Abstract
Aminoglycosides are broad-spectrum antibiotics that are used for the treatment of severe Gram-negative and Gram-positive bacterial infections. While bactericidal effects of aminoglycosides are due to binding to the 30S subunit of the bacterial ribosome, aminoglycosides can affect protein synthesis, intracellular calcium levels and levels of reactive oxygen species (ROS) in eukaryotic cells. While aminoglycosides can be cytotoxic at high concentrations, our results show that at much lower doses, gentamicin can be implemented as a sensitizing agent for the NSCLC cell line NCI-H460, increasing the efficacy of camptothecin, digitoxin and vinblastine in vitro. We have also established that this sensitization is reliant on the ROS response generated by gentamicin.
Keywords: Aminoglycosides, Sensitization, Reactive Oxygen Species, NSCLC
Drug combination therapies have greatly impacted the treatment of cancer and infectious disease. Taking advantage of drug synergy can increase the efficacy of a treatment, reduce side effects (by enabling lower doses) and can help elude resistance.1 Sensitizing agents are drugs that exhibit minimal cytotoxicity at doses in which they improve the efficacy of a chemotherapeutic agent.2,3 In our effort to identify novel sensitizing agents for cancer chemotherapy, we envisioned aminoglycosides as having serious potential. Aminoglycosides were appealing because of their broad therapeutic window, clinical availability and recently validated therapeutic effects on protein synthesis. We sought to leverage this interference with protein synthesis in a way that would stress cancer cells at a concentration below which aminoglycosides are cytotoxic.
At high concentrations (> 5mM) aminoglycoside-induced cytotoxicity can occur and has been attributed to the unfolded protein response/ER stress4, 5 an increase in cytosolic Ca2+,6 and generation of ROS.7 The initiation of a cascade of events to ROS accumulation is thought to be the cause of apoptosis in kidney (nephrotoxicity) and ear hair (ototoxicity) cells. It has been observed in vitro and in-vivo that antioxidants8,9,10 and iron chelators11,12 can be used to mitigate aminoglycoside nephro- and ototoxicity and prevent aminoglycoside-induced lysosome permeabilization.13 In addition to interfering with protein synthesis in mammalian cells, some aminoglycosides (e.g., gentamicin and genticin) have been shown to correct nonsense mutations via readthrough, a placement of a random amino acid for premature stop codons when translating nonsense mutated genes.14,15 This “correction process” can allow for the complete synthesis of a nonsense-mutated protein and has been effective in the clinic. 16 This unique aminoglycoside/ribosome interaction led to the choice of gentamicin for our study. To demonstrate the generalizability of any sensitization effect, a series of cancer drugs that act at various cellular locations with a range of mechanisms of action was chosen for screening: digitoxin17 and its α-L-rhamnoside analogue18,19,20,21,22,23 (extracellular, Na/K-ATPase pump), 24 vinblastine (cytosol, tubulin), 25 5-fluorouracil (nucleus, DNA polymerase), 26 camptothecin (nucleus, Topo I), 27 oxaliplatin (nucleus, DNA), 28 and doxorubicin (nucleus, DNA/TopoII).29
Herein, we describe our successful effort at utilizing gentamicin (GEN) in the sensitization of non-small cell lung cancer cell lines NCI-H460 to a series of anticancer agents at concentrations below which GEN cytotoxicity is observed. In determining the mode of action for the sensitizing effect, H460 serves as the active cell line and A549, unaffected by GEN, serves as a control cell line. This work demonstrates the potential therapeutic use of GEN, which is routinely used as an antibiotic, in a dual-therapy approach to cancer. Furthermore, given the broad acceptance of GEN as a culture medium supplement (e.g., NCI panel of 60 cell lines) to prevent infection, this work also serves as a cautionary tale for its use as a culture media supplement.
RESULTS
Sub-toxic concentrations of gentamicin sensitize H460 cells
Our combination assays for NSCLC cancer cell line (NCI-H460) showed that in a dose dependent manner, gentamicin enhanced the cytotoxicity of several (but not all) anticancer agents: digitoxin, RHA, CPT and VINB (Fig. 1). These four drugs make up three of the six different classes of anticancer drugs evaluated. No measurable sensitization effect was seen for the other anticancer agents: OXA, DOX and 5FU (Fig. 2). The sensitizing effect of GEN for DIG and RHA was first observed at 1 μM GEN, and at 10 μM for CPT and VINB (Fig. 1B). For all four drugs, enhancement of cytotoxicity increased in a dose-dependent manner. The effect of GEN on the drug treatment is synergistic, as no detectable cytotoxicity was observed in the concentration window tested (100 nM to 1 mM). This lack of toxicity enabled us to pretreat cells with GEN (100 nM to 1 mM) for 24 h before drug exposure. Longer exposure times (up to 72 h) also showed no toxicity.
