Abstract
Objectives. We explored the relationship between social isolation and mortality in a nationally representative US sample and compared the predictive power of social isolation with that of traditional clinical risk factors.
Methods. We used data on 16 849 adults from the Third National Health and Nutrition Examination Survey and the National Death Index. Predictor variables were 4 social isolation factors and a composite index. Comparison predictors included smoking, obesity, elevated blood pressure, and high cholesterol. Unadjusted Kaplan–Meier tables and Cox proportional hazards regression models controlling for sociodemographic characteristics were used to predict mortality.
Results. Socially isolated men and women had worse unadjusted survival curves than less socially isolated individuals. Cox models revealed that social isolation predicted mortality for both genders, as did smoking and high blood pressure. Among men, individual social predictors included being unmarried, participating infrequently in religious activities, and lacking club or organization affiliations; among women, significant predictors were being unmarried, infrequent social contact, and participating infrequently in religious activities.
Conclusions. The strength of social isolation as a predictor of mortality is similar to that of well-documented clinical risk factors. Our results suggest the importance of assessing patients’ level of social isolation.
Social isolation is defined as disengagement from social ties, institutional connections, or community participation.1 Socially isolated individuals have been found to have a higher risk of mortality in several studies.2–12 Although a recent meta-analysis suggested that the rate of all-cause mortality among the most socially isolated individuals may be 50% higher than the rate among socially integrated individuals,13 there have been few large-scale studies, and these investigations have yielded mixed findings about the association between social isolation and mortality both in general and according to gender.
Berkman and Syme developed a measure of social isolation and integration, the Berkman-Syme Social Network Index (SNI), that focused on marriage or partnership, frequency of contact with friends and family, frequency of religious participation, and group membership. In their study of 6928 residents of Alameda County, California, they found that SNI scores predicted all-cause mortality among both men and women regardless of health status, socioeconomic status, physical activity, obesity, smoking status, alcohol intake, or health care use.3 However, a study of more than 2000 residents of Evans County, Georgia, that used a measure based on the SNI and controlled for age, chronic disease, blood pressure, cholesterol, smoking, weight and height, heart abnormalities, and social status did not confirm this relationship in adjusted models.4
The Tecumseh (Michigan) Community Health Study, which included a sample of 2754 adults and incorporated measures of isolation including being unmarried and participating infrequently in social activities, confirmed a strong relationship between social isolation and mortality among men but not women (after controlling for age, heart disease risk factors, lung function, smoking status, and employment).5 By contrast, a study of 353 adults in northeastern New York that controlled for baseline health status showed that isolation (as measured with several SNI measures, as well as employment and social role indicators) predicted 7-year mortality among women aged 65 years and older but not among men.6 In a national study that measured individual components of social ties including marital status, social activity, and friends and relatives to count on, only religious attendance and marital status were significant predictors after controlling for age, gender, race, region of residence, health status, and health behaviors.14
The conflicting findings of these studies may represent differences in their populations. To our knowledge, there have been no nationally representative studies on this subject that have stratified by gender, used a combined social isolation measure, and compared the predictive value of social isolation with that of well-validated clinical risk factors such as elevated blood pressure, smoking, obesity, and cholesterol level within the same sample. Although clinicians routinely monitor these biological risk factors, they rarely assess patients’ social isolation or engagement. Understanding the relative predictive value of social isolation with respect to mortality would contribute to a fuller understanding of potentially modifiable risk factors.
METHODS
Data for this study came from the adult component of the Third National Health and Nutrition Examination Survey (NHANES III) and the National Death Index. NHANES III, which is administered by the National Center for Health Statistics (see http://www.cdc.gov/nchs/nhanes.htm), includes detailed social, behavioral, and biological data collected between 1988 and 1994 on 20 050 adult participants ranging in age from 17 years to older than 89 years. The survey includes sampling weights to ensure that it is representative of the noninstitutionalized civilian US population. Follow-up time ranged from 1 month to 18.2 years, with a median follow-up of 14.1 years in our final sample.
The National Death Index is a central index of death record information derived from state vital statistics offices. It was linked to NHANES III through identifying variables provided by NHANES in a publicly released data set.
Variables
Our outcome variable was mortality through December 31, 2006, the final day of available mortality data. We used the SNI to measure social isolation.3 Participants received a score of 0 or 1 for each SNI domain (marital status, frequency of contact with other people, participation in religious activities, and participation in other club or organization activities), an approach used previously for constructing SNI scores for NHANES III participants.15 Participants received 1 point for each of the following: being married or living together with someone in a partnership at the time of their interview, averaging 3 or more interactions per week with other people (assessed with the questions “In a typical week, how many times do you talk on the telephone with family, friends, or neighbors?” and “How often do you get together with friends or relatives?”), reporting that they attended church or religious services 4 or more times per year, and reporting that they belonged to a club or organization such as a church group, union, fraternal or athletic group, or school group.
