Abstract
High-dose (HD) IL-2 therapy in patients with cancer increases the general population of Tregs, which are positive for CD4, CD25, and the Treg-specific marker Foxp3. It is unknown whether specific subsets of Tregs are activated and expanded during HD IL-2 therapy or whether activation of any particular Treg subset correlates with clinical outcome. Here, we evaluated Treg population subsets that were induced in patients with melanoma following HD IL-2 therapy. We identified a Treg population that was positive for CD4, CD25, Foxp3, and the inducible T cell costimulator (ICOS). This Treg population increased more than any other lymphocyte subset during HD IL-2 therapy and had an activated Treg phenotype, as indicated by high levels of CD39, CD73, and TGF-β. ICOS+ Tregs were the most proliferative lymphocyte population in the blood after IL-2 therapy. Patients with melanoma with enhanced expansion of ICOS+ Tregs in blood following the first cycle of HD IL-2 therapy had worse clinical outcomes than patients with fewer ICOS+ Tregs. However, there was no difference in total Treg expansion between HD IL-2 responders and nonresponders. These data suggest that increased expansion of the ICOS+ Treg population following the first cycle of HD IL-2 therapy may be predictive of clinical outcome.
Introduction
High-dose (HD) bolus IL-2 therapy is currently one of the most potent forms of immunotherapy and was approved by the FDA as a single-agent cytokine therapy for metastatic melanoma and renal cell carcinoma (1–3). Typical HD IL-2 therapy consists of bolus infusions of 600,000 or 720,000 IU/kg of aldesleukin (Novartis), and each cycle of therapy is aimed at giving up to 15 bolus infusions every 8 hours or as many as the patient can withstand due to toxicity (1, 4). The therapy cycle is then repeated approximately every 14 to 21 days for up to 6 to 8 cycles, depending on the clinical performance of each patient and toxicities associated with IL-2 therapy. Early single and multicenter clinical trials have consistently shown a 15%–16% partial and complete response rate in patients with stage IIIC or stage IV noncutaneous metastatic melanoma and in patients with renal cell carcinoma, among whom a smaller fraction of patients (about 5%) experience durable long-lasting complete remission for years (1, 2, 5). HD IL-2 has also been combined with other immunotherapies, including adoptive T cell therapy using ex vivo–expanded tumor-infiltrating lymphocytes (6–8) and tumor antigen peptide vaccines (9), where it may enhance antitumor T cell function. IL-2 is known to induce NK cell and CD8+ T cell proliferation, survival, and acquisition of effector function through STAT5 activation (10–12). Increased tumor-infiltrating and circulating perforin+ (PRF1+) NK cells and activated CD8+ T cells have been found in most patients undergoing HD IL-2 therapy, but this finding did not always correlate with tumor regression or clinical response (13–15).
One of the key problems with HD IL-2 therapy, which limits its more widespread use, is its adverse effects, including blood pressure changes, vascular leak syndrome, liver dysfunction, neurological changes (cognitive impairment), and high fever (1, 2). These toxic effects require some patients to withdraw from therapy after a limited number of therapy cycles. Nevertheless, HD IL-2 continues to be a treatment of choice for qualified patients, especially for those with metastatic melanoma, because it is one of the only therapies capable of inducing documented durable clinical remission lasting for many years. Thus, specific biomarkers that can identify subsets of patients who are responsive to HD IL-2, and thereby improve patient selection, are needed to refine this form of therapy and make it more attractive to more clinical centers.
Recently, a number of groups have reported that HD IL-2 markedly expands the classic Treg pool, consisting of CD4+CD25+Foxp3+ Tregs (16–19). Some of these studies have attempted to correlate the extent of Treg expansion during IL-2 therapy with clinical outcome and have suggested a negative correlation between a sustained increase in Tregs during multiple IL-2 therapy cycles and progressive disease (17). Tregs inhibit effector CD8+ and CD4+ T cells by suppressing their proliferation or inducing cell death. Moreover, Tregs can also antagonize NK cell–mediated antitumor activity (20–23). However, the exact role of Tregs in HD IL-2 therapy needs to be further defined.
Tregs exist in two main forms: the so-called natural Tregs, originally derived from the thymus, and induced Tregs, generated from peripheral naive CD4+ T cells in the presence of TGF-β and IL-2 (22, 24, 25). However, the phenotypic markers distinguishing these two main Treg types are still unclear. Although previous studies have tracked the appearance of Tregs during IL-2 therapy by using the classic markers CD25, Foxp3, cytotoxic T lymphocyte antigen 4 (CTLA4), glucocorticoid-induced tumor necrosis factor receptor (GITR), and CD127, Tregs may exist in various states of differentiation and activation that may be discernible with use of additional markers. For example, a subset of Tregs may be tumor antigen specific and activated through the TCR before HD IL-2 therapy; these cells may carry specific activation markers reflecting this ongoing antigenic stimulation that clearly separates them from the bulk circulating Treg population. Thus, these previously “activated” tumor-specific Tregs may be induced to further divide by IL-2 treatment.
In this article, we investigated which lymphoid and myeloid subsets were modulated upon HD IL-2 therapy and their possible association with clinical response to allow preidentification of patients who can benefit from this treatment. We performed comprehensive multiparameter flow cytometry analysis to determine the changes in more than 40 different lymphocyte subsets, including subpopulations of Tregs, DCs, and CD4+, CD8+, NK, and B cells in PBMCs from patients treated with HD IL-2 before IL-2 infusion and 2 days after the last infusion of cycle 1 of HD IL-2 therapy. After analyzing the fold changes of each cell subset, we found that CD4+ICOS+ T cell subset, consisting almost exclusively of CD25hi and Foxp3hi Tregs (ICOS+ Tregs), was one of the most rapidly expanding lymphocyte subsets in response to IL-2. We present data characterizing the phenotype of CD4+ICOS+ and ICOS+ Treg subsets during the first cycle of HD IL-2 therapy and their potential as predictive biomarkers.
Results
Activated T cells within a CD4+ICOS+ subset greatly increase during IL-2 therapy.
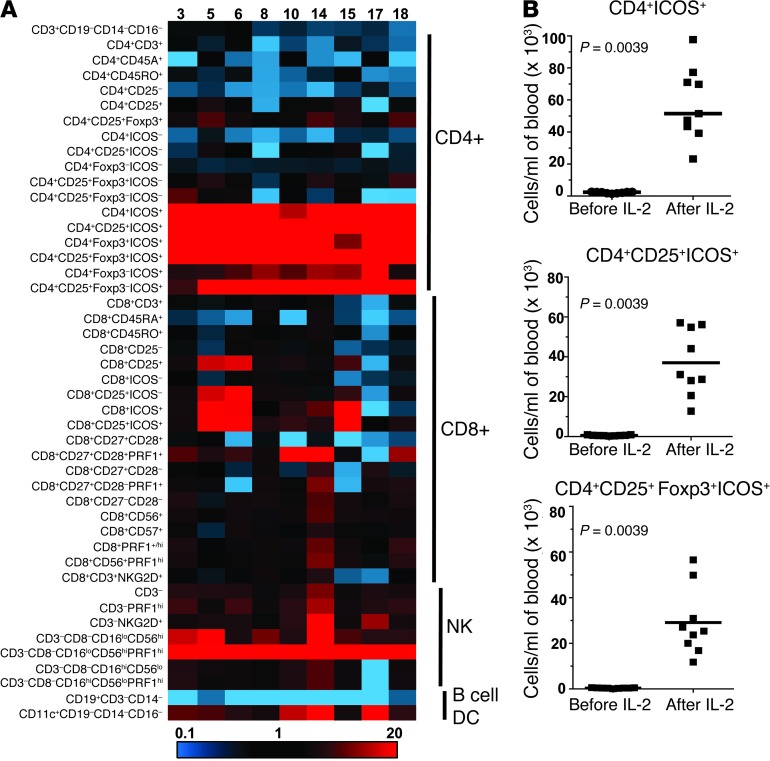
We developed a multicolor FACS staining panel to track changes in multiple lymphocyte subsets in peripheral blood of patients with melanoma during cycle 1 of HD IL-2 therapy. This panel allowed us to track the changes in multiple T cell, B cell, DC, and NK cell lineages before and after HD IL-2 therapy. First, we analyzed the first 9 patients consecutively treated with HD IL-2 (Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI46266DS1). Blood was collected immediately before the first bolus infusion of IL-2 and during the rebound period, which is 2 days after the last IL-2 infusion, when a rapid influx of lymphocytes back into the blood occurs (16, 26, 27). This sample 2 days after IL-2 treatment gives us a “window” into the immediate changes that are induced in patients during the first cycle of IL-2 therapy. A heat diagram of the FACS data shows the fold change in 46 lymphocyte subsets as a percentage of total live lymphocytes (Figure 1A). Strikingly, although minor changes occurred in a number of lymphocyte subsets, some major cell types consistently exhibited markedly high increases in all patients during IL-2 therapy, as indicated by the bright red regions in Figure 1A. One of these cell types was the CD4+ T cells, which consisted of ICOS+, CD25+ICOS+, and CD4+CD25+ICOS+ T cells that coexpressed Foxp3 (Figure 1A).