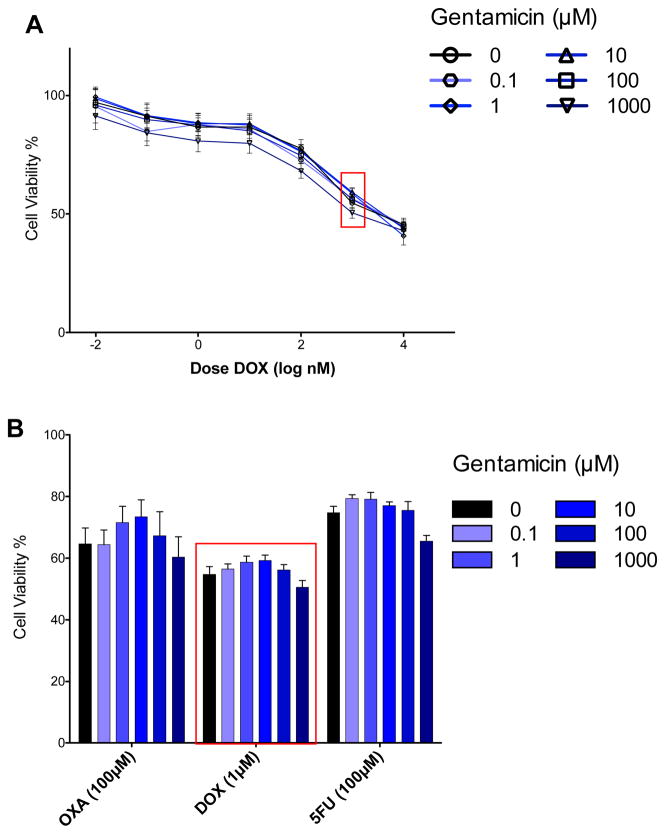
Fig. 1.
Gentamicin sensitizes H460 lung cancer cells to certain anticancer drugs. NCI-H460 cells were treated with GEN (0.1 μM to 1 mM) for 24 h and then cancer drugs (0.1 nM to 100 μM) for an additional 48 h (MTT assay). A Dose-response relationship for CPT at the 5 GEN concentrations (blue lines) plus control (black line). The sensitization effect is most easily observed at 20 μM CPT (red box). B Bar graph depicting dose response relationship for the sensitization effect of gentamicin on the cytotoxicity of the four drugs (DIG, RHA, CPT and VINB) where sensitization would be expected. See the SI for the full dose-response relationship for all drug combinations. (***, P < 0.0001; **, P < 0.001; *, P< .01)
Fig. 2.
Gentamicin does not sensitize H460 to other anticancer drugs. NCI-H460 cells were treated with GEN (0.1 μM to 1 mM) for 24 h and then cancer drugs (0.1 nM to 100 μM) for an additional 48 h followed by MTT. A Dose-response relationship for DOX at the 5 GEN concentrations (blue lines) plus control (black line). B Bar graph depicting dose response relationship at a representative concentration for the three drugs (OXA, DOX and 5FU). See the SI for the full dose-response relationship for all drug combinations.
Selectivity for cell line and anticancer drug
H460 cells were pre-treated with GEN for 24 h and then with anticancer drugs for an additional 48 h. Sensitization was observed for DIG, RHA, CPT and VINB. H460 cells are sensitized to DIG analogues to the largest degree, with 75% and 85% reduction in cell viability at 10 μM GEN for DIG (10 μM) and the α-L-rhamnoside analogue, RHA (10 μM) respectively, compared to the native response of the anticancer drug. Cytotoxicity of CPT is also enhanced, exhibiting a more gradual dose dependence. 100 μM GEN induces a 50% decrease in cell viability compared to CPT alone (20 μM). An increase in cytotoxicity for VINB with GEN treatment is also observed, though to a much lower degree. Enhancement of 5FU, OXA and DOX was not observed after treatment with GEN. Dashed line boxes in Figs. 1A and 2A are to indicate the representative concentration of anticancer drug used to in the single dose data presented in Figs. 1B and 2B. Exposure of A549 to the identical conditions showed no effect, however reducing the cancer drug treatment time to 24 h from 48 h because the inherent cytotoxicity of some anticancer drugs against A549 could mask the effect of GEN. In contrast to H460, the data for A549, regardless of concentration and duration, was never pronounced enough to indicate a similar sensitization effect (Fig. 3). This difference in response of H460 and A549 to GEN pre-treatment was utilized as a means for determining the mode of action for GEN-induced sensitization of H460.