Scores ranged from 0 to 4, with 0 representing the highest level of social isolation and 4 representing the lowest level. To be consistent with previous analyses,15 we combined individuals with a score of 0 or 1 and categorized them as the most socially isolated participants.
The motivation behind using the aforementioned cutoff numbers for determining frequent contact with others and frequent religious service attendance was to be consistent with recent research incorporating NHANES data.15,16 Because this index has been used for decades, its use facilitates comparisons with other studies.
Traditional clinical risk factors were assessed at baseline. They included self-identified current cigarette use (yes or no); obesity (a body mass index [BMI, defined as weight in kilograms divided by the square of height in meters] of 30 kg/m2 or greater) and elevated blood pressure (systolic blood pressure ≥ 140 mm/Hg or diastolic blood pressure ≥ 90 mm/Hg), both assessed during an examination visit; and high cholesterol (total serum cholesterol ≥ 240 mg/dL), assessed with serum collected during an examination visit.
Other covariates included age (continuous), self-reported race/ethnicity (Black non-Hispanic, Mexican American, White non-Hispanic, other), educational level (< 12 years or ≥ 12 years), income level based on the ratio of family income to the poverty threshold defined by the US Census and adjusted by year (poverty [ratio < 1.0], low income [1.0 ≤ ratio < 2.0], middle income [2.0 ≤ ratio < 4.0], and high income [ratio ≥ 4.0]), and self-reported baseline health status (good [excellent, very good, and good] vs poor [fair or poor] health).
Sample
We excluded 3166 participants younger than 25 years because of their low mortality rate, 25 participants with incomplete mortality follow-up data, and 10 who died within the first month after their interview. Thus, the final sample comprised 16 849 participants.
Data Analysis
Because 24% (n = 4052) of the participants in our sample had missing data on at least 1 of the demographic, clinical, or social isolation variables relevant to our analysis, we performed multiple imputation with the Stata (StataCorp LP, College Station, TX) mi impute mvn command, specifying the addition of 5 imputations (estimates were pooled from 5 data sets with imputed values for missing observations). The following variables required imputation (with number of missing values in parentheses): education (186), income (1762), smoking status (16), BMI (1698), blood pressure (338), cholesterol (828), health status (12), marital status (81), social contact (136), religious activity (40), group membership (26), and total SNI score (229).
We constructed unadjusted Kaplan–Meier survival tables to investigate differences in survival time according to social isolation after stratification by SNI score. Because integers are needed to construct Kaplan–Meier tables, and Stata’s multiple imputation program does not necessarily yield integers, we reconstructed a total SNI score based on imputed score values. Dichotomous values were constructed for each SNI score (0 or 1, 2, 3, and 4). We then averaged the imputed value of each dichotomous score, with scores below 0.5 rescored as 0 and those 0.5 or higher rescored as 1. This process yielded 4 dichotomous variables that we used to construct a final summative score of 1 (for an SNI score of 0 or 1), 2 (for an SNI score of 2), 3 (for an SNI score of 3), or 4 (for an SNI score of 4). We performed log-rank tests for equality on the basis of these survival curves.
We used Cox proportional hazards models to predict mortality as of 2006 according to social isolation (with one model using the composite SNI score and one using each SNI component individually), clinical risk factors (smoking, obesity, high blood pressure, and high cholesterol), and covariates (age, race/ethnicity, education, income, and baseline health status). Because some studies have shown gender differences in the influence of social isolation on mortality,5,6 we stratified all models by gender. As a means of accounting for the complex survey design, NHANES sample weights and cluster variables were used in fitting the Cox models. We used the NHANES-provided interview weights rather than the combined interview and physical examination weights because the former are adjusted for the larger sample with complete interview data, mimicking our imputation sample. We used Stata version 12.0 to conduct the analyses.
RESULTS
Data on the characteristics of the sample can be found in Table A (available as a supplement to the online version of this article at http://www.ajph.org). The average age was 48.4 years for women and 46.5 years for men. The majority of the population was White non-Hispanic, had 12 or more years of education, had a middle-level income, and had good baseline health. A total of 17.1% of women were included in the most isolated category compared with 21.3% of men. Each of the traditional clinical risk factors was present in roughly one fifth to one third of both men and women.