Figure 1. CD4+ T cells expressing ICOS with phenotypic characteristics of Tregs increase the most in peripheral blood after HD IL-2 therapy.
PBMCs isolated at baseline and 2 days after the last dose of IL-2 during cycle 1 of HD IL-2 therapy from 9 patients (nonresponders) were stained for multiple T, B, and NK cell and DC markers. The percentage of 46 cell subsets in the live lymphocyte gate were determined, and the fold change in the frequency of each indicated cell subset in the lymphocyte gate was calculated by dividing the frequency of cells before HD IL-2 therapy by the frequency after treatment. (A) Changes in the percentage of indicated cell subsets analyzed for all 9 patients (patient numbers are shown at the top of the heat map) were heat mapped based on the fold changes, with the use of an Excel conditional formatting program, as indicated at the bottom of the figure. The major lymphocyte subpopulations corresponding to the different phenotypic marker subsets (left side) are indicated on the right side of the heat diagram. (B) Total numbers of CD4+ICOS+, CD4+CD25+ICOS+, and CD4+CD25+Foxp3+ICOS+ cells before IL-2 and 2 days after cycle 1 of HD IL-2 therapy (after IL-2) are shown for these 9 patients. Total cell numbers were calculated by multiplying the percentage of each subset in the viable lymphocyte gate by the absolute lymphocyte count. Horizontal bars represent median values. Statistical analyses were performed with 2-tailed Wilcoxon matched paired test.
The second major cell type that showed a high increase in all patients was NK cells, with a predominantly CD56hiCD16loPRF1+ NK phenotype (Figure 1A) and more than an 80-fold increase in these cells in some patients (median, 25-fold increase; n = 9). Expansion of NK cells and acute sensitivity of these cells to IL-2, as these cells constitutively express CD25 (IL-2Rα), as well as perforin-induced expression by IL-2 were expected on the basis of previous studies on IL-2–treated patients (28–31). All other lymphocyte subsets exhibited either small increases after IL-2 therapy (less than 2 fold) or a decrease, as shown by the blue regions in Figure 1A. Increased frequency of CD11c+ DCs was seen in some patients (median, 3.6 fold), whereas the frequency of CD19+ B cells consistently decreased after IL-2 therapy by 3 fold to 4 fold.
The CD4+ICOS+ and CD4+CD25+ICOS+ cell subsets increased by a median of 13 fold and 27 fold, respectively. However, the most consistent and highest increase was in the frequency of CD4+CD25+Foxp3+ICOS+ T cells (median, 37 fold). We calculated the change in total number of ICOS+ cells in the CD4+ subset by multiplying the absolute lymphocyte counts per milliliter of blood by the percentage of each subset in the live lymphocyte gate. As shown in Figure 1B, the total number of CD4+ICOS+, CD4+CD25+ICOS+, and CD4+CD25+Foxp3+ICOS+ T cells increased greatly after HD IL-2 therapy. Similar results were seen with the CD4+CD25+Foxp3–ICOS+ subset (data not shown).
CD4+ICOS+ T cells that increased during HD IL-2 therapy in the peripheral blood reside primarily in the CD25+Foxp3+ subset.
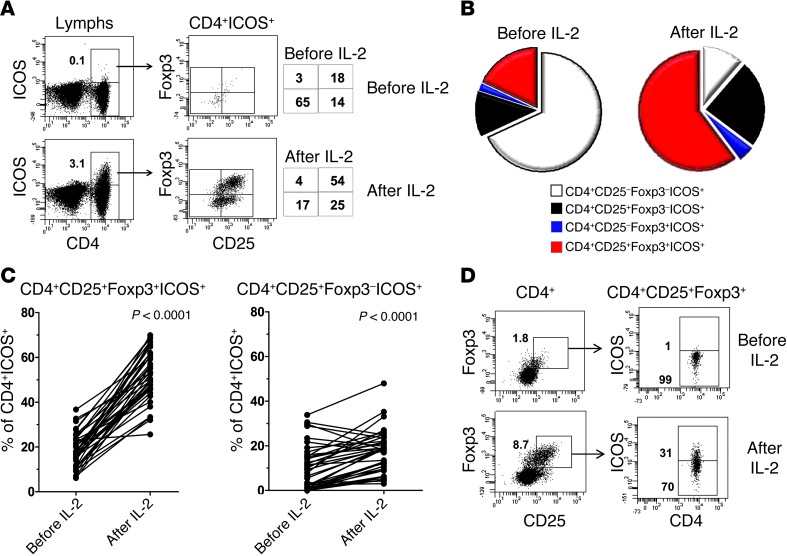
Large increases in ICOS-expressing CD4+ T cells were unexpected and had not been documented before in IL-2–treated patients. We considered this to be a mechanistically important result, based on the emerging role of CD4+ICOS+ in tumor immunotherapy, especially as suppressive Tregs or as activated T cells associated with positive clinical response to anti-CTLA4 therapy (32–37). Moreover, we reported recently that freshly isolated tumor-infiltrating lymphocytes from patients with stage IV metastatic melanoma have a high frequency (up to 50% or more) of ICOS+ cells in the CD4+CD25+Foxp3+ tumor-infiltrating subset (38). Thus, these results led us to further evaluate how CD25+ and Foxp3+ expression changed in the CD4+ICOS+ subset during the first cycle of HD IL-2 therapy. As shown in Figure 2A, CD25+Foxp3+ cells in the activated CD4+ICOS+ subset changed after cycle 1 of HD IL-2 in relation to the other 3 possible types of cells in this subset, namely, CD25+Foxp3–, CD25–Foxp3+, and CD25–Foxp3– cells. Before IL-2 therapy, a minority of CD4+ICOS+ cells were CD25+Foxp3+, whereas the majority of cells were CD25–Foxp3– (Figure 2A). However, after IL-2 therapy, this trend was reversed, with the CD25+Foxp3+ subset becoming the major component and CD25–Foxp3– becoming the minor component of the CD4+ICOS+ cells. The CD25+Foxp3– and CD25–Foxp3+ subsets were also present, with the CD25+Foxp3– subset increasing, but only marginally (Figure 2A). These results are summarized in a pie chart in Figure 2B for a representative patient, illustrating this large increase in the CD25+Foxp3+ subset within the CD4+ICOS+ T cell compartment. In addition, we found this predominance of the CD25+Foxp3+ subset among activated CD4+ICOS+ T cells after IL-2 therapy in all 38 patients analyzed, including both nonresponders and responders (Figure 2C). In contrast, CD25+Foxp3– cells within this subset did not increase as much after IL-2 (Figure 2C). Thus, cells with a CD25+Foxp3+ (Treg-like) phenotype became the major subset in the activated CD4+ICOS+ T cell compartment after the first cycle of HD IL-2 therapy. This dramatic shift in phenotype was also found when we directly analyzed the CD4+CD25+Foxp3+ (Treg) subset before and after HD IL-2. As shown in Figure 2D, we found a large increase in frequency of ICOS+ cells over ICOS– cells in the Treg compartment, indicating a dramatic shift in the phenotype of Tregs induced by repeated HD IL-2 infusions.
Figure 2. Activated CD4+CD25+ICOS+ T cells expanded during HD IL-2 therapy are primarily in the CD4+CD25+Foxp3+ subset.
(A) Dot plots show PBMCs that were isolated from an HD IL-2–treated patient (no. 032) and stained for CD4, CD25, Foxp3, and ICOS before and 2 days after cycle 1 of HD IL-2 therapy. Percentages of CD4+ICOS+ T cells in total lymphocytes (Lymphs) and in the CD25+Foxp3+ subset are as indicated in and beside dot plots. A minimum of 40,000 to 50,000 viable lymphocytes were analyzed at each time point for this patient and for all other patients analyzed. (B) The changes in frequency of CD25–Foxp3–, CD25–Foxp3+, CD25+Foxp3–, and CD25+Foxp3+ cells in the CD4+ICOS+ T cell gate before and after IL-2 therapy in a representative nonresponding patient (no. 014). (C) Graphs depict changes in the frequency of CD25+Foxp3+ T cells in the activated CD4+ICOS+ T cell subset versus the frequency of T cells in the corresponding Foxp3– subpopulation after HD IL-2 therapy (n = 38). (D) A large increase in the ICOS+ cell fraction within the gated CD4+CD25+Foxp3+ subset after cycle 1 of HD IL-2 therapy in a representative patient (no. 006). Percentages of indicated cell subsets are shown in dot plots. Statistical analyses were performed with 2-tailed Wilcoxon matched paired test.