Fig. 3.
Gentamicin does not sensitize A549. A549 cells were treated with GEN (0.1 μM to 1 mM) for 24 h and then cancer drugs (0.1 nM to 100 μM) for an additional 24 h (MTT assay). Bar graph depicting dose response relationship at a representative concentration for the seven drugs (DIG, RHA, CPT, VINB OXA, DOX and 5FU). See the SI for the full dose-response relationship for all subset of drug combinations (DIG, CPT, VINB and OXA). (**, P < 0.001)
Gentamicin/cancer drug synergy
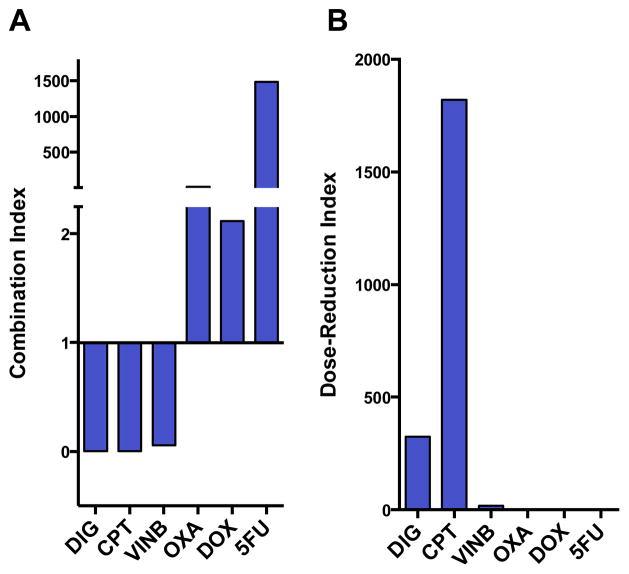
The degree of synergy was quantified by using the Chou-Talalay method (Fig. 4).30,31 Combination index (CI) values less than 1 were found for the three cancer drugs enhanced by gentamicin: digitoxin (0.003), camptothecin (0.001), and vinblastine (0.056) (at 1 μM, 2 μM and 1 μM respectively), whereas CI greater than 1 were found for the non-synergizing cancer drugs oxaliplatin (16.6), doxorubicin (2.12), and 5-fluorouracil (1486) (at 1, μM, 100 nM and 1 μM respectively) (Fig. 4A). The relative magnitudes of the effect are more easily depicted in the dose-response-index (Fig. 4B). However, it should be noted that the absolute values for these indices are overestimates, which result from the lack of cytotoxicity of gentamicin at concentrations in which synergy is noted (GEN IC50 is calculated to be 5.1 mM against H460).
Fig. 4.
Combination and dose-reduction index for GEN in combination with anticancer drugs. The Chou-Talalay synergy formula was used to quantify synergy between gentamicin and anticancer drugs. Data was calculated using Calcusyn (Biosoft, Cambridge, UK). A: Combination index for gentamicin (10 μM) with anticancer drugs (DIG, VINB, OXA, 5FU = 1 μM; CPT = 2 μM; DOX = 100 nM). Values <1 = synergy and > 1 = antagonism. Values close to 0 for DIG, CPT, VINB indicate strong synergism. B: Dose-reduction index is the fold-increase in dose necessary to achieve the same response without gentamicin.
Apoptosis and cell cycle
The effect of GEN (alone and in combination with anticancer drugs) on apoptosis was further explored with flow cytometry. FACS analysis following PI/annexin V co-staining (Fig. 5, A, B) showed that GEN alone does not induce any apoptosis or necrosis across the concentration window (10 to 100 μM). In addition, the enhancement in cytotoxicity of DIG, was shown to occur via apoptosis as opposed to necrosis. The FACS data revealed a similar dose dependency for GEN/DIG co-dosing as with the MTT data. Furthermore, it is important to note that FACS analysis allowed for the detection of sensitization at much lower cancer drug concentrations (150 nM DIG).
Fig. 5.
