Abstract
Likelihood thought–action fusion (TAF-L) refers to a cognitive bias in which individuals believe that the mere thought of a negative event increases its likelihood of occurring in reality. TAF-L is most commonly associated with obsessive–compulsive disorder (OCD) but is also present in depression, generalized anxiety disorder and psychosis. We induced TAF-L in individuals with high (High-OC, N = 23) and low (Low-OC, N = 24) levels of OC traits, and used low resolution electromagnetic tomography (LORETA) to localise the accompanying electrical brain activity patterns. The results showed greater TAF-L in the High-OC than in the Low-OC group (p < .005), which was accompanied by significantly greater upper beta frequency (19–30 Hz) activity in the precuneus (p < .05). Further, the precuneus activity was positively correlated with self-reported magnitude of TAF-L (p < .01), suggesting a specific role of this region in this cognitive bias. Results are discussed with reference to self-referential processing and the default-mode network.
Keywords: Thought–action fusion, Electroencephalography, Precuneus, Default mode network, Obsessive–compulsive disorder
Highlights
-
•
EEG was recorded during TAF induction with High-OC and Low-OC participants.
-
•
High-OCs experienced greater TAF than Low-OCs.
-
•
High-OCs exhibited greater upper beta activity than Low-OCs in the precuneus.
-
•
Precuneus activity was positively correlated with self-reported magnitude of TAF.
1. Introduction
Thought–action fusion, or TAF (Rachman, 1993; Shafran et al., 1996), is a cognitive bias in which an individual believes that thoughts and actions are inextricably linked. This bias takes two forms: Moral TAF (TAF-M) and Likelihood TAF (TAF-L). TAF-M refers to the belief that thinking an unacceptable or immoral thought is the moral equivalent of performing an unacceptable or immoral action (Shafran et al., 1996); for example, if a mother experiences an intrusive thought that she is going to harm her child, she may feel that she is as morally responsible as if she had harmed her child in reality. TAF-L, on the other hand, refers to the belief that thoughts have causal powers. In this form of TAF it is believed that thinking of a negative or disturbing event makes it more likely to occur in reality (Shafran et al., 1996); for example, a husband who experiences a thought of his wife being involved in a car accident may believe that his thought has made the event more likely to transpire. TAF-L can be further divided into Likelihood-self (TAF-LS), referring to the belief that one's thoughts will cause harm to oneself, and Likelihood-other (TAF-LO) – the more pathological of the two (Shafran and Rachman, 2004) – referring to the belief that one's thoughts will be the cause of harm to others (Shafran et al., 1996).
Patients with obsessive–compulsive disorder (OCD) are particularly prone to TAF (Meyer and Brown, 2012; Shafran et al., 1996), which appears to be strongly associated with the inflated sense of responsibility often found in this patient group (see Salkovskis, 1985). However, recent evidence suggests that TAF is not only confined to OCD, but is also found in patient groups with generalized anxiety disorder (GAD) (Thompson-Hollands et al., 2013), major depressive disorder (Hossein et al., 2012), and schizophrenia (Kabakci et al., 2008).
Furthermore, the experience of TAF is correlated with psychopathological symptoms in the normal population; so far there have been accounts of TAF correlating with non-clinical symptoms of OCD (Amir et al., 2001; Muris et al., 2001), GAD (Hazlett-Stevens et al., 2002; Muris et al., 2001), schizotypy (Lee et al., 2005; Muris and Merckelbach, 2003) and depression (Abramowitz et al., 2003; Muris et al., 2001; Rachman et al., 1995; Rassin et al., 2001; Shafran and Rachman, 2004). Reports also suggest that the sub-scale most strongly associated with OCD, anxiety and schizotypy is TAF-L (Abramowitz et al., 2003; Amir et al., 2001; Hazlett-Stevens et al., 2002; Lee et al., 2005; Meyer and Brown, 2012; Thompson-Hollands et al., 2013), with some suggesting that TAF-M may be more strongly related to depression (Abramowitz et al., 2003); correlation coefficients of 0.21, 0.23, and 0.13 between obsessive–compulsive symptoms and TAF-total, TAF-L, and TAF-M have been reported by two separate studies (Rassin et al., 2000a, 2001).