The relationship between social isolation and clinical risk factors is shown in Table 1. In the case of both men and women, smoking prevalence was highest among those who were most socially isolated (P < .001 for both). In addition, the most isolated women were more likely to have high blood pressure (P < .001) and high cholesterol (P = .013). By contrast, obesity was less prevalent among more socially isolated men than among those with more social ties (P = .043).
TABLE 1—
Percentages of Participants With Traditional Clinical Risk Factors, by Social Network Index Score and Gender: Third National Health and Nutrition Examination Survey, United States, 1988–1994
| Social Network Index Score |
|||||
| Gender and Clinical Risk Factor, % | 0/1 | 2 | 3 | 4 | Pa |
| Women (n = 8974) | |||||
| Cigarette use | 33.4 | 31.3 | 19.4 | 13.8 | < .001 |
| Obesity | 28.5 | 27.6 | 25.9 | 25.0 | .174 |
| High blood pressure | 24.0 | 21.8 | 18.6 | 15.8 | < .001 |
| High cholesterol | 33.4 | 31.5 | 30.6 | 26.7 | .013 |
| Men (n = 7875) | |||||
| Cigarette use | 43.6 | 37.0 | 26.3 | 15.2 | < .001 |
| Obesity | 17.4 | 21.8 | 21.3 | 23.9 | .043 |
| High blood pressure | 22.5 | 23.4 | 20.9 | 22.9 | .663 |
| High cholesterol | 28.7 | 27.9 | 27.9 | 24.6 | .083 |
P values determined by 1-way analyses of variance.
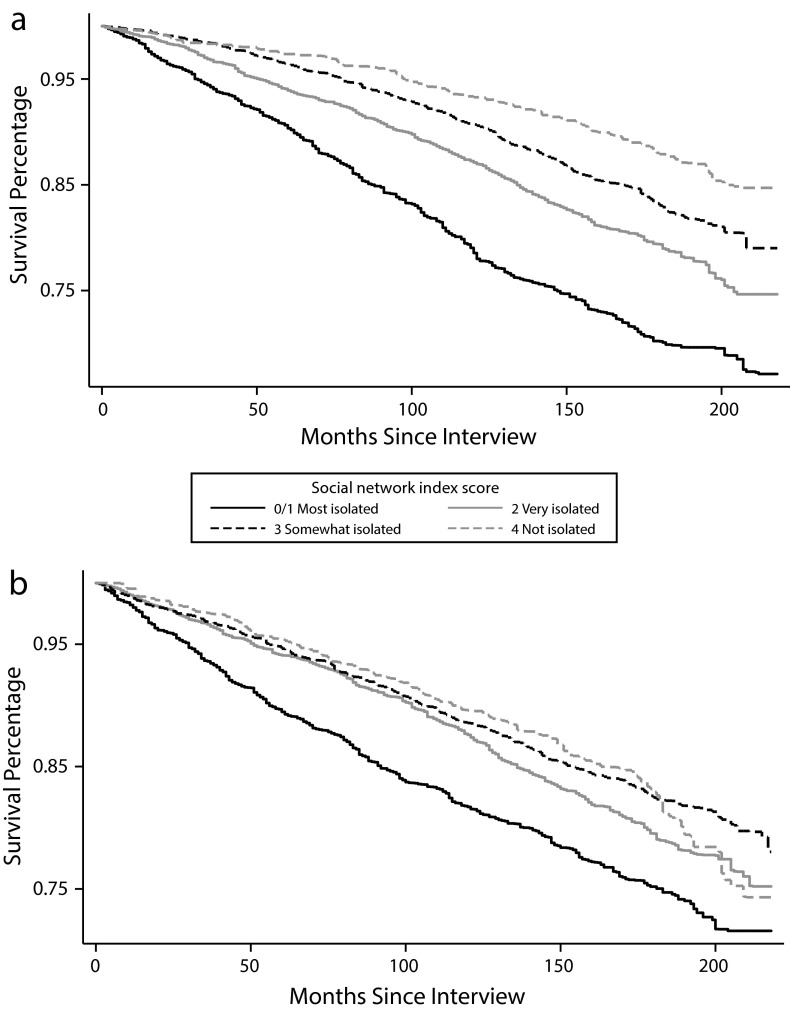
We constructed Kaplan–Meier tables to test whether different levels of social isolation were associated with different survival times. Among both women and men (Figure 1), increasing social isolation was associated with decreased survival time (log-rank P < .001 for both).
FIGURE 1—
Kaplan–Meier survival estimates by Social Network Index score among (a) women and (b) men: Third National Health and Nutrition Examination Survey, United States, 1988–1994.
