion
two of three associated features: BML and/or meniscal damage and/or meniscal extrusion)
three associated features: BML and/or meniscal damage and/or meniscal extrusion)
significant at p < .05
95% CI - 95 % confidence interval
Risk of progressive cartilage loss was increased for subregions with any grade of prevalent cartilage damage at baseline compared with subregions without baseline cartilage surface damage. For both subanalyses – subregions with and without adjacent pathology - focal superficial defects (grade 2 lesions) showed the highest risk of subsequent cartilage loss (subregions without adjacent pathology: aOR 11.3, 95% confidence interval 8.1-16.0; subregions with adjacent pathology: aOR 7.7, 95% confidence interval 5.4-11.1). Subregions with already advanced full thickness cartilage damage at baseline and presence of associated features exhibited the lowest risk, which, however, was still significantly increased (aOR 2.18, 95% confidence interval 1.62-2.92) (Table 3).
Table 3. Risk of cartilage loss at 30 months for subregions with baseline cartilage score of 0 by grade of prevalent baseline damage severity.
| Cartilage morphology status | Subregions without adjacent pathology TF1 | Subregions with adjacent pathology TF1 | |
|---|---|---|---|
| Reference: Grade 0 and 1 combined: Subregions with cartilage loss at follow-up | 180/7286 (2.47) | 172/2461 (7.0) | |
| aOR3 | 1.0 (reference) | 1.0 (reference) | |
| Grade2.0: Subregions with cartilage loss at FU | 66/ 292 (22.6) | 67/184 (36.4) | |
| aOR3 | 11.3 | 7.74 | |
| 95% CI | (8.06,16.0) | (5.43,11.06) | |
| p | <.0001* | <.0001* | |
| Grade 2.5: Subregions with cartilage loss at FU | 11/103 (10.7) | 23/70 (32.9) | |
| aOR3 | 4.83 | 6.49 | |
| 95% CI | (2.49,9.38) | (3.67,11.48) | |
| p | <.0001* | <.0001* | |
| Grade 3: Subregions with cartilage loss at FU | 106/753 (14.1) | 321/1230 (26.1) | |
| aOR3 | 6.53 | 4.38 | |
| 95% CI | (4.78,9.38) | (3.44,5.57) | |
| p | <.0001* | <.0001* | |
| Grade 4: Subregions with cartilage loss at FU | 1/9 (11.1) | 10/40 (25.0) | |
| aOR3 | 4.94 | 3.43 | |
| 95% CI | (0.80,30.58) | (1.62,7.27) | |
| p | 0.09 | 0.01* | |
| Grades 5 and 6 combined: Subregions with cartilage loss at FU | 11/148 (7.4) | 151/948 (15.9) | |
| aOR3 | 3.27 | 2.18 | |
| 95% CI | (1.60,6.71) | (1.62,2.92) | |
| p | 0.001 | <.0001* | |
significant at p <0.05
adjacent pathology: meniscal damage and/or meniscal extrusion and/or bone marrow lesion
adjusted for age, gender, BMI, malalignment, radiographic OA severity, Hoffa-synovitis, effusion-synovitis
TF- tibio-femoral, PF – patello-femoral, FU – follow-up, aOR – adjusted odds ratio, 95% CI – 95% confidence interval
Discussion
For all types of baseline subregional cartilage morphology, including focal and wide-spread full thickness cartilage damage as well as intact articular surface morphology, the risk of future cartilage loss was markedly increased for subregions exposed to presence of associated non-cartilaginous pathology, as we termed the co-localized presence of BMLs, meniscal damage and meniscal extrusion. We demonstrated that this risk is further increased for subregions that exhibit more than one type of associated pathology. In addition, risk of subsequent cartilage loss is increased for subregions with prevalent damage regardless of associated pathology when compared to subregions without prevalent cartilage damage at the baseline visit.