However, evidence suggests that while TAF may be a contributing factor in a variety of other disorders, its strongest influence is in OCD (Muris et al., 2001). As expressed in Rassin et al. (2000b, p.762), TAF “seems to be similar to that of attentional bias, which is also a phenomenon that occurs in a wide range of anxiety disorders, though its ramifications might be greater in some conditions than in others”. Similarly, Starcevic and Berle (2006) suggested that TAF, along with other anxiety-related phenomena such as anxiety sensitivity, pathological worry, and intolerance of uncertainty, may be “pathognomonic”, i.e. common to a number of disorders, rather than isolated to one disorder in particular. These authors also drew a parallel between TAF and psychotic symptoms such as delusions of reference or passivity phenomena.
Further to findings of TAF in psychiatric disorders, it appears that TAF is also found in child populations. It has long been suggested that magical thinking tendencies occur during normal child development (Piaget, 1929/1973), and TAF appears to be one of such tendencies. Evans et al. (2011) found that TAF was endorsed to a greater extent by younger than older children, in an examination of three age groups (mean ages of 7.8, 9.6 and 12 years). Their results also suggested that an extended phase of TAF endorsement in childhood is associated with greater anxiety and ritualistic tendencies. Thus it is suggested that while TAF endorsement appears to be a normal phase of development in childhood, carrying these beliefs into early adolescence may represent a risk factor for later anxiety problems.
Despite this wealth of evidence showing TAF to be an important characteristic in a wide range of psychiatric disorders, and potentially a developmental predictor of psychopathology in later life, to our knowledge there has not been any investigation of its neural basis. Yet finding the neural basis of TAF would increase knowledge of how biological mechanisms influence psychiatric symptoms, and may also have a more practical clinical utility; for example, these regions could be useful as sites for non-invasive stimulation methods such as transcranial direct current stimulation (tDCS). TDCS has proved helpful in the treatment of some psychiatric disorders (see Kuo et al., 2013), and could potentially be used in conjunction with psychotherapy for greater effectiveness; there have been reports of a synergistic effect from combined tDCS and cognitive behavioural therapy (CBT) leading to a long-lasting improvement in drug-resistant depression (D'Urso et al., 2013). As a common step in cognitive therapy for OCD involves addressing the endorsement of TAF by patients (Radomsky et al., 2010), concurrent stimulation may enhance the ‘un-learning’ of this cognitive bias that the therapy hopes to achieve.
The present study aimed to fill this gap by recording EEG signals of participants with high- and low-OC symptoms undergoing a modification of the classic TAF-induction paradigm (Rachman et al., 1996).
In the original TAF-induction experiment (Rachman et al., 1996), participants were asked to keep in mind a close friend or relative, before copying the sentence “I hope that ____ will be in a car accident”, and inserting the name of their chosen loved one into the blank space. Subsequently, they were instructed to close their eyes and imagine the accident for ‘a few seconds’. As a result, participants experienced feelings of anxiety, guilt, the urge to neutralize (or ‘cancel out’ what they had just done), and believed that the likelihood of the event occurring had increased. Since its initial inception, this paradigm has been replicated – with various modifications – a number of times in order to examine the relationship between TAF and thought suppression (Marcks and Woods, 2007; Rassin, 2001), and neutralization (Bocci and Gordon, 2007; Van den Hout et al., 2001, 2002), and the efficacy of anti-TAF interventions (Zucker et al., 2002). The procedure of the current experiment deviated slightly from the original in the two following ways: Firstly we asked our participants to make a short list of their close friends/family prior to TAF-induction. This ensured that participants could think of loved ones for all four trials, and provided a check that participants were using the name of someone they were truly close to. Secondly, while participants were still required to have their eyes closed for a few seconds after repeating the sentence, we did not give any instructions at this point to visualise the event occurring; this allowed spontaneous brain activity occurring in response to the TAF induction – in the absence of any further instructions – to be recorded.