The overall strength at which social isolation predicted mortality was tested in a Cox model with summary SNI score as a predictor (Table 2). When the clinical and SNI score variables were entered simultaneously, low SNI scores were predictive of mortality among men (hazard ratio [HR] = 1.62; 95% confidence interval [CI] = 1.29, 2.02) and were associated with a risk of mortality similar to that of smoking (HR = 1.72; 95% CI = 1.48, 2.00) and higher than that of high blood pressure (HR = 1.16; 95% CI = 1.02, 1.32). Obesity and hypercholesterolemia were not independently significant among men.
TABLE 2—
Mortality Hazard Ratios, by Social Network Index Score, Traditional Clinical Risk Factors, and Gender: Third National Health and Nutrition Examination Survey, United States, 1988–1994
| Women (n = 8974) |
Men (n = 7875) |
|||
| Characteristic | HR (95% CI) | P | HR (95% CI) | P |
| Age, y | 1.09 (1.09, 1.10) | < .001 | 1.10 (1.09, 1.10) | < .001 |
| Race/ethnicity | ||||
| White non-Hispanic (Ref) | 1.00 | 1.00 | ||
| Black non-Hispanic | 1.02 (0.92, 1.13) | .713 | 1.16 (0.98, 1.36) | .078 |
| Mexican American | 0.67 (0.56, 0.81) | < .001 | 0.84 (0.68, 1.03) | .09 |
| Other | 0.61 (0.42, 0.88) | .009 | 0.56 (0.37, 0.84) | .006 |
| Education, y | ||||
| < 12 | 0.98 (0.84, 1.14) | .757 | 0.89 (0.77, 1.04) | .143 |
| ≥ 12 (Ref) | 1.00 | 1.00 | ||
| Income category | ||||
| Poverty | 1.26 (0.97, 1.64) | .078 | 1.65 (1.27, 2.14) | < .001 |
| Low income | 1.15 (0.89, 1.49) | .27 | 1.55 (1.28, 1.88) | < .001 |
| Middle income | 1.03 (0.83, 1.28) | .789 | 1.22 (1.01, 1.48) | .041 |
| High income (Ref) | 1.00 | 1.00 | ||
| Health status | ||||
| Poor | 1.74 (1.51, 2.02) | < .001 | 1.80 (1.55, 2.10) | < .001 |
| Good (Ref) | 1.00 | 1.00 | ||
| Clinical risk factor | ||||
| Cigarette use | 1.86 (1.64, 2.12) | < .001 | 1.72 (1.48, 2.00) | < .001 |
| Obesity | 1.07 (0.92, 1.25) | .355 | 1.09 (0.92, 1.29) | .367 |
| High blood pressure | 1.32 (1.17, 1.48) | < .001 | 1.16 (1.02, 1.32) | .029 |
| High cholesterol | 1.04 (0.92, 1.18) | .528 | 1.10 (0.97, 1.24) | .12 |
| Social Network Index score | ||||
| 0/1 (most isolated) | 1.75 (1.38, 2.23) | < .001 | 1.62 (1.29, 2.02) | < .001 |
| 2 | 1.29 (1.03, 1.61) | .025 | 1.18 (0.97, 1.45) | .103 |
| 3 | 1.14 (0.91, 1.44) | .237 | 1.04 (0.85, 1.27) | .671 |
| 4 (not isolated; Ref) | 1.00 | 1.00 | ||
Note. CI = confidence interval; HR = hazard ratio.
Social isolation was also an important predictor of mortality among women (HR = 1.75; 95% CI = 1.38, 2.23), as were smoking (HR = 1.86; 95% CI = 1.64, 2.12) and high blood pressure (HR = 1.32; 95% CI = 1.17, 1.48). As was the case with men, obesity and hypercholesterolemia were not independent predictors of mortality. Gradients in risk were observed for both women and men, with increasing isolation associated with a greater risk of mortality. We entered SNI score as a linear variable to more formally test for gradient trends; results were significant for both men and women (P < .001; data not shown).