CD4+CD25+Foxp3+ICOS+ T cells are the most highly cycling lymphocyte subset after HD IL-2 therapy.
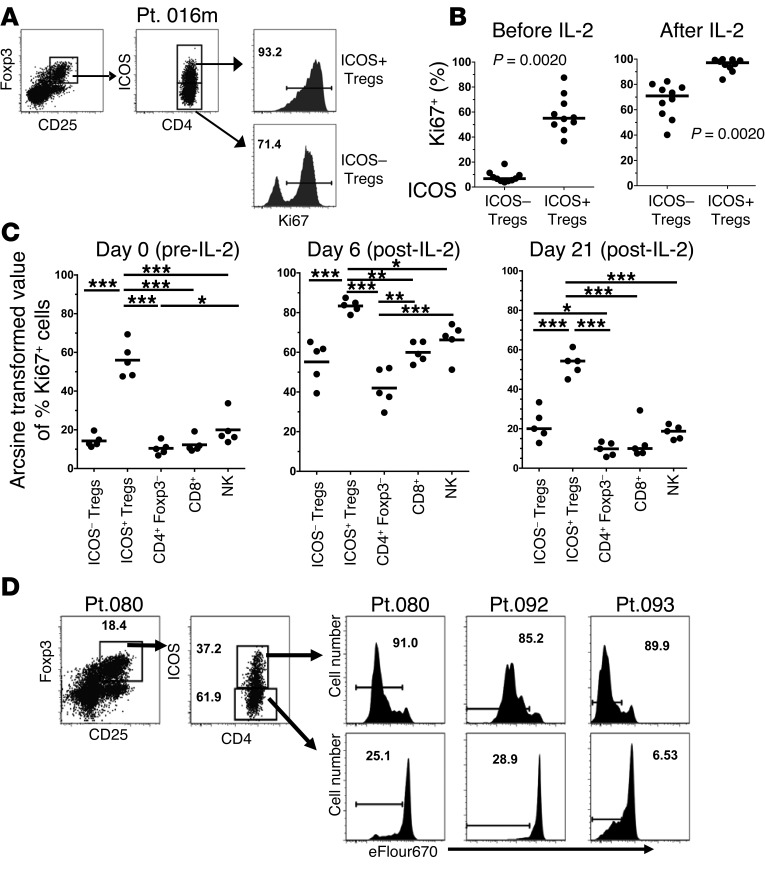
The large increase in CD4+CD25+Foxp3+ICOS+ T cells in most patients after HD IL-2 therapy suggests that the increase in these cells may be due to cell division. Thus, we asked whether the ICOS+ T cells were in the cell cycle. By staining for Ki67 expression, we found that ICOS+ T cells in patients before IL-2 treatment had a much higher proportion of Ki67+ cells (range, 50%–96% Ki67+) than the ICOS– T cell subset (Figure 3, A and B). The fraction of Ki67+ cells in these T cell populations significantly increased after cycle 1 of HD IL-2 and exhibited the highest Ki67 expression, with almost 100% of the cells staining positive, as measured 2 days after the last dose of IL-2 (Figure 3, A and B). HD IL-2 therapy also induces the activation and cell cycle entry of NK cells and other T cell subsets. We were interested in the relative frequency of Ki67 expression in the ICOS+ T cells compared with that in the ICOS– T cell subset and in the CD4+Foxp3– cell, CD8+ T cell, and NK cell subsets before and 2 days after the last IL-2 infusion in cycle 1 of IL-2 therapy. As shown in Figure 3C, the frequency of Ki67+ cells in the ICOS+ T cell subset at baseline was markedly higher than that of any other lymphocyte subsets analyzed. After HD IL-2 therapy, the frequency of Ki67 expression increased in all lymphocyte subsets; however, as before, the ICOS+ T cell subset had the larger proportion of Ki67-expressing cells (Figure 3C). We also tracked Ki67 expression 21 days after cycle 1 of HD IL-2 to determine whether elevated Ki67 persisted for a longer period of time. As shown in Figure 3C, the degree of Ki67 expression in all cell subsets decreased to levels similar to those found before treatment, but the ICOS+ T cell subset was still the highest cycling population, with 50% to 80% of cells staining positive for Ki67. When we performed proliferation assays by using eFluor 670 dye–labeled PBMCs from patients treated with HD IL-2, we observed that ICOS+ T cells proliferated more than ICOS– T cells did after 5 days of culture in the presence of HD IL-2 (Figure 3D), indicating that ICOS+ T cells are more responsive to IL-2–induced proliferation.
Figure 3. ICOS+ Tregs (CD4+CD25+Foxp3+) are more highly activated and more highly cycling than ICOS– Tregs or other lymphocyte subsets before and after HD IL-2 therapy.
(A) Flow cytometry staining for Ki67 expression using intracellular flow cytometry staining in ICOS+ and ICOS– Treg subsets before and after cycle 1 of HD IL-2 therapy. Frequencies of Ki67+ cells are as indicated in dot plots. (B) Summary results from 10 patients are shown in scatter plots. (C) PBMCs from 5 patients were stained for the indicated subsets together with intracellular staining for Ki67 before therapy (day 0), shortly after therapy (day 6), and 3 weeks after therapy (day 21) (see Methods section). Percentages of Ki67+ cells in each lymphocyte subset were determined and then arcsine transformed for 1-way ANOVA and Tukey’s multiple comparison analysis. Arcsine-transformed values were plotted for the indicated subsets. Horizontal bars represent median values. (D) Fresh PBMCs from 3 patients shortly after HD IL-2 therapy (day 6) were isolated and stained with eFluor 670 dye for the dye dilution proliferation assay. Cells were cultured for 5 days in the presence of HD IL-2 (3,000 IU/ml). After 5 days, cells were harvested and stained for Treg markers. The gating strategy and frequencies of indicated cell subsets are as shown in dot plots. Frequencies of proliferative cells in ICOS+ and ICOS– Treg subsets are as indicated in histograms (***P < 0.001, **0.001 < P < 0.01, *0.01 < P < 0.05).
Thus, the ICOS+ Treg subset in patients treated with HD IL-2 is the most highly cycling (Ki67+) lymphocyte population found in the peripheral blood, with almost all cells having entered the cell cycle after cycle 1 of HD IL-2 therapy. The ICOS+ Tregs were also the most highly cycling population 21 days after this first cycle of therapy.
ICOS+ Tregs have phenotypic characteristics of classic suppressive Tregs.
Although Foxp3 expression has been associated primarily with T cells having a regulatory or suppressive function, in some cases transient induction of Foxp3 at low levels can occur during the activation of effector, non-Treg CD4+ T cells (39, 40). We therefore asked whether the CD4+CD25+Foxp3+ICOS+ Tregs (referred to as ICOS+ Tregs herein), which increased substantially during cycle 1 of HD IL-2 therapy, indeed had the properties of Tregs and were not simply activated non-Treg CD4+Foxp3+ T cells with the ICOS activation marker.
