Abstract
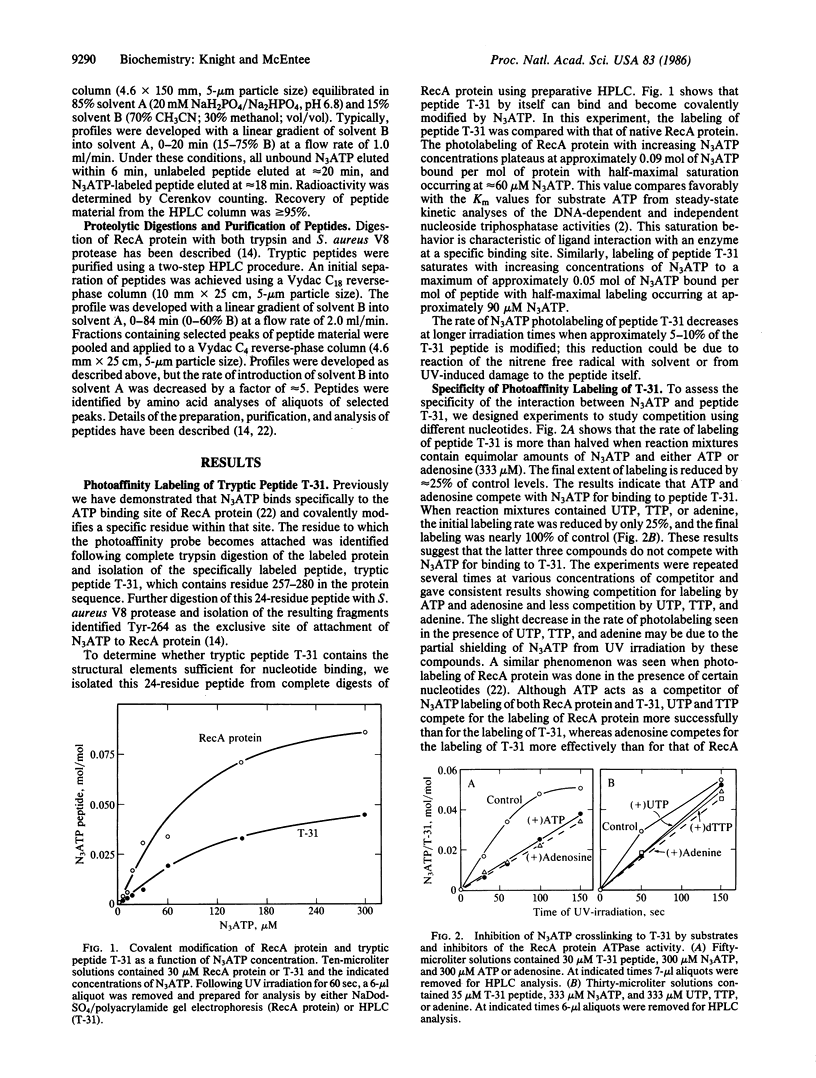
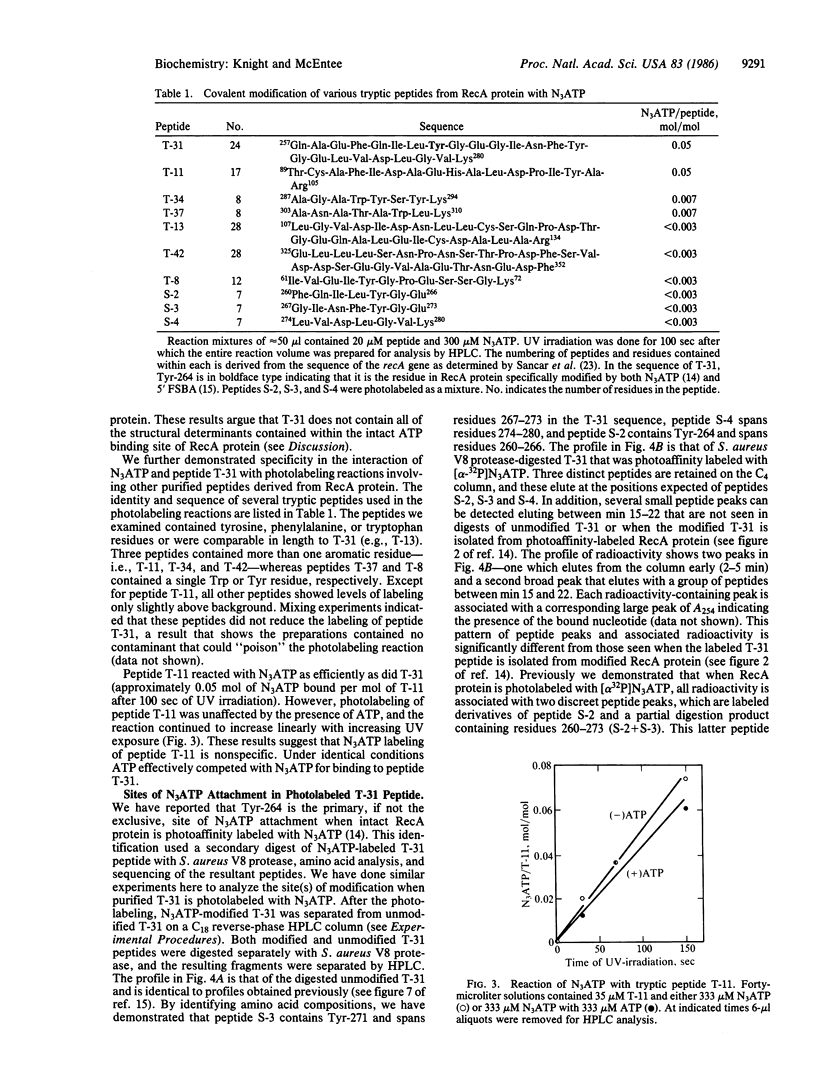
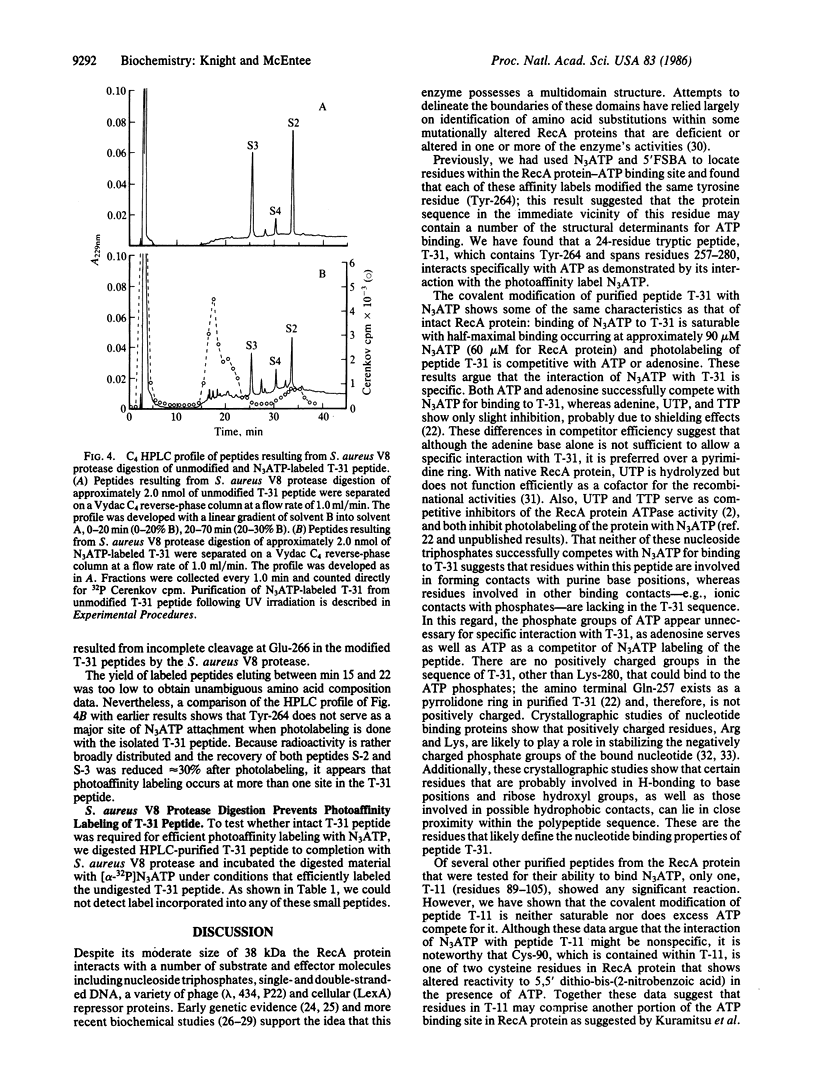
We have recently demonstrated that two ATP analog affinity labels, 8-azidoadenosine 5'-triphosphate (N3ATP) and 5'-p-fluorosulfonylbenzoyladenosine (5'FSBA), covalently modify RecA protein of Escherichia coli at a specific tyrosine residue (Tyr-264) located within a 24-residue tryptic peptide (T-31) spanning residues 257-280. Here we show that N3ATP efficiently modifies purified peptide T-31 and show that the interaction is specific by the following criteria: photolabeling of peptide T-31 is saturable with respect to the N3ATP concentration; photolabeling is competitive with ATP and adenosine but not with adenine, UTP, or TTP; and other peptides derived from RecA protein were poor substrates for photolabeling except for one fragment that showed a nonspecific interaction with the photoaffinity analog. Analysis of N3ATP-modified T-31 shows that the photolabel attaches to more than one site within the peptide. These data argue that peptide T-31 contains some sites of contact for adenine and ribose moieties of ATP when it is bound to RecA protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox M. M., Lehman I. R. recA protein-promoted DNA strand exchange. Stable complexes of recA protein and single-stranded DNA formed in the presence of ATP and single-stranded DNA binding protein. J Biol Chem. 1982 Jul 25;257(14):8523–8532. [PubMed] [Google Scholar]
- Cox M. M., McEntee K., Lehman I. R. A simple and rapid procedure for the large scale purification of the recA protein of Escherichia coli. J Biol Chem. 1981 May 10;256(9):4676–4678. [PubMed] [Google Scholar]
- Cox M. M., Soltis D. A., Lehman I. R., DeBrosse C., Benkovic S. J. ADP-mediated dissociation of stable complexes of recA protein and single-stranded DNA. J Biol Chem. 1983 Feb 25;258(4):2586–2592. [PubMed] [Google Scholar]