To test whether specific aspects of social isolation were independently associated with mortality, we fit Cox models for women and men to predict mortality associated with the 4 individual components of the SNI in addition to clinical risk factors and covariates (Table 3). In these models, current smoking significantly predicted mortality among both men (HR = 1.69; 95% CI = 1.46, 1.97) and women (HR = 1.85; 95% CI = 1.63, 2.11), as did high blood pressure (men: HR = 1.15; 95% CI = 1.01, 1.31; women: HR = 1.32; 95% CI = 1.17, 1.49). Among men, significant individual social isolation factors predictive of mortality included being unmarried (HR = 1.23, 95% CI = 1.08–1.40), infrequently participating in religious activities (HR = 1.27; 95% CI = 1.13, 1.42), and lacking club associations (HR = 1.15; 95% CI = 1.02–1.31). Among women, being unmarried (HR = 1.19; 95% CI = 1.03, 1.37), interacting infrequently with friends and family (HR = 1.25; CI = 1.04, 1.50), and infrequently participating in religious activities (HR = 1.35; 95% CI = 1.17, 1.56) were significant.
TABLE 3—
Mortality Hazard Ratios, by Individual Social Isolation Factors, Traditional Clinical Risk Factors, and Gender: Third National Health and Nutrition Examination Survey, United States, 1988–1994
| Women (n = 8974) |
Men (n = 7875) |
|||
| Characteristic | HR (95% CI) | P | HR (95% CI) | P |
| Age, y | 1.09 (1.09, 1.10) | < .001 | 1.10 (1.09, 1.10) | < .001 |
| Race/ethnicity | ||||
| White non-Hispanic (Ref) | 1.00 | 1.00 | ||
| Black non-Hispanic | 1.04 (0.92, 1.17) | .515 | 1.15 (0.97, 1.35) | .096 |
| Mexican American | 0.70 (0.57, 0.85) | .001 | 0.87 (0.70, 1.07) | .184 |
| Other | 0.61 (0.42, 0.90) | .014 | 0.57 (0.38, 0.86 | .008 |
| Education, y | ||||
| < 12 | 0.99 (0.85, 1.15) | .913 | 0.90 (0.77, 1.05) | .186 |
| ≥ 12 (Ref) | 1.00 | 1.00 | ||
| Income category | ||||
| Poverty | 1.29 (0.98, 1.71) | .071 | 1.66 (1.28, 2.16) | < .001 |
| Low income | 1.17 (0.90, 1.53) | .228 | 1.56 (1.29, 1.89) | < .001 |
| Middle income | 1.04 (0.84, 1.30) | .713 | 1.24 (1.02, 1.50) | .031 |
| High income (Ref) | 1.00 | 1.00 | ||
| Health status | ||||
| Poor | 1.74 (1.50, 2.02) | < .001 | 1.81 (1.56, 2.12) | < .001 |
| Good (Ref) | 1.00 | 1.00 | ||
| Clinical risk factor | ||||
| Cigarette use | 1.85 (1.63, 2.11) | < .001 | 1.69 (1.46, 1.97) | < .001 |
| Obesity | 1.07 (0.92, 1.25) | .371 | 1.09 (0.92, 1.28) | .31 |
| High blood pressure | 1.32 (1.17, 1.49) | < .001 | 1.15 (1.01, 1.31) | .04 |
| High cholesterol | 1.05 (0.92, 1.19) | .488 | 1.10 (0.98, 1.24) | .103 |
| Social isolation factor | ||||
| Unmarried | 1.19 (1.03, 1.37) | .019 | 1.23 (1.08, 1.40) | .002 |
| Infrequent social contact | 1.25 (1.04, 1.50) | .017 | 1.02 (0.85, 1.22) | .831 |
| Infrequent religious activity | 1.35 (1.17, 1.56) | < .001 | 1.27 (1.13, 1.42) | < .001 |
| No club associations | 1.04 (0.91, 1.18) | .607 | 1.15 (1.02, 1.31) | .029 |
Note. CI = confidence interval; HR = hazard ratio.
Because isolation variables were associated with several clinical risk factors, we conducted sensitivity analyses excluding all of these variables to determine whether traditional clinical risk factors would in turn be more predictive of mortality. Four Cox models, each incorporating a single clinical risk factor at a time to predict mortality without the addition of social isolation variables, revealed that (similar to the data presented in Tables 2 and 3) only current smoking (HR = 1.90; 95% CI = 1.66, 2.18) and high blood pressure (HR = 1.30; 95% CI = 1.16, 1.46) were significant predictors of mortality among women (Tables B1 and B2, available as a supplement to the online version of this article at http://www.ajph.org). These models controlled for all other covariates.
Among men (also similar to the data from Tables 2 and 3), both smoking (HR = 1.79; 95% CI = 1.53, 2.09) and high blood pressure (HR = 1.19; 95% CI = 1.04, 1.36) were significant predictors. The only difference was that high cholesterol became a significant predictor when it was examined in the absence of other clinical risk factors and social isolation factors (HR = 1.14; 95% CI = 1.01, 1.29; Tables B1 and B2).