MRI is the only imaging method to directly and non-invasively visualize cartilage and all other non-cartilaginous joint tissues such as the menisci, the subchondral bone and ligaments.[28] Using mainly knee-based approaches, longitudinal studies of knees with OA have suggested that MRI-detected tibiofemoral cartilage loss is associated with older age, female gender, higher BMI, African-American ethnicity, varus malalignment, a high degree of synovitis, large BMLs, anterior cruciate ligament tears, meniscal tears and meniscal extrusion.[6-10, 12, 13,29, 30] However, if concomitant presence of several local risk factors results in a cumulative effect with further increased risk has not been shown previously. A knee-based approach is unlikely to be able to answer the question of the impact of local risk factors on neighboring cartilage, which was reason to use a subregional analytic strategy in the present study.[11] The presented analysis was the first to take into account such a strict subregional approach assessing the impact of neighboring OA features such as cartilage damage, meniscal pathology and BMLs on subseqeuent cartilage loss nearby. This suggests that joint damage in OA evolves in an articular subregion and not more diffusely, with all structures in that subregion affected by disease features. Previous reports applied knee- or compartmental approaches and none of these previous studies took into account different grades of baseline cartilage damage as a risk factor for subsequent cartilage loss.[6,12,14,16,29,30]
Normal articular cartilage has a unique load-support mechanism governed by its high water content and the stiffness and permeability of its collagen–proteoglycan matrix.[31] Interstitial fluid pressurization during loading contributes to more than 90% of the load support, shielding the collagen–proteoglycan matrix from excessive stresses and reducing friction at the articular surfaces.[32,33] Certain alterations in the mechanical environment of the joint adversely affect load distribution. The study of mechanical factors is complicated by the fact that they may be altered further by the disease itself such as malalignment.[34,35] We did not directly analyze the impact of malalignment and other systemic factors that are known to increase joint load such as obesity or proprioceptive deficits.[36, 37] Our focus was the specific local environment of the articular surface and the strong interrelation between different joint structures.
Although our results do not elucidate the possible course of incidence of associated pathologies, i.e. if meniscal damage and extrusion precede BMLs or vice versa, we could clearly show that concomitant presence of these tissue alterations results in a cumulative risk for further cartilage deterioration.[38] Further, we do not know if prevalent cartilage damage increases risk for associated localized pathology, which is likely and has been suggested before for increase in BMLs.[19] The chronological course of events will be elucidated by large ongoing studies with multiple time points such as the Osteoarthritis Initiative (OAI).
A strong association of BMLs and cartilage loss has been shown longitudinally in previous work using subregional semiquantitative approaches.[10, 11] Baseline meniscal extrusion is an established risk factor for consequent cartilage loss in the same compartment for follow-up intervals from 6 to 30 months.[12, 29, 35] Meniscal tears and morphology alterations such as maceration are also important factors that will impact on future cartilage loss.[12,35] We did not assess each risk factor separately but rather grouped these three features into strata of risk factor “load”, i.e. one, two or three risk factors simultaneously present. As multiple combinations of these factors for each subregion are possible, a separate analysis for each combination would have reduced case numbers below a meaningful sample size. In order to avoid dilution of the effects assessed by presence of prevalent cartilage damage, we performed all analyses stratified by degree of baseline cartilage damage.
Risk factor assessment for progression in observational studies of knee OA is challenging and prone to bias as discussed in detail recently.[39] We believe to have accounted for several of these challenges based on our study design and analytic approach and thus, believe that our results are valid in regard to interpretation. It has long been shown that the so-called “horse racing effect” may influence the outcome measure, i.e. further cartilage loss in light of already present cartilage damage, and that baseline adjustment for preexisting cartilage damage may provide biased effect estimates.[40] To account for this phenomenon we have not adjusted but rather stratified our unit of exposure, the articular subregion, according to the degree of prevalent cartilage damage.[41]
The MOST study does not include contrast-enhanced sequences, the gold standard for synovitis assessment.[42,43] A non-specific surrogate of signal changes in Hoffa's fat pad was used as a suurogate measure for synovitis.[44] As associations between high-grade signal changes and subsequent cartilage loss have been reported we decided to include this synovitis surrogate as a potential confounder in our analyses.[9]
We employed 1.0T extremity MRI, which has been questioned to yield inferior image quality when compared to 1.5T or 3T large bore systems. These issues, to the extent they exist, seem not to affect semi-quantitative scoring of knee OA. In a comparative exercise scoring knees of subjects, which had received a 1.0T extremity MRI scan and a 1.5T large bore examination of the same knee on the same day, we could show good agreement, sensitivity and specificity for all assessed features.[24]
In regard to degree of baseline subregional cartilage damage, subregions exhibiting only focal superficial lesions appeared to be at highest risk for subsequent cartilage loss. (The fact that subregions without associated pathology had higher odds than subregions with associated pathology cannot be interpreted easily as these numbers cannot be compared. For these separate analyses the reference groups differed due to stratification according to baseline presence or absence of adjacent pathology.) Given the discussed “horse racing effect” one would expect an increased risk for more advanced damage when compared to small focal defects. This has been shown for example for quantitative approaches that reported highest rates of cartilage loss for knees with definite joint space narrowing and higher degrees of radiographic OA.[45,46] However, it has to be kept in mind that quantitative approaches based on segmentation are not able to depict small focal effects. Given our findings one has to assume that focal lesions are at high risk for progression, which might support the assumption, that treatment of focal cartilage damage is worth consideration in order to avoid progression.[47]
In summary, we showed that associated co-localized joint pathology increases risk of cartilage loss in a defined subregion, which is further increased for subregions with more than one type of associated pathology concomitantly present. This strongly supports the current understanding of OA as a multi-tissue disease process. Our findings confirm that preservation of the integrity of meniscal morphology, meniscal position and subchondral bone marrow seems paramount for continued cartilage preservation. Once cartilage damage has occurred, risk for further progression is increased when compared to areas of intact cartilage regardless of presence or absence of associated pathologies.