Given TAF's pathognomonic nature, we expected activation of those brain regions in which abnormalities are common in multiple psychiatric disorders, spanning OCD, depression, anxiety and psychosis. Additionally, we expected that the neural activity accompanying TAF would reflect its composite cognitive and emotional elements (e.g. agency, causal reasoning, responsibility, guilt, etc.). We therefore hypothesised an activation of either the precuneus or the medial prefrontal cortex (mPFC); recent findings have implicated both of these regions in the self-attribution of cause for (hypothetical) harmful consequences (Cabanis et al., 2012; Morey et al., 2012), a process seemingly very similar to TAF. Furthermore, previous research has provided evidence for the involvement of both the precuneus (Fusar-Poli et al., 2011; Piras et al., 2013; Rotge et al., 2008; Strawn et al., 2013) and the mPFC (Grieve et al., 2013; Piras et al., 2013) in numerous psychiatric disorders.
2. Materials and methods
2.1. Participants
The current analysis combined samples from two separate experiments. Both sample 1 (N = 29, 14 High-OC, 19 females) and sample 2 (N = 18, 9 High-OC, 14 females) began each experimental block with the TAF-induction procedure outlined below, but subsequent block activities differed, with sample 1 continuing to engage in visualisation of the TAF-induction's negative event (Jones and Bhattacharya, 2012), and sample 2 continuing to engage in a low-level sensory perception task (unpublished data). The samples did not differ significantly in age (F(1, 45) = 1.89, p = .18), OCI-R score (F(1, 45) = .86, p = .36), or MIS score (F(1, 45) = .51, p = .48).
In order to account for any influence of these differing activities, sample was included as a covariate in all analyses.
Volunteers were recruited through word of mouth and advertising in Goldsmiths, University of London, and were sent the Obsessive–Compulsive Inventory-Revised (OCI-R) (Foa et al., 2002) by e-mail. Those whose OCI-R scores were below the threshold of the Low-OC cut-off, or above the threshold of the High-OC cut off, were chosen to take part in the EEG study. Participants were chosen on the basis of their scores on the OCI-R. Participants were designated as High-OC if their total score on the OCI-R was equal to or higher than 21, which is the recommended clinical cut-off for OCD (Foa et al., 2002), and has been used as a High-OC boundary in other studies using analogue OCD groups (Cavanagh et al., 2010; Gründler et al., 2009). An arbitrary cut-off of 10 was set as the maximum total score for Low-OCs. Forty-nine participants took part in total, and 12 of them (7 High-OC) received a small cash incentive. Two participants, 1 High-OC and 1 Low-OC, were excluded from the analysis as their OCI-R score had changed considerably from the time of e-mail screening to the time of testing, at which point their scores no longer fit into either group. Therefore the data of 47 participants were included in the final analysis (23 High-OC; 24 Low-OC). The OCI-R score of the High-OC group (32.4 ± 6.8) was significantly higher (F(1, 44) = 302.08, p < .0001) than that of the Low-OC group (5.4 ± 4.0). Participant's demographics and OCI-R subscale scores are shown in Table 1.
Table 1.
Means and standard deviations of High-OC and Low-OC group scores for the Obsessive–Compulsive Inventory-Revised and Magical Ideation Scale, and age and sex.
| High-OC |
Low-OC |
|
|---|---|---|
| N = 23 | N = 24 | |
| Age (years) | 25.4 ± 4.3 | 25.2 ± 3.1 |
| Males/females | 6/17 | 8/16 |
| OCI-R total Score | 32.4 ± 6.8 | 5.4 ± 4.0 |
| Hoarding | 5.7 ± 3.5 | 2.1 ± 2.0 |
| Checking | 6.0 ± 2.5 | 0.8 ± 1.3 |
| Ordering | 7.0 ± 3.0 | 1.0 ± 1.2 |
| Neutralizing | 4.3 ± 3.3 | 0.3 ± 0.7 |
| Cleaning | 3.0 ± 3.1 | 0.3 ± 0.9 |
| Obsessing | 6.5 ± 3.8 | 1.0 ± 1.4 |
| MIS | 9.0 ± 5.2 | 2.6 ± 4.3 |
The majority of participants were female, but there was no significant difference in sex distribution between the High-OC and Low-OC groups (χ2 (2, 47) = .30, p = .59). Three participants (2 High-OC, 1 Low-OC) were undergoing pharmacological treatment with Citalopram for depression; all remaining participants reported that they had not been diagnosed with any mental health condition and were not undergoing treatment.