Combining all clinical risk factors in the model together without any social isolation factors (but including covariates) did not change the significance of any clinical risk factor among women relative to the individual clinical risk factor models (Table B1). However, it did result in the association between high cholesterol and mortality among men becoming nonsignificant (P = .089; Table B2).
Because of similar concerns that individual social isolation factors might be collinear, we ran 4 separate Cox models predicting mortality, each incorporating only one social isolation factor. These models included all clinical variables and covariates and excluded composite SNI score. Among women, when lack of club associations was entered without any other isolation variables, it was a significant predictor of mortality; however, it became insignificant when it was entered with the 3 other individual isolation variables, suggesting its collinearity with these variables. Comparisons with the data presented in Tables 2 and 3 showed that there were no other changes in significance in any of the individual social isolation variables among either men or women (Tables C1 and C2, available as a supplement to the online version of this article at http://www.ajph.org). We also ran a Cox model that included all of the covariates and SNI score but excluded clinical risk factors. This increased the strength of the relationship between SNI score and mortality (Tables B1 and B2).
In addition, we ran the models from Tables 2 and 3 excluding participants who had died within 2 years of follow-up. There were no changes in significance with respect to the data presented in Table 2 (Table D, available as a supplement to the online version of this article at http://www.ajph.org). The only change in significance among isolation variables with respect to the data from Table 3 was that, among men, lack of club associations became a nonsignificant predictor of mortality (P = .078; Table E, available as a supplement to the online version of this article at http://www.ajph.org).
It is worth noting that in models including other covariates, Mexican American participants and participants in the “other” racial/ethnic category were less likely than Whites to die during the follow-up period. Also, although income showed a social gradient, with lower incomes associated with a higher risk of mortality, this association was only significant for men; moreover, education did not have a significant influence on mortality risk (Tables 2 and 3). The fact that more associations were not seen among social factors other than isolation may have been attributable in part to the collinearity of these factors. To test for this possibility, we ran models similar to those shown in Table 2 but also included interaction terms between SNI score and income, education, and race/ethnicity. Although no interactions were found (data not shown), further investigation of the interplay of these factors is warranted given the collinearity among them.
DISCUSSION
Supporting previous investigations, this study documents an increased risk of death among socially isolated men and women in a nationally representative sample.2,3,5–7,11 Importantly, social isolation factors predicted mortality at hazard ratio levels similar to or higher than those of several standard clinical risk factors.
Among both men and women, a low SNI score (a summation of social isolation risks) was associated with a mortality hazard ratio similar to that related to smoking and greater than that related to the other traditional clinical risk factors. Although the overlapping confidence intervals for the hazard ratios associated with the significant traditional clinical risk factors and social isolation factors suggest that isolation factors are not necessarily better predictors than traditional factors in all cases, they are at least equally important. Sensitivity analyses confirmed the strength of the predictive value of social isolation for both men and women.
In models incorporating all of the clinical and individual social variables assessed, unmarried status and infrequent religious activity predicted mortality among both men and women. In addition, lack of group memberships predicted mortality among men, and infrequent social contact predicted mortality among women. In these same models, we found that, of the traditional clinical risk factors, only smoking and high blood pressure were predictive of mortality among both men and women. These results are similar to those of another study in which increased religious involvement and married status were protective against mortality.14
That obesity and high cholesterol do not predict mortality may come as a surprise. However, a recent meta-analysis showed that a BMI of 30 to 35 is not associated with an increased risk of mortality,17 and some studies have shown that obesity can be protective at older ages and that it may provide nutritional reserve for times when the body is stressed, such as during an acute illness or trauma.18,19 Another possible explanation is that 14 years is a relatively short follow-up period, during which time obesity may not exert as strong an effect on mortality as it may on other causes of morbidity and functional outcomes. The lack of association of mortality with total cholesterol level may be attributable in part to the availability of effective medical treatments such as statins. In addition, because cholesterol is routinely monitored (in contrast to social isolation), clinical interventions may have occurred during the follow-up period.
Several mechanisms could account for the impact of social isolation on health. People who are socially integrated may have more access to tangible resources that help promote better health, increase access to knowledge of health-promoting behaviors, or help buffer the body’s negative behavioral and biological responses to stress.20 Berkman and Glass21 suggested that social relationships may affect people’s health by promoting healthy behaviors, increasing self-efficacy, and acting through regulation of biological mechanisms such as decreased allostatic load.