Gentamicin sensitizes cells to apoptosis at sub-toxic doses and does not affect cell cycle progression. A and B NCI-H460 cells were seeded in 6-well plates at a density of 200,000 cells per well. Cells were treated with GEN for 24 h followed by 48 h treatment with 150 nM DIG. Cells were collected via trypsinization, washed 2x with PBS and stained with propidium iodide and annexin V. Fluorescence was read on a flow cytometer followed by quad analysis; data is representative of 10,000 gated events. C H460 cells were treated with 100 μM GEN alone for 24 h followed by treatment of some wells with combination with 50nM DIG or 2 μM CPT. D Cells are treated with 100 nM OXA or 100 nM DOX with and without 24 h GEN pre-treatment. For all cycle analysis, after 4 h of incubation with the anticancer drug, cells were treated with RNAse A and stained with propidium iodide. Cell cycle distribution was measured by FACS.
The effects of co-dosing on cell cycle arrest (Fig. 5C, D) were also measured by flow cytometry. This analysis was performed on cells treated with combinations of GEN with cancer drugs. Drugs that responded to (DIG and CPT) and did respond to (OXA and DOX) sensitization were both used. As with apoptosis, GEN showed no effect on cell cycle. Similarly, no significant effect on cell cycle was observed for the co-dosed cells.
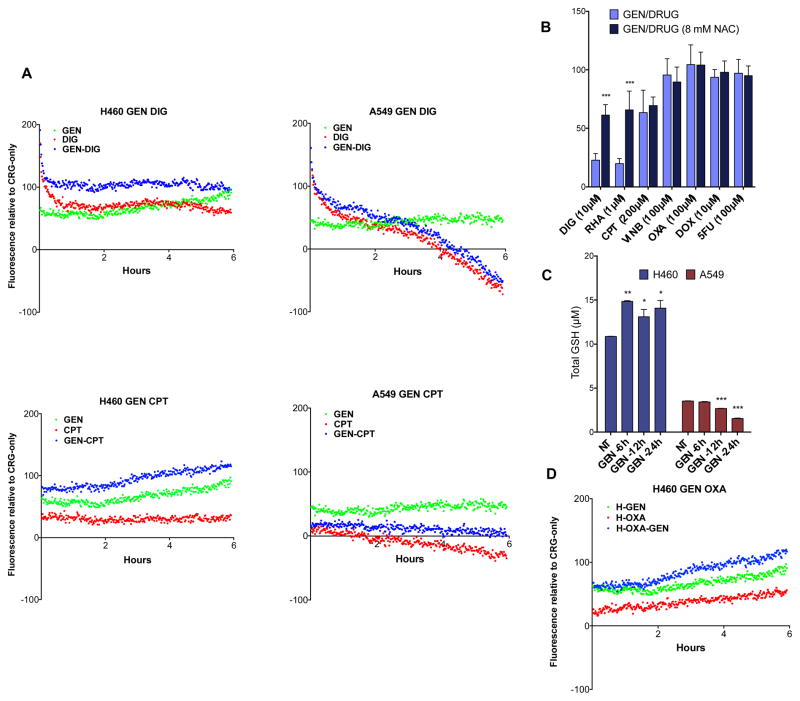
In order to elucidate the mechanism of action for this sensitization effect we investigated the effects of gentamicin on ROS production and the subsequent cellular response. At high concentrations, aminoglycosides are known to cause apoptosis via the accumulation of ROS in models for oto- and nephrotoxicity. To evaluate whether ROS plays a role in GEN-induced sensitization, the antioxidant N-acetylcysteine (NAC) was supplemented into the sensitization assay. H460 cells were treated with NAC 30 min prior to dosing with GEN (10 μM), followed by 24 h incubation. Cells were then treated with anticancer drugs for 48 h, followed by evaluation of cell viability by MTT. At high concentrations of NAC (8 mM), enhancement of DIG cytotoxicity was reduced (Fig. 6B.). This suggests that ROS generation is present where sensitization is observed. However, the effect of NAC on ROS induced by the other cancer drugs (CPT, VINB, OXA, DOX and 5FU) with GEN was inconclusive. This analysis was further complicated by the fact that high concentrations of NAC can result in cytotoxicity and also interferes with the MTT assay (see supplementary information). Thus, two additional means for evaluating gentamicin-induced ROS production were employed; a fluorescent probe for intracellular ROS and direct measurement of total glutathione via an enzyme recycling assay.
Fig. 6.
