Abstract
NADPH oxidases (NOXes) and dual oxidases (DUOXes) generate O2.− and H2O2. Diphenyleneiodonium (DPI) inhibits the activity of these enzymes and is often used as a specific inhibitor. It is shown here that DPI, at concentrations similar to those which inhibit the generation of O2 derivatives, activated the efflux of radioiodide but not of its analog 99mTcO4− nor of the K+ cation mimic 86Rb+ in thyroid cells, in the PCCl3 rat thyroid cell line and in COS cell lines expressing the iodide transporter NIS. Effects obtained with DPI, especially in thyroid cells, should therefore be interpreted with caution.
Keywords: Diphenyleneiodonium, O2 radical generation, NOX, DUOX, Iodide efflux, Thyroid
Abbreviations: DPI, diphenyleneiodonium; DUOXes, dual oxidases; HRP, horse radish peroxidase type II; HVA, homovanillic acid; KRH, Krebs–Ringer Hepes medium; MMI, methylmercaptoimidazole; NIS, sodium/iodide symporter; NOXes, NADPH oxidases
Highlights
-
•
Diphenyleneiodonium (DPI) inhibits H2O2 generation by thyroid DUOXes in the μM range.
-
•
DPI activates iodide efflux from thyroid, thyroid-derived and unrelated cells in the μM range.
-
•
DPI does not activate the efflux of the iodide analog99mTcO4− or of 86Rb+ in the same cells.
-
•
DPI thus behaves as a specific ionophore for iodide.
1. Introduction
Seven specific O2.− and H2O2 generating enzymes have been demonstrated, the NADPH oxidases, NOXes and dual oxidases DUOXes. In many cell types, they generate both intracellular and extracellular O2.− and H2O2. They share many properties, including enzymatic mechanisms, but differ in terms of function, tissue distribution and control [1–3].
Diphenyleneiodonium (DPI) inhibits the activity of these flavoenzymes. It is often considered as a specific inhibitor. Inhibition of any metabolic step by DPI is therefore often attributed to this effect [4–9]. Although caution has been advised against the use of inhibition by DPI as a specific biomarker of NOX and DUOX actions [4], such arguments are still commonly used [10].
In this article we demonstrate another action of DPI which raises new interesting questions. In the course of our study of iodide metabolism in thyroid cells we observed that it increased the release of 125I iodide but not 99mTcO4−, thus behaving as a specific carrier of iodide.
This shows an effect of DPI other than the inhibition of H2O2 generation. It presents an interesting chemical property of this iodinated derivative and may suggest a therapeutic application.
2. Materials and methods
2.1. Reagents
125I as NaI (∼17Ci(629GBq)/mg) and 86Rb+ (19.3 mCi(714.1 MBq)/mg) were purchased from Perkin Elmer Life and Analytical Science (Zaventem, Belgium), 99mTc as Na Pertechnetate (99mTcO4−) was provided to us by the Medical School Hospital. It was prepared daily, carrier free, from a 99mTc generator (Ultra TechneKow, Mallinckrodt Nuclear Medicine). We used around 1 μCi tracer per dish.
Homovanillic acid (HVA), horse radish peroxidase type II (HRP), methylmercaptoimidazole (MMI) and diphenyleneiodonium chloride (DPI) were provided by Sigma–Aldrich BVBA (Diegem, Belgium). Sodium iodide and sodium perchlorate were purchased from Merck KGaA (Darmstadt, Germany).
2.2. Tissue
Pig thyroids were collected at a local slaughterhouse. The fresh tissue was sliced at room temperature with a Stadie-Riggs microtome (Arthur Thomas, Philadelphia, PA, USA). Slices (0.3 mm thick, 45–60 mg wet weight) were incubated in 2 ml incubation medium.