2.2. Measures
2.2.1. Obsessive–Compulsive Inventory-Revised (OCI-R)
As mentioned earlier, the OCI-R (Foa et al., 2002) was used to form two groups of participants, one with low levels of obsessive–compulsive symptoms (Low-OC) and one with high levels of obsessive–compulsive symptoms (High-OC). This is a self-report measure consisting of 18-items assessing distress caused by obsessive–compulsive symptoms in the past month (e.g. “I repeatedly check gas and water taps and light switches after turning them off”). The scale assesses 6 symptom dimensions: hoarding, checking, ordering, cleaning, obsessing, and neutralizing. Participants completed the OCI-R on two separate occasions: first at screening and second at baseline prior to TAF-induction.
2.2.2. Magical Ideation Scale
The Magical Ideation Scale, MIS (Eckblad and Chapman, 1983) was developed to be an indication of schizotypy. The scale is a self-report measure which contains 30 statements to assess individuals' beliefs of causality (e.g. “Some people can make me aware of them just by thinking about me”). The MIS score of the High-OC group was significantly higher than that of the Low-OC group (F(1, 44) = 21.1, p < .001), see Table 1.
2.2.3. Visual Analogue Scales
Four Visual Analogue Scales (VAS) were used during the TAF-induction procedure on which participants indicated their level of anxiety, guilt, their urge to neutralize, and their estimation of the likelihood of the event referred to in each TAF-induction trial occurring in reality. These VAS were presented on screen, and participants used their keypad to move a yellow bar in increasing increments (maximum 10) along the scale, marked from “not at all” (score of 0) up to “very much” (score of 10).
2.3. Procedure
Prior to beginning the study, all participants were informed that the procedure would most likely induce transient feelings of anxiety and guilt, and that they may withdraw from participating at any time. After giving informed consent, participants completed the MIS and the OCI-R, and gave an indication of their baseline level of anxiety on the anxiety VAS. Participants were then asked to write a short list of close friends and family, for later insertion into the sentence stimuli. The study was carried out at Goldsmiths, University of London and was approved by the Ethics Committee of the Department of Psychology at Goldsmiths.
2.3.1. Thought–action fusion induction
The TAF-induction procedure was similar to that of Rachman et al. (1996), in which participants were required to insert the name of a loved one into the sentence “I hope that ___ is in a car accident”. The current manipulation was composed of four trials, and used four TAF-induction sentences — matched for similar negative valence from a larger list by an independent sample of 25 undergraduates from Goldsmiths, University of London (21 females, mean age = 19.4 ± 1.8 years). The sentence stimuli were:
-
1.
I hope that _____ will be in a serious car accident soon
-
2.
I hope that _____ will lose their home soon
-
3.
I hope that _____ will become seriously ill soon
-
4.
I hope that _____ will lose a close family member soon.
The order of presentation of the sentence stimuli was randomized across participants. Each trial began with the presentation of a sentence stimulus on screen. Participants then closed their eyes before repeating the sentence aloud, inserting a name from their list of family and friends. Three seconds after sentence repetition, a beep sounded to inform participants to open their eyes, and VAS scales were completed on screen. VAS scales assessed participant's levels of anxiety, guilt, urge to neutralize and perceived likelihood of the event occurring.