- Dressler D., Potter H. Molecular mechanisms in genetic recombination. Annu Rev Biochem. 1982;51:727–761. doi: 10.1146/annurev.bi.51.070182.003455. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Allison W. S. Identification of a tyrosine residue at a nucleotide binding site in the beta subunit of the mitochondrial ATPase with p-fluorosulfonyl[14C]-benzoyl-5'-adenosine. J Biol Chem. 1978 Sep 10;253(17):6100–6106. [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. ATP-binding site of adenylate kinase: mechanistic implications of its homology with ras-encoded p21, F1-ATPase, and other nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):907–911. doi: 10.1073/pnas.83.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. C., Kuby S. A., Mildvan A. S. NMR studies of the MgATP binding site of adenylate kinase and of a 45-residue peptide fragment of the enzyme. Biochemistry. 1985 Aug 13;24(17):4680–4694. doi: 10.1021/bi00338a030. [DOI] [PubMed] [Google Scholar]
- Haley B. E., Hoffman J. F. Interactions of a photo-affinity ATP analog with cation-stimulated adenosine triphosphatases of human red cell membranes. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3367–3371. doi: 10.1073/pnas.71.9.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M., Palmieri R. H., Russell G. A., Kuby S. A. Studies of adenosine triphosphate transphosphorylases. XIV. Equilibrium binding properties of the crystalline rabbit and calf muscle ATP--AMP transphosphorylase (adenylate kinase) and derived peptide fragments. Arch Biochem Biophys. 1979 Jun;195(1):155–177. doi: 10.1016/0003-9861(79)90338-2. [DOI] [PubMed] [Google Scholar]
- Hollemans M., Runswick M. J., Fearnley I. M., Walker J. E. The sites of labeling of the beta-subunit of bovine mitochondrial F1-ATPase with 8-azido-ATP. J Biol Chem. 1983 Aug 10;258(15):9307–9313. [PubMed] [Google Scholar]