2.3. Cell culture
PCCl3 cells, a rat thyroid cell line, were grown in Coon's modified Ham's F12 medium supplemented with 5% decomplemented fetal bovine serum, bovine TSH (1 mU/ml) transferrin (5 μg/ml), insulin (1 μg/ml), penicillin (100 IU/ml), streptomycin (100 μg/ml) and fungizone (2.5 μg/ml). COS–NIS cells are COS-7 cells transfected with the wild type human sodium/iodide symporter (NIS) cDNA. Stable cell lines were selected by resistance to geneticin [11]. They were cultured in DMEM supplemented with 10% fetal bovine serum, Na pyruvate (1 mM), penicillin (100 IU/ml), streptomycin (100 μg/ml) and fungizone (2.5 μg/ml).
2.4. Incubation
The slices and cells were incubated in Krebs–Ringer Hepes medium (NaCl 124 mM; KCl 5 mM; MgSO4 1.25 mM; CaCl2 1.45 mM; Hepes Buffer 25 mM) supplemented with glucose 8 mM and BSA (bovine serum albumin) 0.5 mg/ml [12].
2.5. Measurement of the H2O2 production
Thyroid slices were preincubated 1 h in KRH medium and then transferred to fresh medium containing 0.1 mg/ml HRP, 440 μM HVA and the tested agonists for 90 min. When H2O2 is produced by the tissue, the HVA is oxidized into a fluorescent dimer by the peroxidase. The fluorescence of the medium was measured at 315 and 425 nm, excitation and emission wavelengths respectively [13]. Generation of H2O2 was expressed as ng H2O2 produced by 100 mg of tissue, or ng H2O2 per dish in the case of cells, during the length of the incubation.
2.6. Measurement of iodide uptake
The thyroid slices were preincubated 1 h in KRH supplemented with 0.1 mM MMI to block organification of iodide, then incubated for 1 h with fresh KRH medium supplemented with 125I− (1 μCi/ml), 10−7 M KI, 0.1 mM MMI and the tested agents, washed and counted. The uptake was evaluated by the ratio: counts per min 125I−/100 mg tissue to counts per min 125I−/100 μl medium [14]. Uptake of investigated cells in culture was divided by the uptake of cells incubated in the presence of NaClO4 1 mM to obtain concentration ratios (cell to medium C/M).
2.7. Measurement of tracer efflux from cells
300,000 cells were seeded in 35-mm-diameter dishes and grown 24 h in the case of COS–NIS cells or 48 h in the case of PCCl3 cells, in their respective culture medium. The cells were then rinsed and incubated under slight agitation in KRH medium supplemented with KI 10−7 M and 1 μCi/ml 125I−, or 1 μCi/ml 99mTcO4−, or 1 μCi/ml 86Rb+. As no iodination takes place in COS or PCCL3 cells, MMI was not used routinely in these experiments. For 86Rb+ uptake, the K+ concentration in the medium was 0.5 mM instead of 5 mM.
After 45 min uptake with 125I− or 99mTcO4− and 2 h with 86Rb+, the cells were rinsed twice with KRH and incubated with fresh medium, which was supplemented with KI (10−7 M) and ClO−4 (1 mM) in the case of 125I− or 99mTcO4− efflux, to avoid tracer re-circulation. DPI (10 μM) is added at the start of this second incubation. Fifty microliter from 1 ml medium was withdrawn at the mentioned times and counted. At the end of incubation, the cells were rinsed twice with KRH medium kept on ice, then dissolved in 1 M NaOH and counted. The efflux was expressed in percent of the total radioactivity taken up by the cells (cells + medium + serial aliquots).
3. Results
DPI, in the same concentration range, inhibits both H2O2 generation and radioiodide uptake in pig thyroid slices (Fig. 1).
Fig. 1.
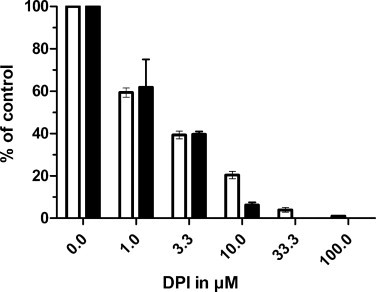
Effect of DPI on iodide uptake level at equilibrium (Empty columns) and H2O2 production (Filled columns) in pig thyroid slices. The incubation lasted 90 min. The results are the mean of 2 pig thyroids. The uptake control value, in absence of DPI, were 23.6 ± 4 and 18.5 ± 3 (mean of duplicates) in pig 1 and 2 respectively. The H2O2 control value, in absence of DPI, were 421 ± 12 and 482 ± 18 (mean of duplicates) ng H2O2/100 mg wet weight tissue for pig 1 and 2 respectively.