2.4. EEG recording
Sixty-four channel EEG signals were continuously recorded by active scalp electrodes according to the extended 10–20 International system of electrode placement (Jasper, 1958). Additional electrodes were placed above and below each eye, and at the outer canthus of each eye, to record vertical and horizontal eye movements, respectively. The EEG signals were amplified by BioSemi Active Two® amplifiers and filtered between 0.6 and 100 Hz. The sampling rate was 512 Hz.
2.5. EEG data analysis
The EEG data was algebraically re-referenced to the average of two earlobes. Notch filter at 50 Hz was applied to remove line-noise interference. Reading phase epochs were extracted from the point immediately following the repetition of the sentence stimuli aloud by the participants (marked with a key press by the experimenter) and lasting for 3 s. Any epochs containing large artefacts due to eye-blinks or muscle movements were rejected based on visual inspection. All data pre-processing and visualisation were carried out using the MATLAB® based toolbox, EEGLAB (Delorme and Makeig, 2004).
As only 4 reading phase epochs were available per participant, these 3-second epochs were subdivided into three 1 second epochs, thus allowing for maximum preservation of data following artefact rejection. In order to ensure that this method did not affect results, analyses were re-run at a later time with the full three second epochs, and no significant differences between the findings using each method were present. A univariate ANOVA, with sample as a covariate, showed no significant difference (F(1, 44) = 2.74, p = .11) between the average number of epochs available for the Low-OC group (10.4 ± 3.3) and those available for the High-OC group (8.9 ± 3.0).
The periodogram of the EEG data was estimated by the Welch method (Welch, 1967) using a 192-point Hanning window with a 50% overlap. The periodogram was separately estimated for each electrode and condition for each participant. Spectral power was then averaged for each of the following frequency bands: theta (2–6 Hz), alpha (8–12 Hz), beta-1 (14–18 Hz), beta-2 (19–21 Hz), and beta-3 (22–30 Hz); spectral power values were log-transformed to reduce variance.
2.6. Statistical analysis of EEG data in electrode space
For regional power analysis, mean band power was calculated for six ROIs: left frontal (Fp1, AF3, AF7, F3, F5, F7), left central (FC5, FC3, FC1, C1, C3, C5, CP5, CP3, CP1), left posterior (P1, P3, P5, P7, PO3, PO7, O1), right frontal (Fp2, AF4, AF8, F2, F4, F6, F8), right central (FC2, FC4, FC6, C2, C4, C6, CP2, CP4, CP6), and right posterior (P2, P4, P6, P8, PO4, PO8, O2). Regional power analysis was carried out using mixed factorial ANCOVAs with Hemisphere (left, right) and Region (frontal, central, and posterior) as within-subjects factor, Group (High-OC, Low-OC) as a between-subjects factor, and Sample as a covariate.
Partial Spearman's correlations were examined between EEG power and VAS responses, controlling for the effect of sample.
2.7. Source localization
Low resolution electromagnetic tomography (LORETA) computes the inverse solution within a three-shell spherical Talairach head model (Talairach and Tourboux, 1988), with the solution space restricted to cortical grey matter and the hippocampus, as determined by the corresponding digitized probability atlas from the Brain Imaging Centre, Montreal Neurologic Institute. A spatial resolution of 7 mm is used, producing 2394 voxels.
The LORETA-KEY software package (Pascual-Marqui, 1999; Pascual-Marqui et al., 1994, 2002) was used to compute average current source density for the two EEG frequency bands in which significant effects were found in electrode space (beta-2, beta-3) for each participant.
The differences between the two groups' brain activity were first analysed using the LORETA-KEY software, through voxel-by-voxel independent t-tests of the log-transformed current source density (Frei et al., 2001). This standard analysis is based on estimating the empirical probability distribution of the maximum t statistic under the null hypothesis, via 5000 randomisations, and corrects for multiple comparisons of all 2394 voxels (see Nicols and Holmes, 2002 for details on this permutation procedure). Further analysis of significant clusters was carried out in IBM SPSS Statistics for Windows, version 20 (2011) using partial Spearman's correlations.