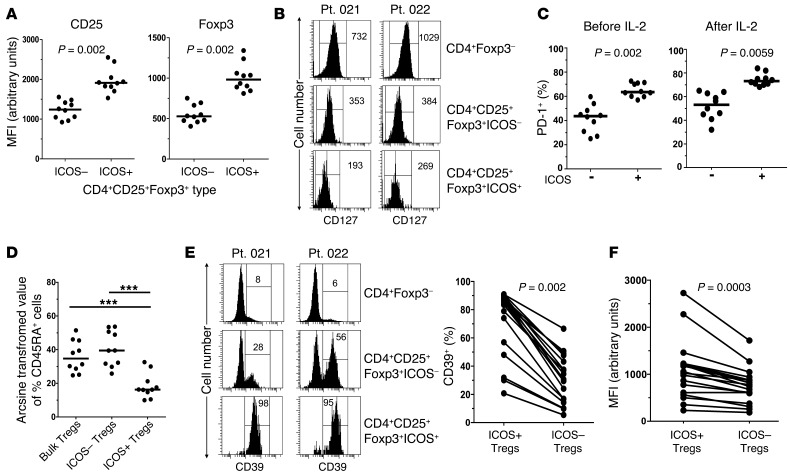
We were limited in these experiments by the volume of blood samples accessible from these patients. We were therefore unable to perform suppressor T cell assays in vitro to directly demonstrate Treg function. However, we analyzed a number of other phenotypic and functional attributes of these cells isolated after cycle 1 of HD IL-2 that had been definitively ascribed to Tregs (18, 41–44). First, we looked at the intensity of CD25 and Foxp3 staining on the ICOS+ and ICOS– cells in the CD4+CD25+Foxp3+ subset and found that the ICOS+ cells had higher levels of CD25 and Foxp3 expression than the ICOS– cells (Figure 4A). Second, consistent with a previous study (45), we found that the ICOS+ Treg subset expressed lower levels of CD127/IL-7Rα than the CD4+Foxp3– non-Treg and ICOS– Treg subsets (Figure 4B). Third, we examined the expression of programmed cell death protein 1 (PD-1) by the ICOS+ Treg subset, since PD-1 induction is another marker for T cell activation (46, 47), and found that ICOS+ Tregs had a significantly higher fraction of PD-1+ cells than the ICOS– Treg subset (Figure 4C). No substantial level of OX40 expression was detected in the CD4 population either before or after HD IL-2 therapy (data not shown). We also analyzed the expression of CD45RA, which has been associated with the Treg activation and ICOS expression (41), and found lower CD45RA expression in the ICOS+ Treg subset than in the ICOS– Treg subset (Figure 4D). Fourth, recent studies have found that highly suppressive Tregs express the CD39 ectonucleotidase on their surface together with CD73, which is also expressed by most CD4+ T cells (48–50). As shown in Figure 4E, we found that non-Tregs (CD4+Foxp3–) were almost exclusively CD39–, whereas a subpopulation of ICOS– Tregs was CD39+. In contrast, the ICOS+ subset was mainly CD39+/hi compared with the corresponding ICOS– subset (Figure 4E) and had a higher expression level of CD39 (Figure 4F). All ICOS+ cells and ICOS– cells expressed high levels of CD73 (Supplemental Figure 1).
Figure 4. CD4+CD25+Foxp3+ICOS+ T cells increased during HD IL-2 therapy and have properties of suppressive Tregs.
PBMCs from patients with melanoma after HD IL-2 therapy (2 days after last IL-2 dose in cycle 1 of therapy) were thawed and stained for various marker attributes to Treg activation and suppression function. (A) Scatter plots depict the intensity of CD25 and Foxp3 expression in the CD4+CD25+Foxp3+ subset based on ICOS expression from 10 representative patients. (B) CD127 expression and (C) frequency of PD-1–expressing ICOS+ and ICOS– Tregs before and after HD IL-2 therapy. Median fluorescence intensity (MFI) is indicated in B. (D) Percentages of CD45RA-expressing cells in bulk Tregs and ICOS+ and ICOS– Tregs from 10 representative patients were determined and arcsine transformed for 1-way ANOVA and Tukey’s multiple comparison analysis. (E) Frequencies of CD39+ cells in CD4+ Foxp3–, CD4+CD25+Foxp3+ICOS–, and CD4+CD25+Foxp3+ICOS+ cells are as indicated in histograms and in the graph. (F) Graph shows the median fluorescence intensity of CD39 expression in ICOS+ and ICOS– Tregs in HD IL-2–treated patients (n = 18). Horizontal bars represent median values. P < 0.05 was considered significant (***P < 0.001).
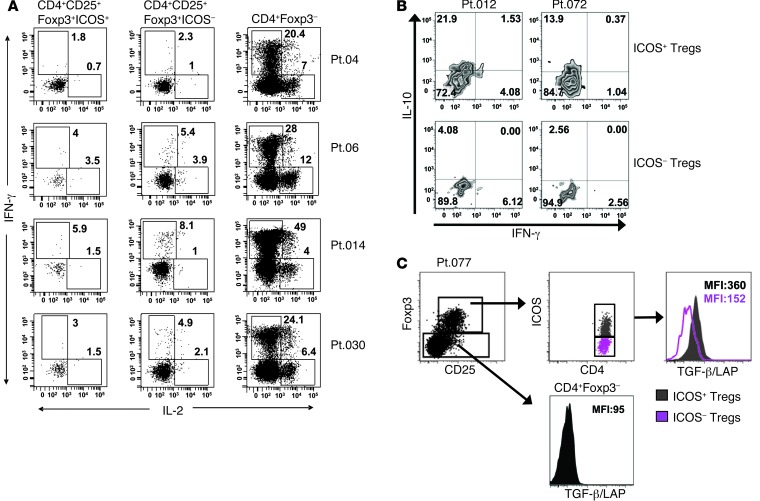
Next, it has been shown that suppressive CD4+ Tregs secrete lesser amounts of IFN-γ and IL-2 in response to stimulation as compared with CD4+Foxp3– T cells (51). When we examined the potential of ICOS+ and ICOS– Tregs to secrete cytokines associated with T effector function, including IFN-γ and IL-2, the intracellular cytokine staining revealed that ICOS+ and ICOS– subsets produced little IFN-γ and IL-2 compared with levels in CD4+Foxp3– cells after 6 hours of stimulation with PMA/ionomycin (Figure 5A). In agreement with the findings of Ito et al. (35), we also found that the ICOS+ Treg subset, but not ICOS– Tregs from HD IL-2–treated patients, was the predominant population that secreted IL-10 upon 24-hour PMA/ionomycin activation (Figure 5B). These results indicate that the expanded ICOS+ Tregs in patients treated with HD IL-2 had definitive attributes of Tregs and not merely an activated non-Treg CD4+ T cell population. Furthermore, ICOS+ Tregs expressed higher levels of the cell surface TGF-β/latency-associated peptide (TGF-β/LAP) than ICOS– T cells (Figure 5C and Supplemental Figure 2). These results indicate that the ICOS+ T cells may be highly suppressive, as described previously (36, 37).
Figure 5. Cytokine production profiles of ICOS+ and ICOS– Tregs.
(A) PBMCs were stimulated with PMA/ionomycin for 6 hours, and the secretion of IFN-γ or IL-2 in CD4+Foxp3– T cells (non-Treg) and in ICOS+ and ICOS– Treg subsets was determined with use of intracellular cytokine staining and measured by using flow cytometry. Results from 4 patients are shown. (B) PBMCs from HD IL-2–treated patients after HD IL-2 (cycle 1 of therapy) were thawed and stimulated with PMA (50 ng/ml) and ionomycin (2 μg/ml) for 24 hours. GolgiStop was added 1 hour after the PMA/ionomycin stimulation and further incubated for the rest of the stimulation period. Cells were harvested and stained for CD4, CD25, Foxp3, ICOS, IFN-γ, and IL-10. The production of IL-10 or IFN-γ by ICOS+ and by ICOS– Tregs is shown for 2 representative patients. Frequencies of cytokine-producing cells in indicated cell subset are shown in A and B. (C) Histograms from 1 representative patient with melanoma show the cell surface expression of TGF-β/LAP in ICOS+ and ICOS– Tregs as well as in CD4+Foxp3– T cells after cycle 1 of HD IL-2 therapy. The median fluorescence intensity values are as indicated in the histograms.
Tregs induce ICOS expression after TCR triggering.
We have previously reported that metastatic melanoma cells express inducible costimulator ligand (ICOSL) and that ICOS costimulation by ICOSL-expressing melanoma cells promotes the expansion of CD4+CD25+Foxp3+ICOS+ T cells after TCR activation (38). The high increase in ICOS+ Tregs during HD IL-2 therapy has been associated with a more activated Treg phenotype of these cells, suggesting that TCR activation may induce high levels of ICOS expression on Tregs and that cooperation of ICOS/ICOSL costimulation with IL-2 signaling may further drive Tregs into cell division. To address this question, we examined how HD IL-2 or TCR stimulation with anti-CD3 antibody affected ICOS and PD-1 (another activation marker) expression on the surface of CD4+CD25+Foxp3+ Tregs in PBMCs from patients with melanoma. As shown in Supplemental Figure 3, A and B, treatment of PBMCs with low-dose or HD IL-2 only marginally affected ICOS and PD-1 expression. However, treatment of the cells with low-dose IL-2 in the presence of anti-CD3 (OKT3) induced CD25 expression and resulted in a large increase in ICOS and PD-1 expression on CD4+CD25+Foxp3+ T cells (Supplemental Figure 2, C and D). Similar results were seen with TCR triggering in the presence of HD IL-2 in PBMCs both from patients with melanoma and from normal donors (data not shown). Thus, upregulation of ICOS on CD4+CD25+Foxp3+ cells was not triggered by IL-2 alone but was dependent on TCR activation.
Changes in the levels of ICOS+ Tregs during cycle 1 of IL-2 therapy correlated with clinical response.