Gentamicin sensitizes H460 in a ROS-mediated pathway. A H460 and A549 cells were treated with gentamicin, digitoxin and camptothecin alone and in the indicated combinations. ROS levels were measured starting immediately after addition of the drug and continued for 6 h. B NCI-H460 cells were plated in a 96-well format. 8 mM NAC was added 30 minutes prior to gentamicin. After 24 h, anticancer agents were added and the cells were incubated for an additional 48 h. Viability was measured via MTT assay. C Gentamicin (100 μM) was added such that points for 6, 12 and 24 h were collected. Absorbance values were compared to a standard GSH curve to obtain total glutathione concentration. Comparisons between ROS curves were determined by fitting a nonlinear curve (cubic) and evaluating significance with a Student’s t-test. NAC and GSH data was analyzed via 2-way ANOVA (***, P < 0.0001; **, P < 0.001; *, P< .01).
CellROX® Green fluorescent reagent (Fig. 6A) was employed for the detection of intracellular ROS. Fluorescence due to ROS is measured in intervals over a 6 h period immediately after exposure to GEN and anticancer drugs. A stable increase in ROS was observed in H460 cells treated with GEN alone and in combination with DIG and CPT. Despite an increase in ROS in A549 with GEN alone, a significant loss in ROS was observed in cells treated with GEN in combination with DIG and CPT.
It has been previously established that the hydrogen peroxide detoxifying enzyme catalase can mitigate GEN-induced toxicity13 and that H460 has very little catalase compared to A549.32 It is therefore possible that the ROS produced by GEN is being actively detoxified by the relatively high level of catalase in A549 cells. To supplement this, we measured the perturbation of total glutathione levels in response to treatment with GEN in H460 and A549 cells (Fig. 6C). H460 and A549 cells were treated with 100 μM GEN and cells were collected by scraping at 6, 12 and 24 h time points. Cells were then lysed, deproteinated and added to a 96-well plate. Addition of a cocktail of glutathione reductase and Ellman’s reagent produced a yellow coloration, and absorbance was read at 405 nm in a microplate reader. A measureable increase in glutathione levels was observed in H460 cells in response to GEN, which may be indicative of the cell’s response to an elevated level of ROS. Although A549 cells have a lower basal level of glutathione, only a decrease in glutathione was observed, indicating that, possibly due to its high level of catalase, A549 does not respond to GEN with glutathione production. Thus, the cell line selectivity of the sensitization effect can be seen in how ROS production in the two cell lines respond to gentamicin/cancer drug exposure.
In addition, differences in ROS production in H460 cells can be seen depending on which drug is co-dosed with gentamicin. For instance, H460 cells respond differently depending upon whether the cells were exposed to the gentamicin sensitive drugs (camptothecin and digitoxin, immediate ROS increase) versus a non-gentamicin sensitive drug (oxaliplatin) (Fig. 6D). Specifically, there was a noticeable 2-hour delay in ROS production from H460 treated with the oxaliplatin/gentamicin drug combination (Fig. 6D/blue) in comparison to gentamicin alone (Fig. 6D/green). In contrast, H460 cells treated with gentamicin and either camptothecin or digitoxin showed an immediate increase in ROS production.
DISCUSSION
While further studies are needed, it is reasonable to imagine the use of gentamicin as a sensitizing agent in future cancer chemotherapies. In fact, gentamicin has been used clinically for the treatment of the infection in patients during cancer chemotherapy.33 In this regard, the gentamicin as well as digitoxin, camptothecin and vinblastine are all approved drugs, so it is reasonable to imagine gentamicin use in cancer therapy as a sensitizing agent. It is also important to note that there are many examples of gentamicin use to prevent bacterial contamination in the culturing of cancer cells.34 Given our findings of a gentamicin/cancer drug sensitization effect, some caution should be taken before deciding to use gentamicin as an antibiotic in the culturing of cancer cells, especially when screening for cytotoxicity.
CONCLUSIONS
Our results show that gentamicin sensitizes NCI-H460 lung cancer cells to digitoxin, camptothecin and vinblastine in a dose-dependent manner. The sensitization is synergetic in that it occurs at concentrations below which cytotoxicity due to gentamicin alone is observed. The cancer drug and cell line specificity of the effect appears to be linked to the production of radical oxygen species (ROS) in the cell. We hypothesize that the sensitizing effect of gentamicin can be attributed to the ROS generated by its interference with protein synthesis. Through direct measurement of ROS as well as changes in total glutathione content, a means by which the cytotoxic effects of certain anticancer drugs can be enhanced by increased ROS levels is proposed.