2.8. Behavioural analysis
Mean VAS responses for the entire sample (24 Low-OC, 23 High-OC) were entered into a mixed factorial ANOVA with VAS (anxiety, guilt, likelihood, urge) as a within-subjects factor, Group (High-OC, Low-OC) as a between-subjects factor, and Sample as a covariate. Partial Spearman's correlations were used to examine the relationship between VAS responses and participants' scores on the OCI-R and MIS.
3. Results
A univariate ANCOVA showed the initial anxiety ratings of the Low-OC group (1.7 ± 1.8) and the High-OC group (2.0 ± 1.6) did not differ significantly (F(1, 44) = .39, p = .54).
3.1. Behavioural effects of TAF-induction
A highly significant main effect of Group (F(1, 44) = 13.6, p = .001) showed that the High-OC group gave higher VAS responses for the four measures combined. No interaction of VAS × Group was present (F(3, 132) = 1.61, p = .19), suggesting the group difference to be consistent across measures. However, analysis of individual VAS measures suggests the group difference to be smaller on the VAS of anxiety (F(1, 44) = 5.00, p = .03,), than those of guilt (F(1, 44) = 11.42, p = .002), likelihood (F(1, 44) = 9.23, p = .004), or urge to neutralize (F(1, 44) = 10.33, p = .002), as shown in Fig. 1.
Fig. 1.
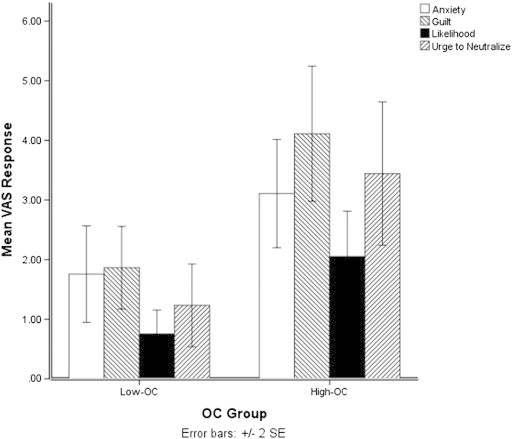
Mean VAS responses for anxiety, guilt, likelihood and urge to neutralize following TAF-induction.
The correlation analysis based on partial Spearman's correlation revealed that participants' VAS responses of guilt, perceived likelihood and urge to neutralize, were positively correlated with their scores on both the OCI-R and MIS (Table 2). The checking subscale of the OCI-R showed the strongest relationship with VAS responses, with correlation coefficients of .43 (p < .01), .47 (p < .01), and .45 (p < .01) with guilt, likelihood and urge, respectively. This was followed by the MIS, which had correlation coefficients of .32 (p < 05), .56 (p < .01) and .41 (p < .01). Total OCI-R score showed a weak relationship only with anxiety (ρ = .30, p < .05).
Table 2.
Partial Spearman's correlations (controlling for sample) between OCI-R total and subscales, MIS and VAS responses following TAF-induction.
| Anxiety | Guilt | Perceived likelihood | Urge to neutralize | |
|---|---|---|---|---|
| Checking | ns | .43⁎⁎ | .47⁎⁎ | .45⁎⁎ |
| Ordering | ns | .35⁎ | .32⁎ | ns |
| Neutralizing | ns | .32⁎ | .36⁎ | .35⁎ |
| Cleaning | ns | ns | .30⁎ | ns |
| Obsessing | ns | ns | .50⁎⁎ | .34⁎ |
| Total | .32⁎ | .43⁎⁎ | .48⁎⁎ | .47⁎⁎ |
| MIS | ns | .32⁎ | .56⁎⁎ | .41⁎⁎ |
ns: non-significant.
p < .05.
p < .01.
3.2. Regional EEG power analysis
No significant group differences were found in the theta, alpha or beta-1 bands. In the beta-2 band, we found a significant interaction of Region × Group (F(2, 88) = 4.31, p = .03). A significant effect of Region in the High-OC group (F(2, 42) = 4.41, p = .02) suggests a greater beta-2 power in posterior than frontal regions, while the Low-OC group showed no topographical differences in beta-2 distribution (p > .05), as shown in Fig. 2a.