The dramatic changes in the CD4+ICOS+ and ICOS+ cells with a high-cycling and suppressive Treg phenotype prompted us to determine whether changes in the frequency of these cell types in the lymphoid compartment during cycle 1 of HD IL-2 therapy correlated with the type of clinical outcome. Clinical responses were determined 21 days after the second cycle of HD IL-2 therapy as part of the normal clinical evaluation process (1, 26). Analysis of patient responses using Response Evaluation Criteria in Solid Tumors (RECIST) criteria at this time (see Supplemental Table 1) revealed that 31 out of 38 analyzed patients were nonresponders (stable disease and progressive disease) and that 7 out of 38 patients were responders (partial response or complete response), as assessed by CT scans after cycle 2 (see Supplemental Tables 1 and 2). A total of 5 out of the 31 nonresponding patients underwent only 1 cycle of HD IL-2 therapy and had to be discontinued from the therapy due to rapid disease progression, whereas all of the other nonresponders (26 out of 31 patients) and all 7 responding patients continued to cycle 2 of therapy (Supplemental Tables 1 and 2). As shown in Supplemental Table 1, the clinical response status in this cohort of patients did not change after assessment following the second cycle of HD IL-2 therapy (data not shown). The average age, percentages of each gender, numbers of IL-2 infusions during cycles 1 and 2 of IL-2 therapy, and absolute lymphocyte, monocyte, and neutrophil counts before and after cycle 1 were summarized in Supplemental Table 2. No significant differences were found in any of the measured leukocyte parameters after IL-2 treatment (Supplemental Table 2). Thus, none of these factors can account for the differences in patient clinical response.
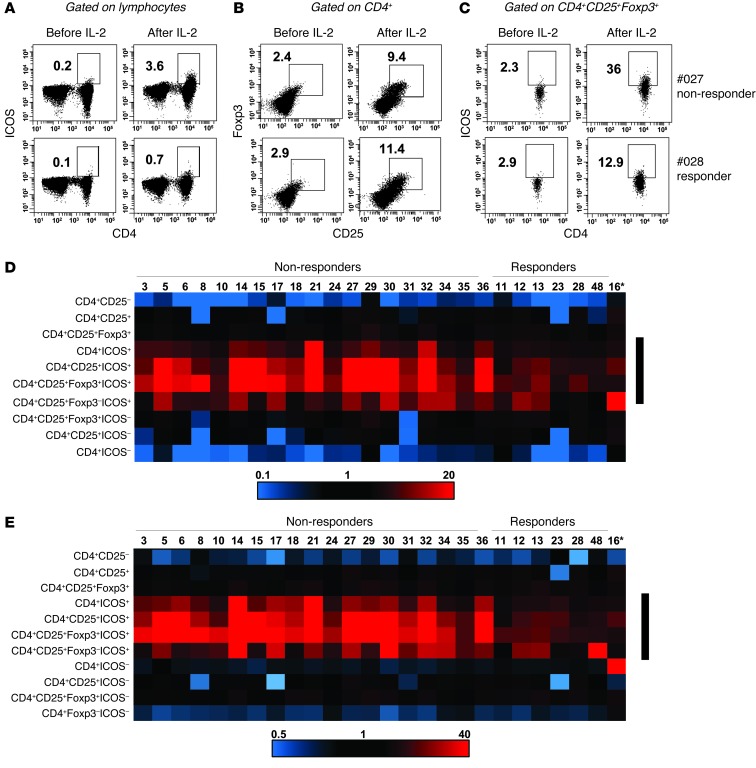
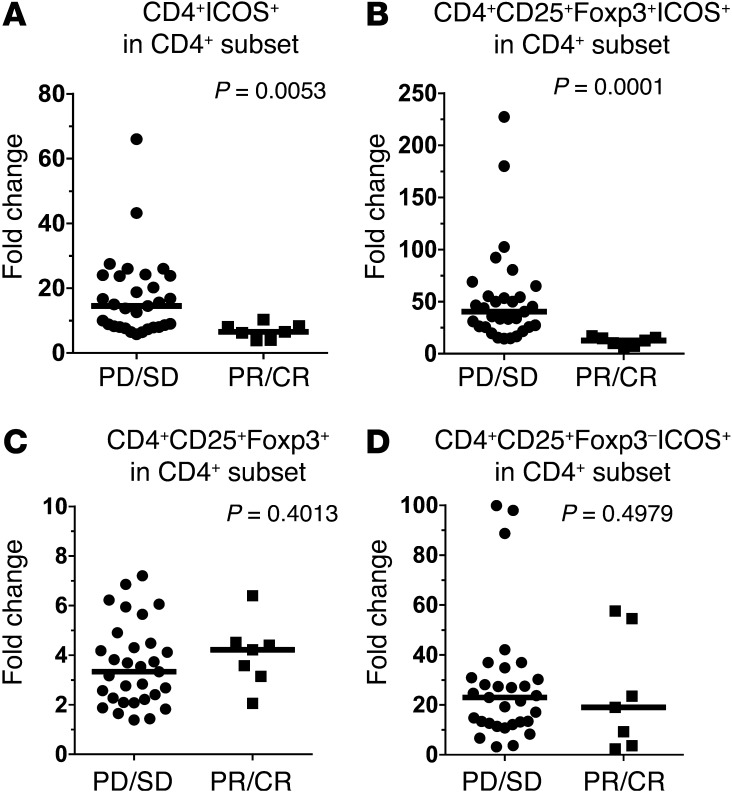
However, we noticed that responding patients had a markedly lower increase in the frequency of CD4+ICOS+ cells, as found by gating on total viable lymphocytes (Figure 6A) or on CD4+CD25+Foxp3+ cells (Figure 6C) before and after the last dose of IL-2 in cycle 1. The overall CD4+CD25+Foxp3+ (Treg) bulk subset (Figure 6B) did not show significant differences between responders and nonresponders. Next, we tracked the changes in 10 or 11 different CD4+ subsets in responders and nonresponders by heat mapping these multiple subsets in the first 19 nonresponders and 7 responders. Our analysis revealed that a substantial difference emerged between responders and nonresponders within the ICOS-expressing CD4+ cells (CD4+ICOS+, CD4+CD25+ICOS+, and CD4+CD25+Foxp3+ICOS+ cells) but not within the other subsets, with nonresponders having a clearly more intense increase in these ICOS+ subsets, which were analyzed as a percentage of the total viable lymphocyte gate (Figure 6D) as well as the CD4+ subpopulation (Figure 6E). The fold changes of CD4+ICOS+ (Figure 7A and Supplemental Figure 4A) and CD4+CD25+Foxp3+ICOS+ cells (Figure 7B) within the CD4+ T cell subset or in total lymphocytes of all 31 nonresponders and 7 responders showed that the nonresponder patient group had a significantly higher increase in both subsets.
Figure 6. Changes in CD4+ICOS+ and CD4+CD25+Foxp3+ICOS+ T cells during HD IL-2 therapy in responding and nonresponding patients.
(A) Frequencies of CD4+ICOS+ T cells in the total viable lymphocyte gate are shown before and after cycle 1 of HD IL-2 therapy for a representative nonresponder (no. 027) and responder (no. 028). (B) Changes in the frequency of CD25+Foxp3+ in the gated CD4+ T cell subpopulation and (C) ICOS+ cells within the gated CD4+CD25+Foxp3+ subpopulation before and after cycle 1 of HD IL-2 therapy for the same nonresponder and responder. Frequencies of indicated cell subsets are indicated in A–C. (D and E) Analysis of the fold changes in different ICOS+ and ICOS– subsets during HD IL-2 therapy in nonresponding (n = 19) and responding (n = 7) patients. The indicated cell subsets were analyzed by flow cytometry before and 2 days after cycle 1 of therapy, and the fold change of each population was heat mapped by using an Excel conditional formatting program. Heat diagrams show the fold changes in the indicated T cell subsets as (D) the percentage of total peripheral blood lymphocytes in nonresponder and responders and (E) a percentage of the CD4+ subpopulation in nonresponders and responders (patient numbers are shown at the top of the heat map). The black bars on the right of each heat diagram indicate the CD4+ICOS+ subsets showing the greatest differences between responding and nonresponding patients. The asterisk denotes patient number 16 who received prior vaccination with recombinant MAGE-A3 vaccine before HD IL-2 therapy.
Figure 7. CD4+CD25+Foxp3+ICOS+ Tregs in patients who eventually respond to therapy increased to a lesser extent after HD IL-2 cycle 1.