METHODS
Materials
CellROX™ was obtained from Life Technologies (Carlsbad, CA). Glutathione assay kit was obtained from Cayman Chemical Company (Ann Arbor, MI). Propidium iodide/Annexin V apoptosis assay kit was obtained from BD Biosciences (Franklin Lakes, NJ). Drugs were obtained from commercial sources and used without purification, with the exception of rhamno-digitoxin (RHA), which was synthesized in our labs. Gentamicin (GEN) was obtained from Indofine Chemical Company (Hillsborough, NJ), Camptothecin (CPT) was obtained from TCI America (Portland, OR), Vinblastine (VINB) was obtained from Eli Lilly and Co. (Indianapolis, IN), Oxaliplatin (OXA) and Doxorubicin (DOX) were obtained from Selleck Chemicals (Houston, TX). Digitoxin (DIG) and 5-fluorouracil (5FU) were obtained from Sigma-Aldrich Co. (St. Louis, MO). MTT and metaphosphoric acid were obtained from Alfa Aesar (Ward Hill, MA). N-Acetylcysteine was obtained from Avantor Performance Materials (Center Valley, PA) and triethanolamine was obtained from Fisher Scientific (Waltham, MA).
Cell culture and reagents
NCI-H460 and A549 cell lines were provided by the labs of Yon Rojanasakul (West Virginia University) and cultured in RPMI-1640 media supplanted with 10% fetal bovine serum, 1 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were cultured in an incubator set to 37 °C and conditioned with 5% CO2.
Combination assays
H460 or A549 cells were seeded in full media in 96-well plates at a density of 5000 cells per well. Drug dilutions were performed in serum-free RPMI-1640 (SFM). After seeding for 18 h, cells were dosed with GEN (5 concentrations, log 10) or SFM for 24 h. Cells were then dosed with an anticancer agent (7 concentrations, log 10) across all GEN concentrations. Plates were then incubated for 48 h (H460) or 24 h (A549) at 37 °C. All treatments were performed in duplicate on each plate and repeated for a total of 3 independent experiments. Cell viability was measured via MTT assay. Briefly, 10 μL of 5 mg/mL MTT solution was added to each well. After 4 h, media was evacuated and 100 μL DMSO was added. Absorbance was read at 570 nm in a BioTek® Synergy™ plate reader and data was processed using Graphpad Prism. Reported data is the average of 3 independent experiments; error bars correspond to mean ± SEM.
Apoptosis and cell cycle
Cells were seeded for 18 h in 6-well plates at a density of 200,000 cells per well. For apoptosis/necrosis studies, cells were treated with GEN for 24 h followed by DIG for 48 h. Detached and attached cells were collected (attached via trypsinization) and stained with propidium iodide/annexin V. For cell cycle analysis, cells were collected by trypsinization/centrifugation and incubated with RNAse A for 30 minutes, followed by addition of propidium iodide. For both experiments, fluorescence was read on a BD Biosciences® flow cytometer; data is representative of 10,000 gated events.
Measurement of reactive oxygen species
H460 and A549 cells were seeded in 96-well plates at a density of 5000 cells per well and incubated for 24 h. Growth media was then removed and wells were washed twice with PBS. Wells were filled with phenol red-free media (with 10% FBS) containing CellROX™ green fluorescent reagent (CRG). Plates were then incubated for 30 minutes. Drugs were then added to the appropriate wells and fluorescence readings were immediately started. Fluorescence was read in 1 min 13 sec intervals a BioTek® microplate reader (485ex/520em). Data is presented as fluorescence units with probe-only signal subtracted out. Presented data is representative of three independent experiments.
Total glutathione quantification
Total glutathione was measured with an enzyme recycling assay.35 Concentrations of glutathione were established from a standard assay with known GSH quantities. Briefly: GEN (100 μM) was added at specified time points. Upon experiment completion, cells were washed with PBS and harvested by scraping. Cells were pelleted, lysed with 300 μL RIPA buffer and deproteinated with 300 μL of 100mg/mL metaphosphoric acid. 5 μL of 4 M triethanolamine was added to 100 μL of deproteinated lysate. Deproteinated lysate for each sample was added into a 96-well plate in duplicate followed by glutathione reductase recycling assay cocktail. Samples were briefly placed on a shaker in the dark, followed by microplate analysis for absorbance at 405 nm in 5 minute intervals. GSH measurements at the 25 min time point are presented. Protein lysate prior to deproteination was analyzed via Bradford36 assay to confirm that similar amounts of cell lysate were obtained from the two cell lines (see supplementary information).
Statistical analyses
Cytotoxicity data were expressed as mean ± SEM. Comparisons were made using 2-way ANOVA.