Supplementary Material
A. Sagittal proton-density weighted image illustrates the subregional division for the lateral compartment. The lateral femur is subdivided into an anterior, central and posterior subregion defined by the menisci and the middle of the connection between the anterior and posterior osteochondral junctions. The anterior subregion is not part of the tibio-femoral joint but the patello-femoral joint and was no considered in the analyses. The tibia is divided into three subregions defined by the meniscus. Image depicts full-thickness cartilage loss in the central subregion of the lateral tibia (arrow). The medial compartment is divided in identical fashion into 6 subregions. B. Coronal STIR image shows division into medial and lateral. The femoral notch is part of the medial compartment. The S region , which is not covered by articular cartilage was not considered in the analyses. Cartilage defect of the central lateral tibial plateau is also visualized in coronal image (no arrow).
Acknowledgments
We would like to thank the participants and staff of the MOST study at the clinical sites in Birmingham, AL and Iowa City, IA and at the Coordinating Center at UCSF, San Francisco, CA. We acknowledge the valuable contributions of Dr. Burton Sack and Dr. Piran Aliabadi, both Boston, MA, USA, who were expert reviewers of the knee radiographs.
Funding: Supported by NIH grants from the National Institute of Aging to Drs. Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820).
Footnotes
Competing interest statement: Dr. Guermazi has received consultancies, speaking fees, and/or honoraria from Facet Solutions, Genzyme, Stryker, Merck Serono, Novartis and Astra Zeneca and is the President of Boston Imaging Core Lab (BICL), a company providing image assessment services. He received a research grant from General Electric Healthcare. Dr. Roemer is Vice President and shareholder of BICL. Dr. Roemer has received consultancies, speaking fees, and/or honoraria from Merck Serono and the National Institutes of Health. Dr. Crema and Dr. Marra are shareholders of BICL.
Ethics approval: The study was conducted in compliance with the ethical principles derived from the Declaration of Helsinki and in compliance with local Institutional Review Boards, informed consent regulations, and International Conference on Harmonization Good Clinical Practices Guidelines.
Copyright: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Group and co-owners or contracting owning societies (where published by the BMJ Group on their behalf), and its Licensees to permit this article (if accepted) to be published in ARD and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence at: (http://ard.bmj.com/site/about/licence.pdf)
References
- 1.Felson DT, McLaughlin S, Goggins J, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139:330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 2.Englund M, Lohmander LS. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheum. 2004;50:2811–9. doi: 10.1002/art.20489. [DOI] [PubMed] [Google Scholar]
- 3.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Peterfy CG, Guermazi A, Zaim S, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Bijlsma JW, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 6.Davies-Tuck ML, Wluka AE, Wang Y, et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337–42. doi: 10.1016/j.joca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis Rheum. 2004;50:94–7. doi: 10.1002/art.11483. [DOI] [PubMed] [Google Scholar]
- 9.Hill CL, Hunter DJ, Niu J, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter DJ, Zhang Y, Niu J, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 11.Roemer FW, Guermazi A, Javaid MK, et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann Rheum Dis. 2009;68:1461–5. doi: 10.1136/ard.2008.096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 13.Hunter DJ, Zhang YQ, Tu X, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–95. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 14.Ding C, Cicuttini F, Scott F, Boon C, Jones G. Association of prevalent and incident knee cartilage defects with loss of tibial and patellar cartilage: a longitudinal study. Arthritis Rheum. 2005;52:3918–27. doi: 10.1002/art.21474. [DOI] [PubMed] [Google Scholar]
- 15.Wluka AE, Ding C, Jones G, Cicuttini FM. The clinical correlates of articular cartilage defects in symptomatic knee osteoarthritis: a prospective study. Rheumatology (Oxford) 2005;44:1311–6. doi: 10.1093/rheumatology/kei018. [DOI] [PubMed] [Google Scholar]
- 16.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46:2884–92. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 17.Roemer FW, Kwoh CK, Hannon MJ, et al. Risk factors for MRI-detected patello-femoral and tibio-femoral cartilage loss over a 6-month period: the JOG study. Arthritis Rheum. 2011 Dec 27; doi: 10.1002/art.34353. Epub ahead of print. [DOI] [Google Scholar]