Cole et al.,22 examining the biological mechanisms that could explain the effects of social isolation on health, found that gene expression differed between those who were more socially isolated and those who were not. Isolated individuals had increased expression of genes related to proinflammatory cytokine signaling and prostaglandin synthesis, as well as underexpression of genes involved in antiviral resistance, antibody production, and lymphocyte function. An understanding of the biological mechanisms through which social isolation affects health could inform clinical interventions targeting isolated individuals. Examining the pathways through which social ties affect mortality is a compelling research topic.
Our results emphasize the value of identifying social isolation as a potentially modifiable risk factor. Researchers are now investigating how to modify social isolation. A recent clinical trial23 used psychotherapy to increase social support among patients after a myocardial infarction, but the study’s results did not reveal mortality differences between these patients and a control group. The researchers speculated that the 6-month intervention may have provided an inadequate “dose” and that social support may be more beneficial as a primary intervention than following an event such as a myocardial infarction. Future trials should examine the effects of longer term as well as primary prevention interventions designed to increase social integration.
Although a patient's social history is often inadequately explored in health care encounters,24,25 our results indicate the potential importance of assessing social isolation. The brief 4 questions included in the modified SNI scale, or a similar set of questions, could possibly help clinicians identify individuals at higher risk of mortality. In a busy clinical setting, adding these items to standardized screening questions administered electronically or by nonmedical clinic staff and highlighting patients’ responses for the physician when a threshold is reached would not add substantially to clinician burden, and it could potentially help in discerning which patients have worse health outcomes and targeting those patients for increased surveillance.
Comparing assessments of social isolation with assessments of traditional clinical risk factors is not meant to downplay the importance of the latter. Rather, our analysis shows that social isolation is also an important independent risk factor to assess. The development of interventions designed to modify social isolation merits increased attention given the continued struggle to reduce modifiable clinical risk factors.
Limitations
Our study involved several limitations. For example, most of our data were derived from participants’ self-reports. Such data may not capture true levels of social activity, and, although we controlled for a variety of possible confounders, unmeasured confounders may have affected the relationship between social isolation and mortality.
In addition, although we attempted to control for reverse causality by taking baseline health status into account and excluding individuals who had died within 2 years of follow-up from our sensitivity analyses, it is still possible that health problems contributed to social isolation, particularly in the case of chronic conditions that may have limited social integration over longer periods of time. Finally, only baseline measures of social isolation were available. Although social isolation seems to remain stable over time,26 we cannot confirm that it did so in our study.
Conclusions
Our results clarify the relationship between social isolation and mortality. Of particular importance, this relationship was found in a well-powered study with a national sample representative of the US civilian noninstitutionalized population. Given that the relationship remained robust after a variety of sensitivity analyses, the power of isolation as a marker of poor health cannot be ignored. Our findings highlight the value of isolation as a risk factor for mortality and emphasize the clinical importance of understanding a patient’s social integration and support.
Acknowledgments
This study was funded by a Dean’s Summer Research Fellowship grant from the School of Medicine, University of California, San Francisco (UCSF); a UCSF Clinical and Translational Science Institute (CTSI) Pathways to Careers in Clinical and Translational Research Quarterly Fellowship grant, which was supported by the National Center for Research Resources, the National Center for Advancing Translational Sciences, and the Office of the Director, National Institutes of Health, through UCSF-CTSI (grant TL1 RR024129); and a thesis research grant from the University of California, Berkeley–UCSF Joint Medical Program.
Preliminary findings from this study were presented at the 2009 and 2011 annual meetings of the American Public Health Association (November 7-11, 2009; October 29–November 2, 2011).
We thank Robert Pantell, MD, for his helpful input.
Note. The funders did not contribute in any way to the design, conduct, collection, management, analysis, or interpretation of data in this study, nor did they contribute to the preparation, review, or approval of the article. The authors are solely responsible for the content of this article, which does not necessarily represent the official views of the funders.
Human Participant Protection
This study was approved by the institutional review board of the University of California, San Francisco. Documented consent was obtained from participants in the Third National Health and Nutrition Examination Survey.