Supplementary Material
Acknowledgments
Funding was provided by NIH (GM090259) and NSF (CHE-1213596 and DGE-0965843)
Footnotes
The authors declare no competing financial interests
Supporting Information. The supporting material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kummar S, Chen HX, Wright J, Holbeck S, Millin MD, Tomaszewski J, et al. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nature Reviews Drug Discovery. 2010;9:843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- 2.Kim J-H, Yoo H-I, Kang HS, Ro J, Yoon S. Salinomycin sensitizes antimitotic drugs-treated cancer cells by increasing apoptosis via the prevention of G2 arrest. Biochemical and biophysical research communications. 2012;418:98–103. doi: 10.1016/j.bbrc.2011.12.141. [DOI] [PubMed] [Google Scholar]
- 3.Jung EM, Park J-W, Choi KS, Park J-W, Lee HI, Lee KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis. 2006;27:2008–2017. doi: 10.1093/carcin/bgl026. [DOI] [PubMed] [Google Scholar]
- 4.Quiros Y, Vicente-Vicente L, Morales AI, López-Novoa JM, López-Hernández FJ. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicological Sciences. 2011;119:245–256. doi: 10.1093/toxsci/kfq267. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura M. Endoplasmic reticulum stress in the kidney. Clin Exp Nephrol. 2008;12:317–325. doi: 10.1007/s10157-008-0060-7. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen ARB, Hvidtfeldt M, Bräuner-Osborne H. Biased agonism of the calcium-sensing receptor. Cell Calcium. 2012;51:107–116. doi: 10.1016/j.ceca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Priuska EM, Schacht J. Formation of Free Radicals by Gentamicin and Iron and Evidence for an Iron/Gentamicin Complex. Biochem Pharmacol. 1995;50:1749–1752. doi: 10.1016/0006-2952(95)02160-4. [DOI] [PubMed] [Google Scholar]
- 8.Maniu A, Perde-Schrepler M, Cosgarea M. Protective effect of L-N-acetylcysteine against gentamycin ototoxicity in the organ cultures of the rat cochlea. Romanian journal of morphology and embryology. 2011;52:159–164. [PubMed] [Google Scholar]
- 9.Yang TH, Young YH, Liu SH. EGb 761 (Ginkgo Biloba) Protects Cochlear Hair Cells Against Ototoxicity Induced by Gentamicin via Reducing Reactive Oxygen Species and Nitric Oxide-Related Apoptosis. The Journal of Nutritional Biochemistry. 2011;22:886–894. doi: 10.1016/j.jnutbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Peyrou M, Hanna PE, Cribb AE. Cisplatin, Gentamicin, and p-Aminophenol Induce Markers of Endoplasmic Reticulum Stress in the Rat Kidneys. Toxicological Sciences. 2007;99:346–353. doi: 10.1093/toxsci/kfm152. [DOI] [PubMed] [Google Scholar]
- 11.Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL, and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- 12.Mostafa BE, Tawfik S, Hefnawi NG, Hassan MA, Ismail FA. The role of deferoxamine in the prevention of gentamicin ototoxicity: a histological and audiological study in guinea pigs. Acta Otolaryngol. 2007;127:234–239. doi: 10.1080/00016480600794495. [DOI] [PubMed] [Google Scholar]
- 13.Denamur S, Tyteca D, Marchand-Brynaert J, Van Bambeke F, Tulkens PM, Courtoy PJ, Mingeot-Leclercq M-P. Role of oxidative stress in lysosomal membrane permeabilization and apoptosis induced by gentamicin, an aminoglycoside antibiotic. Free Radical Biology and Medicine. 2011;51:1656–1665. doi: 10.1016/j.freeradbiomed.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Floquet C, Deforges J, Rousset JP, Bidou L. Rescue of non-sense mutated p53 tumor suppressor gene by aminoglycosides. Nucleic Acids Research. 2011;39:3350–3362. doi: 10.1093/nar/gkq1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brendel C, Belakhov V, Werner H, Wegener E, Gärtner J, Nudelman I, et al. Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. Journal of Molecular Medicine. 2010;89:389–398. doi: 10.1007/s00109-010-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilschanski M, Yahav Y, Yaacov Y, Blau H, Bentur L, Rivlin J, Aviram M, Bdolah-Abram T, Bebok Z, Shushi L, Kerem B, Kerem E. Gentamicin-Induced Correction of CFTR Function in Patients with Cystic Fibrosis and CFTR Stop Mutations. New England Journal of Medicine. 