Fig. 2.
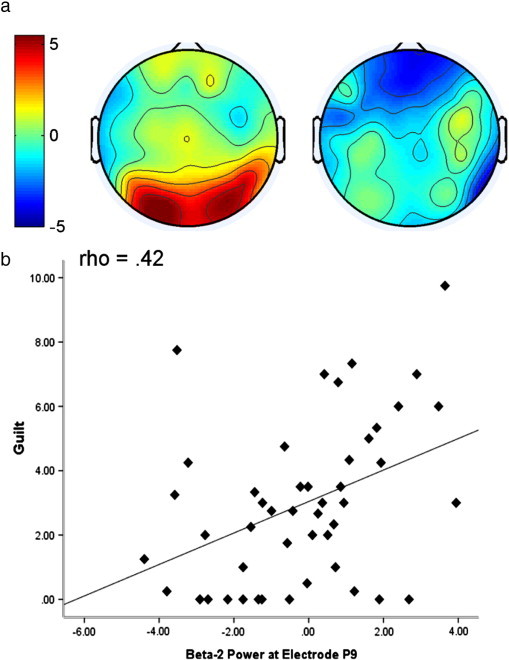
a. Topographical maps showing log-transformed beta-2 power in the High-OC group (left) and the Low-OC group (right) following TAF-induction. b. Scatterplot showing the correlation between log-transformed beta-2 power at electrode P9 and self-reported guilt following TAF-induction.
Similar results were found in the beta-3 band where the interaction of Region × Group was significant in centro-posterior regions (F(1, 44) = 4.23, p = .05). In the Low-OC group this reflected lower beta-3 power in posterior region than in central region (F(1, 22) = 7.94, p = .01), while the High-OC group once again exhibited greater beta-3 power in posterior region than in frontal region (F(1, 21) = 4.76, p = .04).
Spearman's correlations revealed a significant positive relationship between beta-2 and beta-3 power in parietal electrodes, and participants' VAS responses following TAF-induction (see Table 3). The strongest relationships were observed with VAS responses for guilt (Fig. 2b), while anxiety only showed a weak relationship with activity in electrode P5 (ρ = .29, p < .05).
Table 3.
Partial Spearman's correlations (controlling for sample) between beta 2 and beta 3 powers in parietal electrodes, and VAS responses following TAF-induction.
| P5 | P7 | P9 | P6 | P8 | P10 | PO8 | |
|---|---|---|---|---|---|---|---|
| Beta 2 | |||||||
| Anxiety | .29⁎ | ||||||
| Guilt | .29⁎ | .33⁎ | .42⁎⁎ | .37⁎ | .36⁎ | ||
| Likelihood | .40⁎⁎ | .37⁎ | .33⁎ | .37⁎ | .32⁎ | .29⁎ | |
| Urge to neutralize | .36⁎ | ||||||
| Beta 3 | |||||||
| Anxiety | |||||||
| Guilt | .29⁎ | .33⁎ | .39⁎⁎ | .36⁎ | .30⁎ | .29⁎ | |
| Likelihood | .32⁎ | .33⁎ | .29⁎ | ||||
| Urge to neutralize | .37⁎ | .30⁎ | |||||
p < .05.
p < .01.
3.3. Source localisation results
Whole-brain analysis revealed significantly greater beta-2 and beta-3 activity in parieto-occipital regions of the High-OC group compared with the Low-OC group (see Fig. 3a). Significant differences in both bands were found primarily in the precuneus, and in the beta-2 band there were also significant differences in the cingulate gyrus, posterior cingulate, and cuneus. Table 4 provides Talairach co-ordinates and t values for the voxel showing the greatest group difference in each cluster.
Fig. 3.