- Kawashima H., Horii T., Ogawa T., Ogawa H. Functional domains of Escherichia coli recA protein deduced from the mutational sites in the gene. Mol Gen Genet. 1984;193(2):288–292. doi: 10.1007/BF00330682. [DOI] [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Affinity labeling of a tyrosine residue in the ATP binding site of the recA protein from Escherichia coli with 5'-p-fluorosulfonylbenzoyladenosine. J Biol Chem. 1985 Aug 25;260(18):10177–10184. [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Covalent modification of the recA protein from Escherichia coli with the photoaffinity label 8-azidoadenosine 5'-triphosphate. J Biol Chem. 1985 Jan 25;260(2):867–872. [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Tyrosine 264 in the recA protein from Escherichia coli is the site of modification by the photoaffinity label 8-azidoadenosine 5'-triphosphate. J Biol Chem. 1985 Aug 25;260(18):10185–10191. [PubMed] [Google Scholar]
- Kuramitsu S., Hamaguchi K., Tachibana H., Horii T., Ogawa T., Ogawa H. Cysteinyl residues of Escherichia coli recA protein. Biochemistry. 1984 May 22;23(11):2363–2367. doi: 10.1021/bi00306a006. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Low B. Some genetic consequences of changes in the level of recA gene function in Escherichia coli K-12. Genetics. 1976 Dec;84(4):675–695. doi: 10.1093/genetics/84.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino O., Shibata Y., Maeda H., Shibata T., Ando T. Monoclonal antibodies with specific effects on partial activities of recA protein of Escherichia coli. J Biol Chem. 1985 Dec 15;260(29):15402–15405. [PubMed] [Google Scholar]
- McEntee K. Kinetics of DNA renaturation catalyzed by the RecA protein of Escherichia coli. Biochemistry. 1985 Jul 30;24(16):4345–4351. doi: 10.1021/bi00337a014. [DOI] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Binding of the recA protein of Escherichia coli to single- and double-stranded DNA. J Biol Chem. 1981 Aug 25;256(16):8835–8844. [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Initiation of general recombination catalyzed in vitro by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2615–2619. doi: 10.1073/pnas.76.6.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand P., Blanco M., Devoret R. Characterization of lexB mutations in Escherichia coli K-12. J Bacteriol. 