The effect of DPI on iodide uptake level at equilibrium results from the balance between the continuous uptake by NIS and the release. The inhibition of iodide uptake by DPI takes place at a lower concentration (IC50 1–4 μM) than the effect of competing iodide (IC50 40 μM) (Fig. 2). Moreover the C–I bond of the iodine to the benzene ring of DPI is not labile [9]. The inhibition therefore is not due to iodide release from DPI.
Fig. 2.
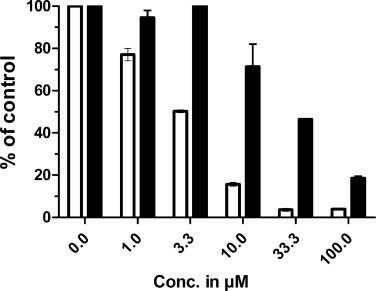
Comparison between the effect of the same range of DPI (Empty columns) and iodide (Filled columns) concentrations on iodide uptake value at equilibrium in pig thyroid slices. The incubation with DPI or KI lasted 1 h. The results presented are the mean of 2 pig thyroids. The uptake control value were 12.05 ± 1.4 and 7.1 ± 0.1 (means of duplicates) in pig 1 and 2 respectively. (Ratio of tissue vs medium radioactivity)
For the release experiments (Fig. 3, 4, 5), DPI is added after the labeling of the cells and the release is measured immediately after; it is, in effect, quasi instantaneous. The effect of DPI does not correspond to a general membrane permeabilization as preloaded 86Rb+, used as analog of K+, is not discharged from PCCl3 nor COS–NIS cells (Fig. 3).
Fig. 3.
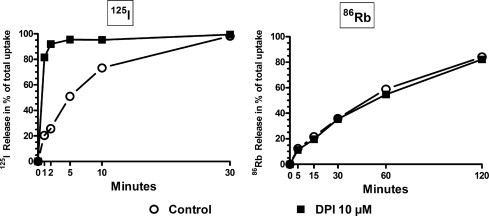
Comparison of DPI effect on 125I− and 86Rb+ efflux from COS–NIS preloaded cells. The efflux of the tracer from the cell to the medium is expressed as percentage of total uptake by the cells. The 125I− uptake at equilibrium was 24.6 in this experiment. (Ratio of control vs NaClO4 treated cells). Empty circles: control. Filled square: DPI 10 μM.
Moreover, DPI is a carrier more specific than NIS, the transporter of iodide (Na+/I− symporter) as it does not release 99mTcO4− from prelabeled cells. Thus, contrary to the NIS [15], DPI does not recognize 99mTcO4− as radioiodide (Fig. 4).
Fig. 4.
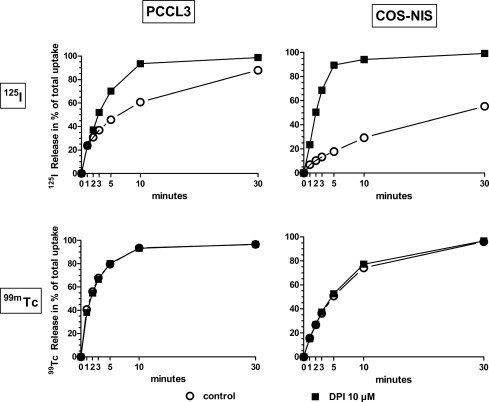
Comparison of DPI effect on 125I− and 99mTcO4− efflux from PCCl3 and COS–NIS preloaded cells. DPI was added at the beginning of the efflux. The uptake values at equilibrium for 125I− were 24 ± 1 and 24 ± 0.05 in PCCl3 and COS–NIS cells respectively. The uptake values at equilibrium for 99mTcO4− were 216 ± 6 and 228 ± 9 in PCCl3 and COS–NIS cells respectively (mean of duplicates). The results of 125I− and 99mTcO4− efflux are expressed as percentage of total uptake by the cells. Empty circles: control. Filled square: DPI 10 μM.