2003;349:1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 17.Iyer A, Zhou M, Azad N, Elbaz H, Wang H-Y, Rogalsky DK, Rojanasakul Y, O’Doherty GA, Langenhan JM. A Direct Comparison of the Anticancer Activities of Digitoxin MeON-Neoglycosides and O-Glycosides: Oligosaccharide Chain Length-Dependant Induction of Caspase-9-Mediated Apoptosis. ACS Med Chem Lett. 2010;1:326–330. doi: 10.1021/ml1000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H-Y, Wu B, Zhang Q, Rojanasakul Y, O’Doherty GA. C5′-Alkyl Substitution Effects on Digitoxigenin α-L-Glycoside Epithelial Human Lung Cancer Cells Cytotoxicity. ACS Med Chem Lett. 2011;2:259–263. doi: 10.1021/ml100291n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou M, O’Doherty GA. The De Novo Synthesis of Oligosaccharides: Application to the Medicinal Chemical Study of Digitoxin. Current Topics in Medicinal Chemistry. 2008;8:114–125. doi: 10.2174/156802608783378828. [DOI] [PubMed] [Google Scholar]
- 20.Wang H-Y, Rojanasakul Y, O’Doherty GA. Synthesis and Evaluation of the α-D-/α-L-Rhamnosyl and Amicetosyl Digitoxigenin Oligomers as Antitumor Agents. ACS Medicinal Chemistry Letters. 2011;2:264–269. doi: 10.1021/ml100290d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pongrakhannon V, Stueckle TA, Wang H-Y, O’Doherty GA, Dinu CZ, Rojanasakul Y. Monosaccharide digitoxin derivatives sensitize human non-small cell lung cancer cells to anoikis through Mcl-1 proteasomal degradation. doi: 10.1016/j.bcp.2013.10.027. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elbaz H, Stueckle TA, Wang H-Y, O’Doherty GA, Lowry DT, Sargent LM, Wang L, Dinu CZ, Rojanasakul Y. Digitoxin and a Synthetic Monosaccharide Analog Inhibit Cell Viability in Lung Cancer Cells. Toxicol Appl Pharmacol. 2012;258:51–60. doi: 10.1016/j.taap.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinds JW, McKenna SB, Sharif EU, Wang H-Y, Akhmedov NG, O’Doherty GA. C3′/C4′-Stereochemical Effects of Digitoxigenin α-L-/α-D-Glycoside in Cancer Cytotoxicity. Chem Med Chem. 2013;8:63–69. doi: 10.1002/cmdc.201200465. [DOI] [PubMed] [Google Scholar]
- 24.Wang H-Y, O’Doherty GA. Modulators of Na/K-ATPase: a patent review. Expert Opin Theraputic Patents. 2012;22:587–605. doi: 10.1517/13543776.2012.690033. [DOI] [PubMed] [Google Scholar]
- 25.Lobert S, Frankfurter A, Correia JJ. Binding of Vinblastine to Phosphocellulose-Purified and ap-Class III Tubulin: The Role of Nucleotides and 3-Tubulin Isotypes. Biochemistry. 1995;34:8050–8060. doi: 10.1021/bi00025a011. [DOI] [PubMed] [Google Scholar]
- 26.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 27.Pommier Y, Redon C, Rao VA, Seiler JA, Sordet O, Takemura H, Antony S, Meng LH, Liao ZY, Kohlhagen G, Zhang HL, Kohn KW. Repair of and checkpoint response to topoisomerase I-mediated DNA damage. Mutation Research. 2001;532:173–203. doi: 10.1016/j.mrfmmm.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 29.Momparler RL, Karon M, Siegel SE, et al. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36:2891–2895. [PubMed] [Google Scholar]
- 30.Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–4. [Google Scholar]
- 31.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 32.Choi C-H, Jung Y-K, Oh SH. Selective induction of catalase-mediated autophagy by dihydrocapsaicin in lung cell lines. Free Radical Biology and Medicine. 2010;49:245–257. doi: 10.1016/j.freeradbiomed.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Bodey GP, Middleman E, Umsawadi T, Rodriguez V. Infections In Cancer Patients-Results with Gentamicin Sulfate Therapy. Cancer. 1972;6:1697–1701. doi: 10.1002/1097-0142(197206)29:6<1697::aid-cncr2820290638>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.NCI panel of 60 cell lines. http://dtp.nci.nih.gov/branches/btb/ivclsp.html.
- 35.Eyer P, Podhradsky D. Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman’s reagent. Anal Biochem. 1986;153:57–66. doi: 10.1016/0003-2697(86)90061-8. [DOI] [PubMed] [Google Scholar]
- 36.Bradford MM. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.