Comparison of the fold change of (A) CD4+ICOS+ cells, (B) CD4+CD25+Foxp3+ICOS+ cells, (C) CD4+CD25+Foxp3+ cells, and (D) CD4+CD25+Foxp3–ICOS+ cells as a percentage of the total CD4+ compartment between nonresponders (progressive disease/stable disease [PD/SD]) (n = 31) and responders (partial response/complete response [PR/CR]) (n = 7). Fold change was calculated by dividing the percentage of each indicated subset before HD IL-2 therapy by the percentage after therapy. The horizontal bar in each scatter plot represents the median value of the fold change. Statistical analyses were performed with 2-tailed Mann-Whitney test for unpaired data. P < 0.05 was considered significant.
In contrast, although the overall bulk Treg (CD4+CD25+Foxp3+) (Figure 7C) and non-Treg (CD4+CD25+Foxp3–ICOS+) subsets (Figure 7D) increased within the CD4+ subset or within the total lymphocyte gate, the fold change was not correlated to clinical response after HD IL-2 therapy. In addition, the CD4+CD25+ICOS+ subset had a significantly lower increase in responders after cycle 1 of therapy (Supplemental Figure 4B). A similar change was observed in ICOS+ Tregs but not in CD4+CD25+Foxp3–ICOS+ T cells when we plotted the fold change as an absolute number in the blood (data not shown). The fold increases in ICOS+ Tregs in nonresponders and responders were 63.2 fold and 20.1 fold, respectively (P = 0.0129) (Supplemental Figure 4, C and D). Notably, no statistically significant differences in the absolute numbers of ICOS+ Tregs and CD4+CD25+Foxp3–ICOS+ cells at baseline before therapy were found between responders and nonresponders (data not shown). Thus, these changes in ICOS+ Tregs associated with the type of clinical response were not due to differences in baseline levels of these cells in responders, but rather to a higher rate of expansion of the cells in nonresponding patients.
Furthermore, we found that the differences in ICOS+ Treg fold change were not related to the total number of doses in cycle 1 or 2 given to either group of patients (Supplemental Table 2 and Supplemental Figure 5). After cycle 1, the absolute number of ICOS+ Tregs and the frequency of these cells within the CD4+ T cell subset or the total lymphocyte population remained statistically significant, even when 5 out of the 31 nonresponding patients who had received only 1 cycle of HD IL-2 were excluded from analysis (data not shown). Although nonresponding patients had a higher increase in CD56+CD16–PRF1+ NK cells (median, 25 fold) over responders (median, 15 fold), this was not statistically significant (data not shown). Interestingly, a significantly higher fold increase in the CD8+ICOS+ cells (mainly Foxp3– cells) but not CD8+Foxp3+ population was observed in nonresponders compared with that in responders (Supplemental Figure 6).
Finally, we looked at changes in the ratio of ICOS+ non-Tregs to ICOS+ Tregs, as the percentage of CD4+ T cell subset, and did not find significant differences between responders and nonresponders before and after HD IL-2 therapy (Supplemental Figure 7). The ratios of CD4+CD25+Foxp3–ICOS+ to CD4+CD25–Foxp3+ICOS+ cells and CD4+CD25+Foxp3– to CD4+CD25–Foxp3+ cells at baseline and after HD IL-2 therapy were also not correlated with clinical outcome (Supplemental Figure 8, A and B). We also looked at the ratio of CD56+CD16–PRF1+ NK cells and CD8+ICOS+ cells to ICOS+ Tregs during HD IL-2 cycle 1 but did not find any differences between responders and nonresponders (data not shown). Thus, only the magnitude of changes in CD4+CD25+Foxp3+ICOS T cells during the first cycle of HD IL-2 therapy was indicative of the type of response to therapy.
Discussion
In this article, we have shown that one of the most significant immunological changes induced by HD IL-2 therapy in patients with metastatic melanoma is a large expansion of the CD4+ICOS+ subset, consisting predominantly of CD4+CD25+Foxp3+ICOS+ T cells having the hallmarks of highly suppressive Tregs, including high levels of CD39, TGF-β expression, and an ability to produce IL-10. Importantly, our findings indicate that monitoring the CD4+ICOS+ T cell population, especially a subset of ICOS+CD39+ Tregs, in the peripheral blood during the initial cycle of HD IL-2 therapy is a predictive biomarker to discern which patients will eventually benefit from HD IL-2 therapy after further cycles of therapy needed to induce meaningful long-term clinical responses. This could provide a powerful clinical management tool to select patients to continue or not with additional cycles of therapy given the toxicities associated with HD IL-2 therapy. In addition, the CD4+ICOS+ and ICOS+ Treg markers, including CD39, could also be combined with other potential IL-2 therapy biomarkers (52) to yield a biomarker “fingerprint” that may improve predictive power even further. Verification of the predictive value of ICOS+ Tregs will require a multicenter format in which a larger set of patients than is possible at a single center can be studied using standardized sample collection time points and blood processing and staining methods similar to those used here.
Interestingly, an increase of CD4+ICOS+ effector cells and the ratio of ICOS+ effector cells to ICOS+ Tregs have been described in patients with breast and bladder cancer after anti-CTLA4 treatment (33, 53). Here, we also noticed a similar increase of this ratio in HD IL-2–treated patients; however, it did not associate with clinical outcome. The expanded CD4+ICOS+ cells within the Treg subset, as described here in patients treated with HD IL-2, had unique features that clearly distinguished these cells from the “bulk” Treg population or ICOS– Tregs. These include the expression of higher levels of CD25, Foxp3, and cell surface TGF-β/LAP complexes but a lower level of CD127. Moreover, this ICOS+ Treg subset is mostly CD39+CD45RA–/lo and capable of producing IL-10 (49, 50, 54–56). When we stained Tregs with Helios, a marker that has been shown to be expressed by activated and proliferating Tregs (57), we found that approximately 80%–90% of the ICOS+ Tregs expressed Helios. Interestingly, there was a trend of higher Helios expression levels in ICOS+ Tregs than in ICOS– Tregs (data not shown). These findings indicate that the highly cycling ICOS+ Tregs induced by HD IL-2 therapy represent a more activated form of Treg, with more potent suppressive properties than ICOS– Tregs.
Although we were unable to study the suppressive function of these IL-2–induced ICOS+ Tregs on T or NK cells ex vivo due to the limitations in the size of blood samples available from our patients, the finding that higher levels of expansion of this Treg subset with the aforementioned phenotype during IL-2 therapy and its correlation with poor clinical response strongly support the immunosuppressive nature of these cells. Moreover, our previous study showed that CD4+CD25+ T cells expanded after TCR stimulation and together with ICOS/ICOSL ligation were able to suppress the proliferation of activated autologous CD4+CD25– T cells in classic in vitro suppression assays (38). Similar results have also been described in Tregs isolated from metastatic melanomas in which ICOS+ Tregs showed a more potent suppressive function than other Treg subsets (36). One marker in particular that emerged to be significant was CD39, which has recently been reported as a marker distinguishing suppressive Tregs from nonsuppressive Tregs in patients with HIV and in patients with remitting-relapsing multiple sclerosis (48, 58). CD39 is an ecto-5′-nucleotidase on the surface of some activated CD4+ T cell subsets that converts ATP to ADP and AMP, thereby removing available ATP in the microenvironment, which deprives T cells from a potent stimulatory signal. Moreover, CD39 acts in concert with CD73, another ecto-5′-nucleotidase usually coexpressed with CD39 that cleaves ADP and AMP releasing adenosine and phosphate, the former of which is a highly immunosuppressive molecule in effector T cells (48–50). In a preliminary study of 5 IL-2 responders and 15 nonresponders, we found that a lower expansion of ICOS+CD39+ Tregs correlated with clinical response, while ICOS+CD39– Tregs did not (G.C. Sim et al., unpublished observations). Thus, the higher expression of CD39 (together with CD73) on ICOS+ Tregs is emerging to be another key biomarker that needs further investigation.
Another marker that is more highly expressed on the highly cycling ICOS+ Tregs in the patients treated with IL-2 than their ICOS– counterparts was PD-1. Due to unavailable tumor materials, we were unable to determine the expression of programmed cell death 1 ligand 1 (PD-L1) in the tumor in this cohort of patients. It would be of interest in future studies to investigate whether higher fractions of PD-1+ICOS+ Tregs were correlated to the PD-L1 expression of tumor and whether PD-1 ligation by PD-L1 on these PD-1hiICOS+ Tregs positively or negatively regulates their expansion and suppressive function. This is especially relevant, since, as with other activated T cell subsets, PD-1 may actually negatively regulate the function of highly activated Tregs, as recently found in chronic hepatitis C virus–infected patients (47).