Spontaneous iodide release is much decreased when the temperature is lowered from 37 to 22 °C and 15 °C (Fig. 5). However, the relative effect of DPI is as important at the three temperatures.
Fig. 5.
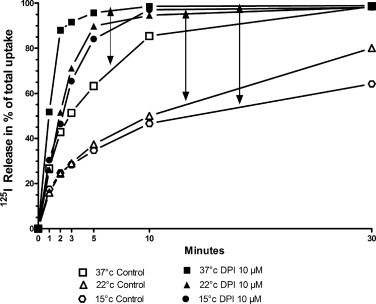
Effect of temperature on DPI action on 125I− efflux from PCCl3 preloaded cells. The cells were loaded with 125I− at 37 °C for 45 min, then quickly rinsed with 2 × 1 ml KRH at 37 °C or 22 °C or 15 °C. One milliliter of KRH at 37 °C (□) or 22 °C (Δ) or 15 °C (○) was then added and efflux measured at those temperatures. The arrows bind the basal and DPI stimulated release curves at each temperature. Empty symbols: basal release. Filled symbols: DPI stimulated release.
To check the reversible or permanent character of DPI effect on iodide uptake (through its effect on efflux) and on H2O2 production we designed a protocol made of 3 sequential incubations called A, B and C. The measurement of iodide uptake (in the presence of 125I and 10−7 M KI, for 45 min) and of H2O2 production (in the presence of 440 μM HVA and 100 μg/ml Peroxidase II for 90 min), took place in the last incubation C. Incubation A and B lasted 45 min each for either parameter. No DPI was added in any of these incubations, for the control. To test if DPI effect was short or long lasting we added it at 3 different times: In A only, or in B only, or in C only. The results presented in Fig. 6 show that the effect of DPI on iodide uptake is strongest when added in C, but fade away if it is added in B only and more so if added in A only, which suggests that the DPI involved in iodide release is rapidly washed in the absence of DPI.
Fig. 6.
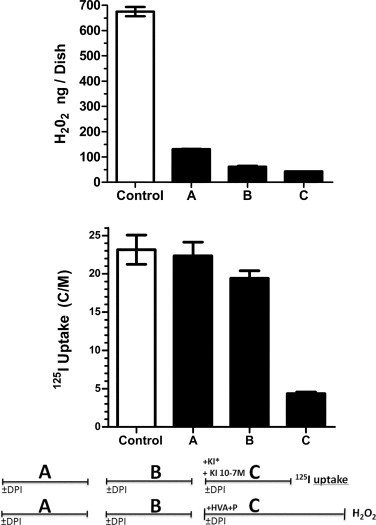
Study of the reversibility of DPI effect on H2O2 production or iodide uptake (C/M) in PCCl3 cells. The experimental protocol is made up of 3 sequential incubations called A, B or C. 125I− for uptake (C/M) measurements or HVA and peroxydase II for H2O2 measurements were added in incubation C only, which lasted 45 min for 125I− uptake and 90 min for H2O2 production. Incubation A and B lasted 45 min each. DPI 10 μM was added either in A alone or in B alone, or in C alone. Empty columns: control. Filled columns: DPI 10 μM.
The effect of DPI on H2O2 is much more persistent. Indeed, if added previously, even in the first incubation A only, it works as well. This suggests a more stable binding of the DPI involved in this inhibition.
4. Discussion
We have shown that DPI, at concentrations of the same order as those that inhibit H2O2 generation by thyroid cells, also causes an immediate specific release of iodide from thyroid and non-thyroid cells.
The effect of NaClO4 or NaSCN which only compete with the uptake of iodide and decrease by this mechanism the cell/medium equilibrium level [15] is relatively slow. The immediate effect of DPI on iodide efflux carried out in the presence of perchlorate is therefore not due to an inhibitory role on iodide uptake by NIS. It does not seem to require the specific iodide channel(s) involved in the efflux of iodide at the apical membrane of the thyrocyte [16,17], as it is reproduced in COS–NIS cells i.e. in non-hyroid cells.
