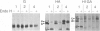Abstract
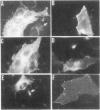
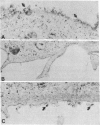
In polarized epithelial cells, influenza virus buds exclusively from the apical domain of the plasma membrane, whereas vesicular stomatitis virus (VSV) buds exclusively from the basolateral domain. In virus-infected cells, the envelope proteins, influenza hemagglutinin (HA) and vesicular stomatitis virus G (VSV G), are likewise transported to and localized in the same domain of the plasma membrane from which the viruses bud. Previous studies have shown that influenza HA and VSV G proteins, when expressed from cloned cDNAs, are accumulated preferentially on the proper domains (apical and basolateral, respectively), indicating that the signal(s) for polarized transport resides in the polypeptide backbone of the proteins. To further elucidate the structural features required for apical vs. basolateral transport, we have constructed a gene that encodes a chimeric protein (H1GA) containing the external domain of HA and the transmembrane and cytoplasmic domains of VSV G. When the chimeric protein (H1GA) is expressed in CV1 cells using a simian virus 40 late expression vector, it is transported to the cell surface with kinetics similar to that of the native HA protein. Further, the chimeric protein, when expressed in polarized MDCK cells using a vaccinia virus early expression vector, is transported only to the apical surface, suggesting that the ectodomain of HA contains a signal for apical transport.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso-Caplen F. V., Compans R. W. Modulation of glycosylation and transport of viral membrane glycoproteins by a sodium ionophore. J Cell Biol. 1983 Sep;97(3):659–668. doi: 10.1083/jcb.97.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. R., Bos T. J., Nayak D. P. Active influenza virus neuraminidase is expressed in monkey cells from cDNA cloned in simian virus 40 vectors. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3976–3980. doi: 10.1073/pnas.80.13.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Cell-surface expression of influenza haemagglutinin from a cloned DNA copy of the RNA gene. Nature. 1981 Oct 22;293(5834):620–625. doi: 10.1038/293620a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Hartman J. R., Nayak D. P., Fareed G. C. Human influenza virus hemagglutinin is expressed in monkey cells using simian virus 40 vectors. Proc Natl Acad Sci U S A. 1982 Jan;79(2):233–237. doi: 10.1073/pnas.79.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. V., Compans R. W., Davis A. R., Bos T. J., Nayak D. P. Surface expression of influenza virus neuraminidase, an amino-terminally anchored viral membrane glycoprotein, in polarized epithelial cells. Mol Cell Biol. 1985 Sep;5(9):2181–2189. doi: 10.1128/mcb.5.9.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- McQueen N. L., Nayak D. P., Jones L. V., Compans R. W. Chimeric influenza virus hemagglutinin containing either the NH2 terminus or the COOH terminus of G protein of vesicular stomatitis virus is defective in transport to the cell surface. Proc Natl Acad Sci U S A. 1984 Jan;81(2):395–399. doi: 10.1073/pnas.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Misek D. E., Bard E., Rodriguez-Boulan E. Biogenesis of epithelial cell polarity: intracellular sorting and vectorial exocytosis of an apical plasma membrane glycoprotein. Cell. 1984 Dec;39(3 Pt 2):537–546. doi: 10.1016/0092-8674(84)90460-4. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Tobita K., Janda J. M., Davis A. R., De B. K. Homologous interference mediated by defective interfering influenza virus derived from a temperature-sensitive mutant of influenza virus. J Virol. 1978 Oct;28(1):375–386. doi: 10.1128/jvi.28.1.375-386.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Machamer C. E., Rose J. K. Cytoplasmic domains of cellular and viral integral membrane proteins substitute for the cytoplasmic domain of the vesicular stomatitis virus glycoprotein in transport to the plasma membrane. J Cell Biol. 1986 Jun;102(6):2147–2157. doi: 10.1083/jcb.102.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler M. J., Ivanov I. E., Plesken H., Rodriguez-Boulan E., Sabatini D. D. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J Cell Biol. 1984 Apr;98(4):1304–1319. doi: 10.1083/jcb.98.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo L. J., Finidori J., Gonzalez A., Arpin M., Ivanov I. E., Adesnik M., Sabatini D. D. Biosynthesis and intracellular sorting of growth hormone-viral envelope glycoprotein hybrids. J Cell Biol. 1985 Oct;101(4):1351–1362. doi: 10.1083/jcb.101.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W., Giusti L., Davis A. R., Nayak D. P., Gething M. J., Sambrook J. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell. 1983 Jun;33(2):435–443. doi: 10.1016/0092-8674(83)90425-7. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Fitzpatrick J. P., Compans R. W. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Srinivas R. V., Compans R. W. Basolateral maturation of retroviruses in polarized epithelial cells. J Virol. 1983 Mar;45(3):1065–1073. doi: 10.1128/jvi.45.3.1065-1073.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F. Acylation of viral spike glycoproteins: a feature of enveloped RNA viruses. Virology. 1982 Jan 15;116(1):327–338. doi: 10.1016/0042-6822(82)90424-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W., Earl P., Moss B. Surface expression of viral glycoproteins is polarized in epithelial cells infected with recombinant vaccinia viral vectors. EMBO J. 1986 Feb;5(2):237–245. doi: 10.1002/j.1460-2075.1986.tb04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Lai C. J. Functional expression in primate cells of cloned DNA coding for the hemagglutinin surface glycoprotein of influenza virus. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5488–5492. doi: 10.1073/pnas.78.9.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Gilmore R., Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984 Aug;38(1):5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Srinivas R. V., Hunter E. Mutations of the Rous sarcoma virus env gene that affect the transport and subcellular location of the glycoprotein products. J Cell Biol. 1984 Dec;99(6):2011–2023. doi: 10.1083/jcb.99.6.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]