At present, how ICOS+ Tregs arise in IL-2–treated patients with melanoma is unclear. However, our data raise a number of possibilities. First, the detection of a high percentage of ICOS+ Tregs in melanoma metastases from stage IV patients by others (36) and us (38) suggests that these cells could be a preexisting activated Treg subset that were possibly recruited into the tumor microenvironment and/or activated and expanded in situ in the tumor microenvironment. HD IL-2 therapy may further induce these cells to divide and recirculate back into the bloodstream after cessation of IL-2 therapy. Second, ICOS+ Tregs may arise from the “bulk” Treg (mostly ICOS–) population upon TCR activation after contact with self antigens and tumor antigens during IL-2 therapy and induce an activation phenotype of Tregs, as mentioned earlier. Indeed, as shown above, we noted changes in ICOS, PD-1, CD25, Foxp3, CD45RA, and CD127 according to what would be expected after additional activation of a Treg. A recent study by Miyara et al. (41) showed that ICOS expression is associated with an activated CD4+CD25hiFoxp3hiCD45RA–/lo subset in normal donors. These phenotypic properties closely resemble the ICOS+ Tregs found in our patients treated with IL-2 here. It is also possible that a fraction of ICOS+ Tregs may have arisen from CD4+Foxp3– T cells (induced Tregs). In this case, activated CD4+CD25+ICOS+ T cells may have trafficked into tumors then converted by TGF-β into suppressive Foxp3+ cells that spilled back out into the peripheral blood after cessation of IL-2 therapy. It remains unknown whether these ICOS+ Tregs that exhibit a highly activated and suppressive phenotype could be HLA-DR–expressing terminally differentiated effector Tregs (59). It is possible that these HLA-DR+ effector Tregs are similar to the ICOS+ Tregs we found here, since both of these cells are able to express higher CD25 and are more suppressive than other Tregs.
Last, Tregs have been recently categorized into 2 subsets in healthy donors and in patients with cancer, and these subsets can be distinguished by their ICOS expression (33, 35). ICOS ligation was found to be critical to inducing the expansion and survival of the ICOS+ Treg subset (35). Indeed, we have shown that ICOS/ICOSL ligation induces activation and expansion of a Treg subset that is capable of producing IL-10 (38). Furthermore, these ICOS+ Tregs can also express higher levels of cell surface TGF-β/LAP, suggesting this Treg subset is similar to those ICOS- and TGF-β/LAP–expressing cells found in melanoma metastases (36). Thus, all of the available evidence indicates that the ICOS+ Tregs that expanded during HD IL-2 therapy are highly suppressive cells that could curtail the antitumor immune response in these patients. Furthermore, several studies have shown that ICOSL-expressing plasmacytoid DCs in the tumor microenvironment can promote the immunosuppressive function and accumulation of Tregs via ICOS costimulation (59, 60). Together with these findings, our data point to a plausible model in which Treg subsets in the tumor undergo activation and express ICOS, followed by ICOS costimulation via the interaction with ICOSL+ plasmacytoid DCs (60, 61) and/or melanoma cells in the tumor microenvironment that further drives ICOS+ Treg activation (38). Upon HD IL-2 therapy, these activated ICOS+ Tregs are then driven to divide rapidly. In addition, IL-2 itself may further drive TCR-independent activation of ICOS+ Tregs through the transcriptional activation of Foxp3 and STAT5 (62, 63). It is also possible that a significant fraction of ICOS+ Tregs is already present in tissue and that these Tregs are then “pushed” back into circulation along with other lymphocytes, as part of the “lymphocytosis” that occurs in these patients after cessation of IL-2 therapy (26).
Another critical question raised by our data is why patients responding to HD IL-2 therapy exhibited lower increases in the frequency of ICOS+ Tregs using all of the different measurement parameters used in our studies. Subtle differences in IL-2 signaling mediated by polymorphisms in the IL-2R complex or its associated downstream signaling molecules as a hard-wired genetic mechanism may be responsible. Alternatively, the ICOS/ICOSL pathways driving gene expression in Tregs and their activation may be responsible for the differential ICOS+ Treg expansion between IL-2 responders and nonresponders regulating the activity of antitumor effector cells (especially NK cells) that may counterbalance the “Treg surge” during the initial cycles of HD IL-2 therapy. About 50% of tumors from patients with metastatic melanoma expressed ICOSL (38), suggesting that differential expression of this and other costimulatory molecules by tumor cells or APC may drive ICOS+ Treg expansion to different extents (60–65). Tracking ICOSL and ICOS+ Tregs in tumor biopsies in future studies should help shed light on this possibility. Another possibility for the differing levels of ICOS+ Tregs after IL-2 in these patients is that responding patients may have lower baseline frequencies of ICOS+ and bulk Treg populations. However, the baseline frequencies of CD4+ICOS+ and ICOS+ Tregs were not significantly different between responders and nonresponders. Nevertheless, it is possible that nonresponding patients have higher starting numbers and frequencies of ICOS+ Tregs in their tumors and other tissues (undetectable using assays on peripheral blood). This higher number of tumor- or tissue-resident Tregs would be further expanded during the first IL-2 therapy cycle, with many migrating into the blood after cessation of IL-2 dosing. This would further elevate the baseline pool of Tregs for the next IL-2 therapy cycle and also predicts that successive HD IL-2 therapy cycles may incrementally increase the ICOS+ Treg pool, leading to increased immunosuppression. This notion will need to be tested in future studies and may be relevant to other forms of immunotherapies that modulate Treg levels.
Methods
Patient characteristics, treatment, and response evaluation.
Patients with stage IIIC or IV (M1a-M1c) metastatic melanoma were treated with HD recombinant human IL-2 (720,000 IU/kg; Proleukin, Novartis) administered by 15-minute bolus infusion every 8 hours to a maximum of 15 total doses or until nonresolving grade 4 toxicity. Each course consisted of 2 cycles of therapy at 3-week intervals (1, 17, 27). Clinical response was determined by radiologists after the second cycle of therapy with use of CT scans of the chest, abdomen, and pelvis and magnetic resonance imaging of the brain based on National Cancer Institute (NCI) RECIST criteria (66). Patients underwent imaging within 4 weeks before starting therapy. Exclusion criteria for patients included an age of less than 18 years and any prior IL-2 therapy. Thirty-eight patients (7 responders and 31 nonresponders) were evaluated for changes in CD4+ T cell and Treg phenotypic markers immediately before the first bolus IL-2 infusion and 2 days after the first cycle of HD IL-2 therapy during the rebound period after IL-2 therapy, when a large influx of lymphocytes into the blood occurs (1, 17, 27). The pretreatment demographics and clinical status of these patients as well as a summary of their clinical response to HD IL-2 therapy are shown in Supplemental Table 1.
Blood sampling and processing.
Blood samples were collected before and after the first cycle of HD IL-2 therapy (HD IL-2 cycle 1) 1 day before the start of therapy and 2 days after the last bolus IL-2 infusion from patients undergoing HD IL-2 therapy between April 2007 and December 2012. In some cases, an additional blood sample was collected 1 day before the second cycle of HD IL-2 (HD IL-2 cycle 2). Whole blood (40–50 ml total) was collected, and PBMCs were isolated using Ficoll-Hypaque (Sigma-Aldrich) within 2 hours after collection. PBMCs were cryopreserved in 10% DMSO, 90% human AB serum (Valley Biomedical) until analysis. Absolute lymphocyte, monocyte, and granulocyte (neutrophil, eosinophil, basophil) counts were obtained with the use of an automated cell counter (Beckman Coulter) in the Department of Pathology and Laboratory Medicine at MD Anderson Cancer Center.
FACS analysis of PBMC and Treg populations.
PBMCs were thawed and stained with a set of previously validated fluorochrome-conjugated antibodies against markers distinguishing the different lymphocyte lineages (T, B, and NK cell) and markers distinguishing different states of T cell differentiation, memory, and effector cell subsets. These markers included CD4, CD8, CD3, CD45RA, CD45RO, CD14, CD11c, CD16, CD19, CD56, CD27, CD28, CD62L, CD25, CD122, CD57, NKG2D, ICOS, granzyme B, and perforin (all from BD Biosciences). Tregs were stained with use of anti-CD4, anti-CD8, anti-CD25, ICOS, and Foxp3 (all from eBioscience) as well as other indicated markers such as CD45RA, OX-40, CD73, CD39, and Ki67 (all from BD Biosciences); PD-1 and TGF-β/LAP (both from BioLegend); and Helios (eBioscience). All stained cells were washed and resuspended in 300 μl D-PBS, 1% p-formaldehyde, and 0.25% ethanol and stored for a maximum of 24 hours before acquisition on a FACSCanto II flow cytometer using FACSDiva software (Becton Dickinson). Acquired samples were analyzed as described in Supplemental Methods with the use of FACSDiva or FlowJo software (Treestar).