Besides, an effect on NIS would similarly affect the efflux of 99mTcO4− [15,18]. The absence of effect on 99mTcO4− or 86Rb+ release, excludes a general permeabilization of the cells by DPI.
Other unspecific effects of DPI have been observed but at higher concentrations than those effective on NOXes. For example, DPI (10 μM) also directly inhibits cholinesterase activity and to a lesser extent an internal Ca++ pump [19]. The effect of DPI on iodide efflux is not due to the inhibition of H2O2 generation as it occurs in COS–NIS cells without DUOX and is, contrary to the effect on DUOX, relieved by incubation without the drug.
We have no explanation for this effect on iodide. Certainly because the covalent link in DPI between iodine and the other part of the DPI molecule is not labile, there is no dynamic exchange with medium iodide. The molecule is stable under stringent conditions [9].
Moreover, higher concentrations of iodide than DPI are needed to reach the same inhibition of C/M.
We can therefore only speculate on the mechanism of the DPI effect. A first explanation would be the rapid activation of an anion channel in the cell [20]. However DPI also acts on COS–NIS cells [11] which do not express any iodide specific channel, especially a channel distinguishing iodide and 99mTcO4−. A second explanation could be that DPI behaves as a membrane ionophore specific for iodide. Catalysis of an anion/hydroxyl ion exchange across mitochondrial membrane by DPI has been reported [21]. The fact that depressing membranes fluidity by decreasing the temperature of the cells slows down both basal and the increased efflux, does not distinguish the hypothesis of DPI acting as a shuttle for iodide from the concept that it acts as an intramembrane channel as is the case for valinomycin and K+ [22,23]. An ionic type of carrier mechanism would be similar to the mitochondrial protein carrier mechanism which is believed to account for the uncoupling effect on mitochondrial oxidative phosphorylation by dinitrophenol. Both hypotheses of channel and shuttle, would suggest a specific affinity of DPI molecule for iodide.
Nevertheless the present data confirm that DPI cannot be considered as a specific inhibitor, of NOXes and DUOXes. They suggest that DPI or an analog with similar properties could therefore be considered as drugs for iodine or amiodarone induced hyperthyroidism as they would combine in one molecule the inhibition of thyroid hormone synthesis and iodide uptake (in fact retention) as a combination of antithyroperoxidase drugs and perchlorate.
Acknowledgments
The authors thank Dr. J. Wolff (NIH, Bethesda) for critical reading of the manuscript, the Fondation Solvay and the Fondation Roi Baudouin “Fonds Yvonne Smits” and the Fonds de la Recherche Scientifique Médicale (FRSM) for their support and Mrs. D. Leemans for the preparation of the manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Cross A.R., Segal A.W. The NADPH oxidase of professional phagocytes – prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsuyama M., Matsuno K, Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J. Clin. Biochem. Nutr. 2012;50:9–22. doi: 10.3164/jcbn.11-06SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 4.Aldieri E., Riganti C., Polimeni M., Gazzano E., Lussiana C., Campia I., Ghigo D. Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr. Drug Metab. 2008;9:686–696. doi: 10.2174/138920008786049285. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind F., Bartok E., Rieger A., Franchi L., Nunez G., Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross A.R., Jones O.T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem. J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doussiere J., Vignais P.V. Inhibition of O2-. generating oxidase of neutrophils by iodonium biphenyl in a cell free system: effect of the redox state of the oxidase complex. Biochem. Biophys. Res. Commun. 1991;175:143–151. doi: 10.1016/s0006-291x(05)81212-4. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell V.B., Smith G.C., Jones O.T. Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Mol. Pharmacol. 1994;46:778–785. [PubMed] [Google Scholar]