- 18.Englund M, Guermazi A, Roemer FW, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–9. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dore D, Martens A, Quinn S, et al. Bone marrow lesions predict site-specific cartilage defect development and volume loss: a prospective study in older adults. Arthritis Res Ther. 2010;12(6):R222. doi: 10.1186/ar3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlson EW, Sanchez-Guerrero J, Wright EA, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, Niu J, Guermazi A, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 22.Peterfy CG, Guermazi A, Zaim PF. Non-fluoroscopic method for flexed radiography of the knee that allows reproducible joint-space width measurement. Arthritis and Rheumatism. 1998;41:S361. [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roemer FW, Lynch JA, Niu J, et al. A comparison of dedicated 1.0 T extremity MRI vs large-bore 1.5 T MRI for semiquantitative whole organ assessment of osteoarthritis: the MOST study. Osteoarthritis Cartilage. 18:168–74. doi: 10.1016/j.joca.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roemer FW, Nevitt MC, Felson DT, et al. Validity of within-grade scoring of longitudinal changes of MRI-based cartilage morphology and bone marrow lesion assessment - the MOST Study. Arthritis Rheum. 2011;63(Suppl):S779. doi: 10.1016/j.joca.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergman AG, Willen HK, Lindstrand AL, Pettersson HT. Osteoarthritis of the knee: correlation of subchondral MR signal abnormalities with histopathologic and radiographic features. Skeletal Radiol. 1994;23:445–8. doi: 10.1007/BF00204605. [DOI] [PubMed] [Google Scholar]
- 27.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–40. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 28.Roemer FW, Crema MD, Trattnig S, Guermazi A. State of the Art: Advances in Osteoarthritis and Cartilage Imaging. Radiology. 2010;260:332–54. doi: 10.1148/radiol.11101359. [DOI] [PubMed] [Google Scholar]
- 29.Roemer FW, Zhang Y, Niu J, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–80. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin S, Guermazi A, Lavalley MP, et al. Complete anterior cruciate ligament tear and the risk for cartilage loss and progression of symptoms in men and women with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:897–902. doi: 10.1016/j.joca.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 32.Ateshian GA, Wang H, Lai WM. The role of interstitial fluid pressurization and surface porosities on the boundary friction of articular cartilage. Journal of Tribology. 1998;120:241–251. [Google Scholar]
- 33.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 34.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. Jama. 2001;286:188–95. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 35.Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–26. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 36.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 37.Petrella RJ, Lattanzio PJ, Nelson MG. Effect of age and activity on knee joint proprioception. Am J Phys Med Rehabil. 1997;76:235–241. doi: 10.1097/00002060-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Englund M, Guermazi A, Roemer FW, et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST Study. Ann Rheum Dis. 2010;69:1796–802. doi: 10.1136/ard.2009.121681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt MC, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:1527–32. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burrows B, Knudson RJ, Camilli AE, Lyle SK, Lebowitz MD. The “horse-racing effect” and predicting decline in forced expiratory volume in one second from screening spirometry. Am Rev Respir Dis. 1987;135:788–793. doi: 10.1164/arrd.1987.135.4.788. [DOI] [PubMed] [Google Scholar]
- 41.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–78. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 42.Loeuille D, Sauliere N, Champigneulle J, et al. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011;19(12):1433–9. doi: 10.1016/j.joca.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Roemer FW, Kassim Javaid M, Guermazi A, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18:1269–74. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Madrid F, Karvonen RL, Teitge RA, et al. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–183. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 45.Eckstein F, Nevitt M, Gimona A, et al. Rates of change and sensitivity to change in cartilage morphology in healthy knees and in knees with mild, moderate, and end stage radiographic osteoarthritis. Arthritis Care Res (Hoboken) 2010. 2010 Oct 18; doi: 10.1002/acr.20370. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirth W, Buck R, Nevitt M, et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography--data from the OA initiative. Osteoarthritis Cartilage. 2011;19:689–699. doi: 10.1016/j.joca.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:881–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Sagittal proton-density weighted image illustrates the subregional division for the lateral compartment. The lateral femur is subdivided into an anterior, central and posterior subregion defined by the menisci and the middle of the connection between the anterior and posterior osteochondral junctions. The anterior subregion is not part of the tibio-femoral joint but the patello-femoral joint and was no considered in the analyses. The tibia is divided into three subregions defined by the meniscus. Image depicts full-thickness cartilage loss in the central subregion of the lateral tibia (arrow). The medial compartment is divided in identical fashion into 6 subregions. B. Coronal STIR image shows division into medial and lateral. The femoral notch is part of the medial compartment. The S region , which is not covered by articular cartilage was not considered in the analyses. Cartilage defect of the central lateral tibial plateau is also visualized in coronal image (no arrow).