References
- 1.Seeman T. Social ties and health: the benefits of social integration. Ann Epidemiol. 1996;6(5):442–451. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- 2.Eng P, Rimm E, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol. 2002;155(8):700–709. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- 3.Berkman L, Syme S. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 4.Schoenbach V, Kaplan B, Fredman L, Kleinbaum D. Social ties and mortality in Evans County, Georgia. Am J Epidemiol. 1986;123(4):577–591. doi: 10.1093/oxfordjournals.aje.a114278. [DOI] [PubMed] [Google Scholar]
- 5.House J, Robbins C, Metzner H. The association of social relationship and activities with mortality: prospective evidence from the Tecumseh Community Health Study. Am J Epidemiol. 1982;116(1):123–140. doi: 10.1093/oxfordjournals.aje.a113387. [DOI] [PubMed] [Google Scholar]
- 6.Forster L, Stoller E. The impact of social support on mortality: a seven-year follow-up of older men and women. J Appl Gerontol. 1992;11(2):173–186. [Google Scholar]
- 7.Kawachi I, Ascherio A, Rimm E, Giovannucci E, Stampfer M, Willett W. A prospective study of social networks in relation to mortality and cardiovascular disease in men in the USA. J Epidemiol Community Health. 1996;50(3):245–251. doi: 10.1136/jech.50.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuda N, Zimmerman S, Hawkes W, Fredman L, Hebel J, Magaziner J. Relation of social network characteristics to 5-year mortality among young-old versus old-old white women in an urban community. Am J Epidemiol. 1997;145(6):516–523. doi: 10.1093/oxfordjournals.aje.a009139. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Norris S, Gregg E, Beckles G. Social support and mortality among older persons with diabetes. Diabetes Educ. 2007;33(2):273–281. doi: 10.1177/0145721707299265. [DOI] [PubMed] [Google Scholar]
- 10.Horsten M, Mittleman M, Wamala S, Schenck-Gustafsson K, Orth-Gomer K. Depressive symptoms and lack of social integration in relation to prognosis of CHD in middle-aged women: the Stockholm Female Coronary Risk Study. Eur Heart J. 2000;21(13):1072–1080. doi: 10.1053/euhj.1999.2012. [DOI] [PubMed] [Google Scholar]
- 11.Berkman L, Melchior M, Chastang J, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France. Am J Epidemiol. 2004;159(2):167–174. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- 12.Giles L, Glonek G, Luszcz M, Andres G. Effect of social networks on 10 year survival in very old Australians: the Australian Longitudinal Study of Aging. J Epidemiol Community Health. 2005;59(7):574–579. doi: 10.1136/jech.2004.025429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt-Lunstad J, Smith T, Layton B. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hummer R, Rogers R, Nam C, Ellison C. Religious involvement and US adult mortality. Demography. 1999;36(2):273–285. [PubMed] [Google Scholar]
- 15.Ford E, Loucks E, Berkman L. Social integration and concentrations of C-reactive protein among US adults. Ann Epidemiol. 2006;16(2):78–84. doi: 10.1016/j.annepidem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Rees C, Karter A, Young B. Race/ethnicity, social support, and associations with diabetes self-care and clinical outcomes in NHANES. Diabetes Educ. 2010;36(3):435–445. doi: 10.1177/0145721710364419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flegal K, Kit B, Orpana H, Graubard B. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabowski D, Ellis J. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. J Am Geriatr Soc. 2001;49(7):968–979. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 19.Diehr P, Bild D, Harris T, Duxbury A, Siscovick D, Rossi M. Body mass index and mortality in nonsmoking older adults: the Cardiovascular Health Study. Am J Public Health. 1998;88(4):623–629. doi: 10.2105/ajph.88.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen S. Psychosocial models of the role of social support in the etiology of physical disease. Health Psychol. 1988;7(3):269–297. doi: 10.1037//0278-6133.7.3.269. [DOI] [PubMed] [Google Scholar]
- 21.Berkman L, Glass T. Social integration, social networks, social support, and health. In: Berkman L, Kawachi I, editors. Social Epidemiology. New York, NY: Oxford University Press; 2000. pp. 137–173. [Google Scholar]
- 22.Cole S, Hawkley L, Arevalo J, Sung C, Rose R, Cacioppo J. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8(9):R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkman LF, Blumenthal J, Burg M et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Disease Patients (ENRICHS) randomized trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 24.Griffith C, Rich E, Wilson J. Housestaff’s knowledge of their patients’ social histories. Acad Med. 1995;70(1):64–66. doi: 10.1097/00001888-199501000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Duffy D, Hamerman D, Cohen M. Communication skills of house officers: a study in a medical clinic. Ann Intern Med. 1980;93(2):354–357. doi: 10.7326/0003-4819-93-2-354. [DOI] [PubMed] [Google Scholar]
- 26.Brissette I, Cohen S, Seeman T. Measuring social integration and social networks. In: Cohen S, Underwood LG, Gottlieb BH, editors. Social Support Measurements and Interventions: A Guide for Social and Health Scientists. New York, NY: Oxford University Press; 2000. pp. 53–85. [Google Scholar]