PBMC activation and intracellular cytokine staining.
PBMCs were cultured as described in Supplemental Methods and stimulated with 50 ng/ml PMA plus 2 μg/ml ionomycin for IFN-γ/IL-2 or IL-10 detection for 6 hours or 24 hours, respectively. Cells were then stained and acquired on a FACScanto II flow cytometer using FACSDiva software (Supplemental Methods).
Proliferation assay.
For the dye dilution proliferation assay, PBMCs were freshly isolated from blood collected from HD IL-2–treated patients 2 days after their last dose of therapy. 1 × 106 cells were labeled with Cell Proliferation Dye eFluor 670 according to the manufacturer’s instructions (eBioscience) (see Supplemental Methods) and then cultured in the presence of IL-2 at 3,000 IU/ml for 5 days. Cells were then harvested and stained for markers of Tregs as indicated. Flow cytometric data were acquired on a FACSCanto II (Becton Dickinson) and analyzed using FlowJo software (Treestar).
Statistics.
Two-tailed Mann-Whitney U test, 2-tailed Wilcoxon matched paired test, or 1-way ANOVA in conjunction with Tukey’s test was used to determine statistical significance (95% confidence interval), and a P value of less than 0.05 was considered statistically significant. Arcsine transformation was performed on percentage values of cell populations for multiple group statistical analysis.
Study approval.
All patients enrolled in this study provided informed consent, and the collection and use of patient blood samples for laboratory analysis was approved by the Institutional Review Board at MD Anderson Cancer Center (protocol no. LAB06-0762).
Supplementary Material
Acknowledgments
We thank Prometheus Therapeutics and Diagnostics and Novartis for their generous support of this study through an unrestricted research grant (CPRL002AUS08/10PLK02) and the NCI for their Melanoma SPORE Developmental Grant (5 P50 CA093459-05-DRP14). We are also grateful for the postdoctoral fellowship from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation to Geok Choo Sim. Additional support came from infrastructure funds supporting the Immunomonitoring Core Lab as part of the MD Anderson Cancer Center Support Grant (5P30 CA016672 31) from the NCI. We thank Willem Overwijk, Chen Dong, and Tamara Locke for reviewing the manuscript. We are also grateful to Diane Kentor, Tejal Patel, Minerva Griffin, and Jennifer Perez for processing and cryopreservation of patient blood samples. We wish to dedicate this work to the memory of Diane Kentor, who worked tirelessly in the lab for our patients over many years and succumbed to cancer recently.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2014;124(1):99–110. doi:10.1172/JCI46266.
References
- 1.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 2.Dutcher J. Current status of interleukin-2 therapy for metastatic renal cell carcinoma and metastatic melanoma. Oncology (Williston Park). 2002;16(11 suppl 13):4–10. [PubMed] [Google Scholar]
- 3.Kirkwood JM, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26(20):3445–3455. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 4.Whittington R, Faulds D. Interleukin-2. A review of its pharmacological properties and therapeutic use in patients with cancer. Drugs. 1993;46(3):446–514. doi: 10.2165/00003495-199346030-00009. [DOI] [PubMed] [Google Scholar]
- 5.Fyfe G, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28(3):258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartzentruber DJ, et al. gp100 peptide vaccine interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiSanto JP. Cytokines: shared receptors, distinct functions. Curr Biol. 1997;7(7):R424–R426. doi: 10.1016/S0960-9822(06)00208-9. [DOI] [PubMed] [Google Scholar]
- 11.Imada K, et al. Stat5b is essential for natural killer cell-mediated proliferation cytolytic activity. J Exp Med. 1998;188(11):2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriggl R, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10(2):249–259. doi: 10.1016/S1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 13.Harlin H, et al. Tumor progression despite massive influx of activated CD8(+) T cells in a patient with malignant melanoma ascites. Cancer Immunol Immunother. 2006;55(10):1185–1197. doi: 10.1007/s00262-005-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letsch A, et al. High frequencies of circulating melanoma-reactive CD8+ T cells in patients with advanced melanoma. Int J Cancer. 2000;87(5):659–664. doi: 10.1002/1097-0215(20000901)87:5<659::AID-IJC7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.McMannis JD, et al. In vivo effects of recombinant IL-2. I. Isolation of circulating Leu-19+ lymphokine-activated killer effector cells from cancer patients receiving recombinant IL-2. J Immunol. 1988;140(4):1335–1340. [PubMed] [Google Scholar]
- 16.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cesana GC, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24(7):1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67(15):7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 19.Brandenburg S, et al. IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38(6):1643–1653. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 20.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, interleukin-2. J Immunother. 2005;28(2):120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaput N, et al. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol. 2007;179(8):4969–4978. doi: 10.4049/jimmunol.179.8.4969. [DOI] [PubMed] [Google Scholar]
- 22.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32(1–3):155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 23.Ghiringhelli F, Menard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 24.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20(2):241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royal RE, et al. Correlates of response to IL-2 therapy in patients treated for metastatic renal cancer melanoma. Cancer J Sci Am. 1996;2(2):91–98. [PubMed] [Google Scholar]
- 27.Phan GQ, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19(15):3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 28.Caligiuri MA, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91(1):123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehniger TA, et al. Potential mechanisms of human natural killer cell expansion in vivo during low-dose IL-2 therapy. J Clin Invest. 2000;106(1):117–124. doi: 10.1172/JCI6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SK, Gasser S. The role of natural killer cells in cancer therapy. Front Biosci (Elite Ed). 2010;2:380–391. doi: 10.2741/e98. [DOI] [PubMed] [Google Scholar]
- 31.Poli A, et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coquerelle C, et al. Anti-CTLA-4 treatment induces IL-10-producing ICOS+ regulatory T cells displaying IDO-dependent anti-inflammatory properties in a mouse model of colitis. Gut. 2009;58(10):1363–1373. doi: 10.1136/gut.2008.162842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199(11):1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vonderheide RH, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 35.Ito T, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus periphery. Immunity. 2008;28(6):870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss L, et al. Expression of ICOS on human melanoma-infiltrating CD4+CD25highFoxp3+ T regulatory cells: implications impact on tumor-mediated immune suppression. J Immunol. 2008;180(5):2967–2980. doi: 10.4049/jimmunol.180.5.2967. [DOI] [PubMed] [Google Scholar]
- 37.Vocanson M, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of T(h)17/T(h)1 and regulatory T cells. J Allergy Clin Immunol. 2010;126(2):280–289. doi: 10.1016/j.jaci.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Orozco N, et al. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Cancer Res. 2010;70(23):9581–9590. doi: 10.1158/0008-5472.CAN-10-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, et al. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37(1):129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler SF. FOXP3: not just for regulatory T cells anymore. Eur J Immunol. 2007;37(1):21–23. doi: 10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- 41.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in self-tolerance autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Wing K, Miyara M. Regulatory T cells — a brief history and perspective. Eur J Immunol. 2007;37(suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 44.Venken K, et al. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180(9):6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- 45.Liu W, et al. CD127 expression inversely correlates with FoxP3 suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franceschini D, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119(3):551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radziewicz H, Dunham RM, Grakoui A. PD-1 tempers Tregs in chronic HCV infection. J Clin Invest. 2009;119(3):450–453. doi: 10.1172/JCI38661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borsellino G, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP immune suppression. Blood. 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher JM, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells are impaired in multiple sclerosis. J Immunol. 2009;183(11):7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 50.Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346(1–2):55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kryczek I, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69(9):3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 52.Sabatino M, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27(16):2645–2652. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liakou CI, et al. CTLA-4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105(39):14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin D, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70(6):2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70(16):6407–6411. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dwyer KM, et al. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3(1–2):171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akimova T, et al. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6(8):e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulze Zur Wiesch J, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of Foxp3+ T regulatory cells correlates with progressive disease. J Virol. 2011;85(3):1287–1297. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 60.Conrad C, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012;72(20):5240–5249. doi: 10.1158/0008-5472.CAN-12-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faget J, et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4+ T cells. Cancer Res. 2012;72(23):6130–6141. doi: 10.1158/0008-5472.CAN-12-2409. [DOI] [PubMed] [Google Scholar]
- 62.Eckerstorfer P, et al. Proximal human FOXP3 promoter transactivated by NF-kappaB negatively controlled by feedback loop SP3. Mol Immunol. 2010;47(11–12):2094–2102. doi: 10.1016/j.molimm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ito T, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204(1):105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janke M, et al. Eminent role of ICOS costimulation for T cells interacting with plasmacytoid dendritic cells. Immunology. 2006;118(3):353–360. doi: 10.1111/j.1365-2567.2006.02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.