- 9.O’Donnell B.V., Tew D.G., Jones O.T., England P.J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 1993;290(Pt 1):41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doroshow J.H., Gaur S., Markel S., Lu J., van Balgooy J., Synold T.W., Xi B., Wu X., Juhasz A. Effects of iodonium-class flavin dehydrogenase inhibitors on growth, reactive oxygen production, cell cycle progression, NADPH oxidase 1 levels, and gene expression in human colon cancer cells and xenografts. Free Radic. Biol. Med. 2013;57:162–175. doi: 10.1016/j.freeradbiomed.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohlenz J., Duprez L., Weiss R.E., Vassart G., Refetoff S., Costagliola S. Failure of membrane targeting causes the functional defect of two mutant sodium iodide symporters. J. Clin. Endocrinol. Metab. 2000;85:2366–2369. doi: 10.1210/jcem.85.7.6700. [DOI] [PubMed] [Google Scholar]
- 12.Corvilain B., Van Sande J., Laurent E., Dumont J.E. The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology. 1991;128:779–785. doi: 10.1210/endo-128-2-779. [DOI] [PubMed] [Google Scholar]
- 13.Benard B, Brault J. Production of peroxide in the thyroid. Union Med. Can. 1971;100:701–705. [PubMed] [Google Scholar]
- 14.Van Sande J., Lamy F., Lecocq R., Mirkine N., Rocmans P., Cochaux P., Mockel J., Dumont J.E. Pathogenesis of autonomous thyroid nodules: in vitro study of iodine and adenosine 3’,5’-monophosphate metabolism. J. Clin. Endocrinol. Metab. 1988;66:570–579. doi: 10.1210/jcem-66-3-570. [DOI] [PubMed] [Google Scholar]
- 15.Van Sande J., Massart C., Beauwens R., Schoutens A., Costagliola S., Dumont J.E., Wolff J. Anion selectivity by the sodium iodide symporter. Endocrinology. 2003;144:247–252. doi: 10.1210/en.2002-220744. [DOI] [PubMed] [Google Scholar]
- 16.Bizhanova A., Kopp P. Controversies concerning the role of pendrin as an apical iodide transporter in thyroid follicular cells. Cell Physiol. Biochem. 2011;28:485–490. doi: 10.1159/000335103. [DOI] [PubMed] [Google Scholar]
- 17.Twyffels L., Massart C., Golstein P.E., Raspe E., Van Sande J., Dumont J.E., Beauwens R., Kruys V. Pendrin: the thyrocyte apical membrane iodide transporter? Cell Physiol. Biochem. 2011;28:491–496. doi: 10.1159/000335110. [DOI] [PubMed] [Google Scholar]
- 18.Dohan O., Portulano C., Basquin C., Reyna-Neyra A., Amzel L.M., Carrasco N. The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20250–20255. doi: 10.1073/pnas.0707207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tazzeo T., Worek F., Janssen L. The NADPH oxidase inhibitor diphenyleneiodonium is also a potent inhibitor of cholinesterases and the internal Ca(2+) pump. Br. J. Pharmacol. 2009;158:790–796. doi: 10.1111/j.1476-5381.2009.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatley S.J., Martin J.L. Some aspects of the pharmacology of diphenyleneiodonium, a bivalent iodine compound. Xenobiotica. 1979;9:539–546. doi: 10.3109/00498257909042319. [DOI] [PubMed] [Google Scholar]
- 21.Holland P.C., Sherratt H.S. Biochemical effects of the hypoglycaemic compound diphenyleneiodonnium. Catalysis of anion-hydroxyl ion exchange across the inner membrane of rat liver mitochondria and effects on oxygen uptake. Biochem. J. 1972;129:39–54. doi: 10.1042/bj1290039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreoli T.E., Tieffenberg M., Tosteson D.C. The effect of valinomycin on the ionic permeability of thin lipid membranes. J. Gen. Physiol. 1967;50:2527–2545. doi: 10.1085/jgp.50.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golstein P., Abramow M., Dumont J.E., Beauwens R. The iodide channel of the thyroid: a plasma membrane vesicle study. Am. J. Physiol. 1992;263:C590–C597. doi: 10.1152/ajpcell.1992.263.3.C590. [DOI] [PubMed] [Google Scholar]