1977 Aug;131(2):572–582. doi: 10.1128/jb.131.2.572-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moras D., Olsen K. W., Sabesan M. N., Buehner M., Ford G. C., Rossmann M. G. Studies of asymmetry in the three-dimensional structure of lobster D-glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1975 Dec 10;250(23):9137–9162. doi: 10.2210/pdb1gpd/pdb. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Wabiko H., Tsurimoto T., Horii T., Masukata H., Ogawa H. Characteristics of purified recA protein and the regulation of its synthesis in vivo. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):909–915. doi: 10.1101/sqb.1979.043.01.099. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Konigsberg W., Howard-Flanders P. Isolation of altered recA polypeptides and interaction with ATP and DNA. J Biol Chem. 1985 Jan 25;260(2):949–955. [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Yarranton G. T. Cloned truncated recA genes in E. coli. I. Effect on radiosensitivity and recA+ dependent processes. Mol Gen Genet. 1982;185(1):93–98. doi: 10.1007/BF00333796. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. Hydrolysis of nucleoside triphosphates catalyzed by the recA protein of Escherichia coli. Characterization of ATP hydrolysis. J Biol Chem. 1981 Aug 25;256(16):8829–8834. [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. Hydrolysis of nucleoside triphosphates catalyzed by the recA protein of Escherichia coli. Hydrolysis of UTP. J Biol Chem. 1981 Aug 25;256(16):8856–8858. [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. Hydrolysis of nucleoside triphosphates catalyzed by the recA protein of Escherichia coli. Steady state kinetic analysis of ATP hydrolysis. J Biol Chem. 1981 Aug 25;256(16):8845–8849. [PubMed] [Google Scholar]
- Yarranton G. T., Sedgwick S. G. Cloned truncated recA genes in E. coli II. Effects of truncated gene products on in vivo recA+ protein activity. Mol Gen Genet. 1982;185(1):99–104. doi: 10.1007/BF00333797. [DOI] [PubMed] [Google Scholar]


