Abstract
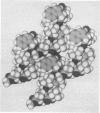
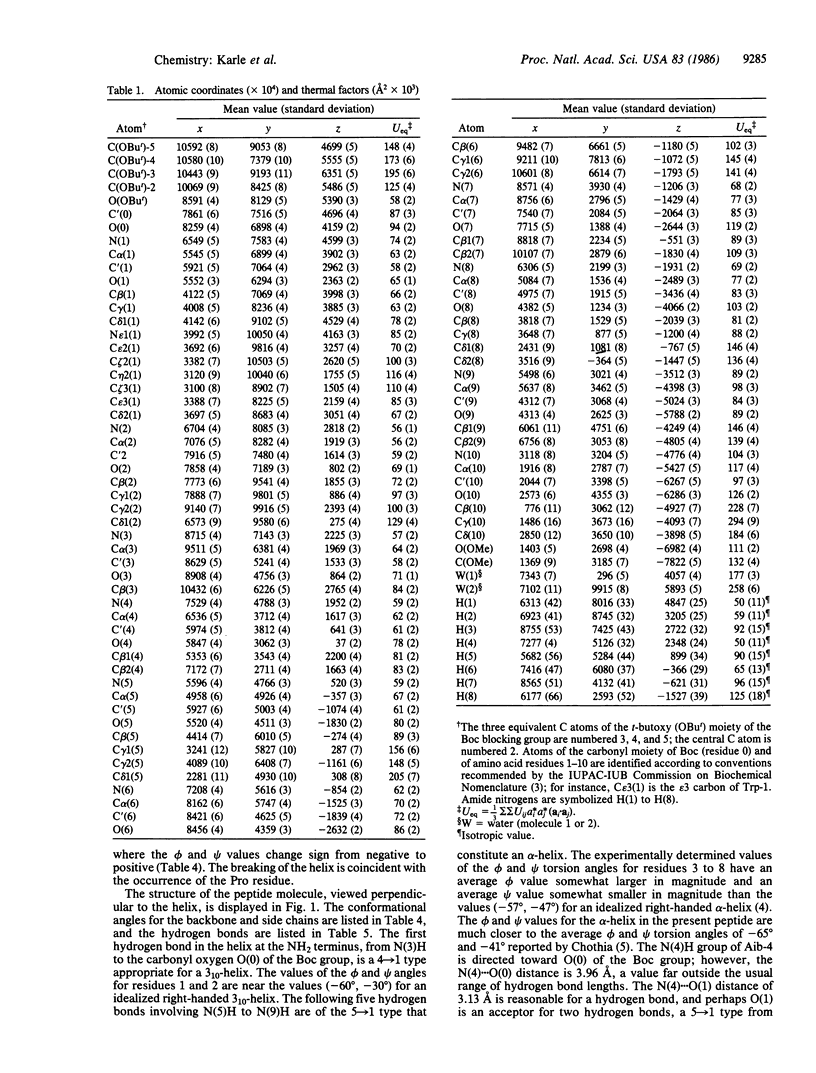
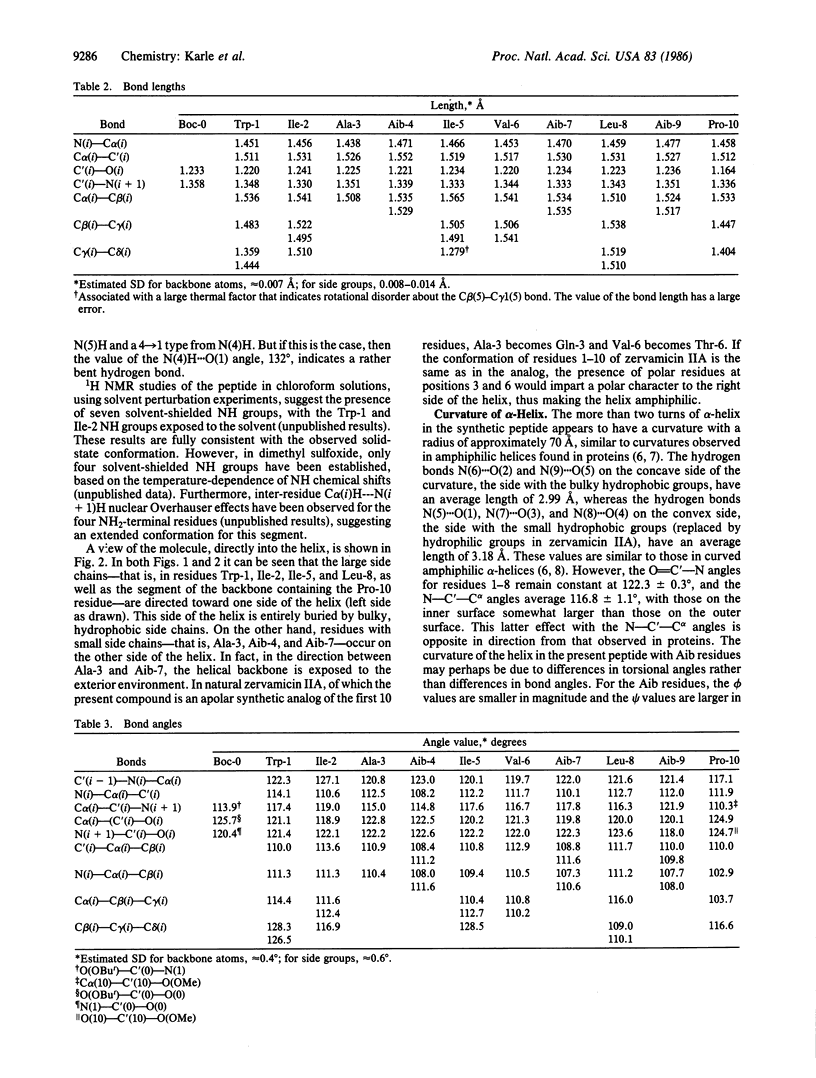
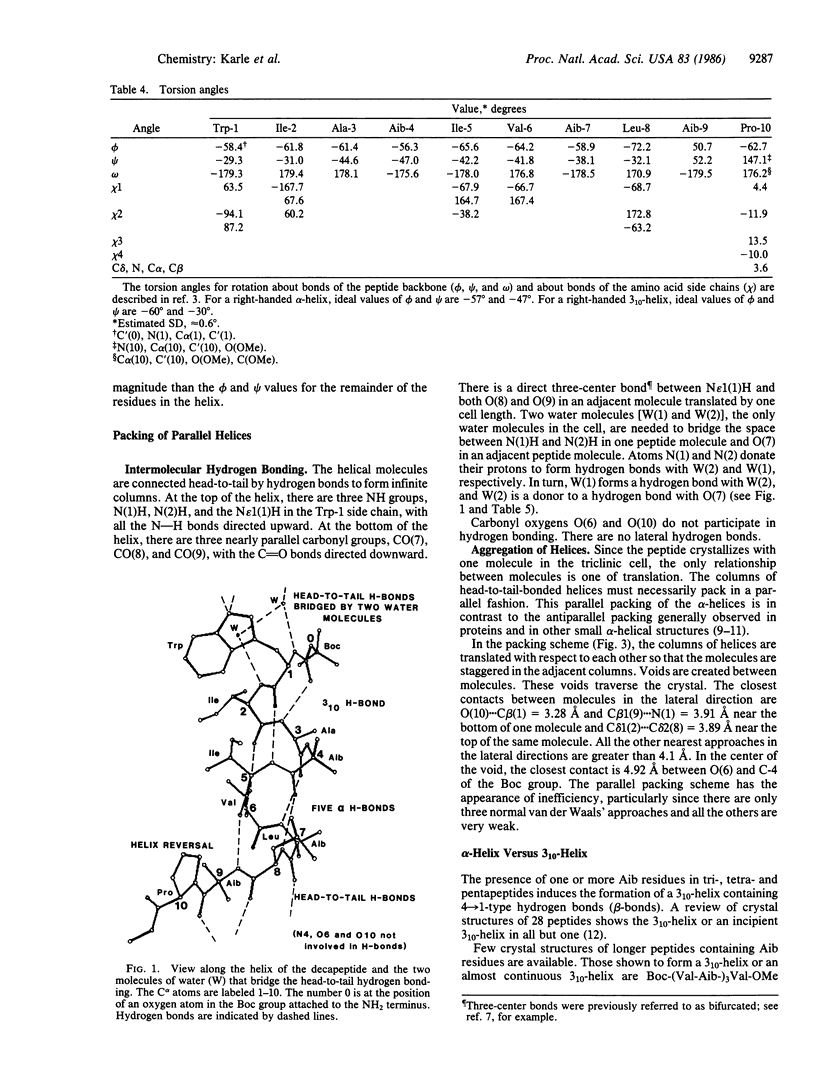
An apolar synthetic analog of the first 10 residues at the NH2-terminal end of zervamicin IIA crystallizes in the triclinic space group P1 with cell dimensions a = 10.206 +/- 0.002 A, b = 12.244 +/- 0.002 A, c = 15.049 +/- 0.002 A, alpha = 93.94 +/- 0.01 degrees, beta = 95.10 +/- 0.01 degrees, gamma = 104.56 +/- 0.01 degrees, Z = 1, C60H97N11O13 X 2H2O. Despite the relatively few alpha-aminoisobutyric acid residues, the peptide maintains a helical form. The first intrahelical hydrogen bond is of the 3(10) type between N(3) and O(0), followed by five alpha-helix-type hydrogen bonds. Solution 1H NMR studies in chloroform also favor a helical conformation, with seven solvent-shielded NH groups. Continuous columns are formed by head-to-tail hydrogen bonds between the helical molecules along the helix axis. The absence of polar side chains precludes any lateral hydrogen bonds. Since the peptide crystallizes with one molecule in a triclinic space group, aggregation of the helical columns must necessarily be parallel rather than antiparallel. The packing of the columns is rather inefficient, as indicated by very few good van der Waals' contacts and the occurrence of voids between the molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bavoso A., Benedetti E., Di Blasio B., Pavone V., Pedone C., Toniolo C., Bonora G. M. Long polypeptide 3(10)-helices at atomic resolution. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1988–1992. doi: 10.1073/pnas.83.7.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T., Barlow D., Borkakoti N., Thornton J. Solvent-induced distortions and the curvature of alpha-helices. Nature. 1983 Nov 17;306(5940):281–283. doi: 10.1038/306281a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti P., Bernard M., Rees D. C. Peptide-bond distortions and the curvature of alpha-helices. Biopolymers. 1986 Jun;25(6):1087–1093. doi: 10.1002/bip.360250609. [DOI] [PubMed] [Google Scholar]
- Chothia C. Principles that determine the structure of proteins. Annu Rev Biochem. 1984;53:537–572. doi: 10.1146/annurev.bi.53.070184.002541. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]