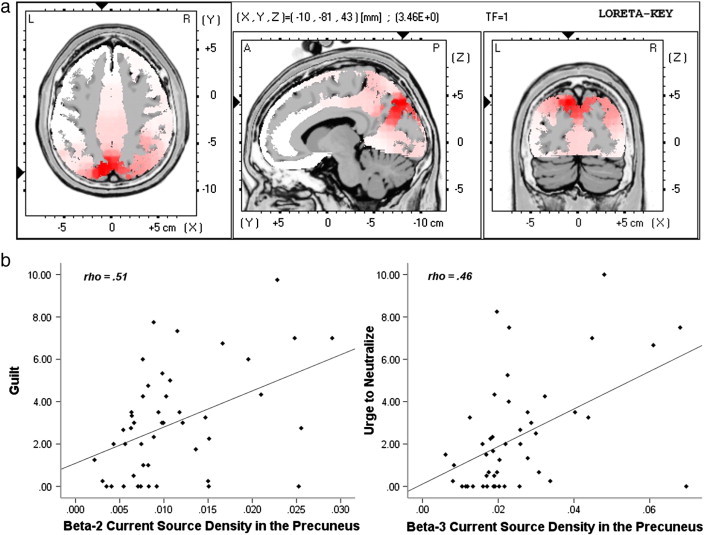
a. Beta-2 current source density in a contrast of High-OC vs. Low-OC participants following TAF-induction. b. Scatterplots showing correlations between TAF-induced guilt and beta-2 current source density in the precuneus (left) and between TAF-induced urge to neutralize and beta-3 current source density in the precuneus (right).
Table 4.
Source localization of current source density comparisons between High-OC and Low-OC groups following TAF-induction.
| Primary lobe | Region | Cluster size |
Brodmann's area(s) |
Peak voxel (Talairach coordinates) |
||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | BA | t | x | y | z | ||
| Beta 2 | ||||||||
| Occipital | Cuneus | 4 | 2 | 7, 18, 19 | t = 3.11 | − 3 | − 67 | 29 |
| Limbic | Cingulate gyrus | 1 | 1 | 31 | t = 3.04 | − 3 | − 60 | 29 |
| Parietal | Precuneus | 19 | 22 | 7, 19, 31 | t = 3.46 | − 10 | − 74 | 43 |
| Beta 3 | ||||||||
| Parietal | Precuneus | 9 | 2 | 7, 19 | t = 3.00 | − 3 | − 74 | 43 |
CG = cingulate gyrus; Cu = cuneus; PCu = precuneus.
Partial Spearman's correlations revealed highly significant positive relationships between this beta-2 and beta-3 activity and VAS responses of participants following TAF-induction, with the strongest relationships observed between guilt and precuneus beta-2 (ρ = .51, p < .001) and beta-3 (ρ = .52, p < .001), and urge to neutralize and precuneus beta-3 (ρ = .54, p < .001), as shown in Fig. 3b. Positive relationships were also seen between this activity and participants' questionnaire scores (see Table 5), with particularly strong correlations between MIS score and precuneus beta-3 (ρ = .54, p < .001), and total OCI-R score and precuneus beta-2 (ρ = .51, p < .001).
Table 5.
Partial Spearman's correlations (controlling for sample) between Beta 2 and Beta 3 current source density and VAS responses following TAF induction, and participants' scores on the Obsessive–Compulsive Inventory-Revised and Magical Ideation Scale.
| Cingulate Beta 2 | Cuneus Beta 2 | Precuneus Beta 2 | Precuneus Beta 3 | |
|---|---|---|---|---|
| VAS responses | ||||
| Anxiety | .43⁎⁎ | .46⁎⁎ | .46⁎⁎ | .45⁎⁎ |
| Guilt | .45⁎⁎ | .50⁎⁎ | .51⁎⁎⁎ | .52⁎⁎⁎ |
| Likelihood | .40⁎⁎ | .43⁎⁎ | .48⁎⁎ | .44⁎⁎ |
| Urge to neutralize | .44⁎⁎ | .46⁎⁎ | .46⁎⁎ | .54⁎⁎⁎ |
| MIS | .49⁎⁎ | .46⁎⁎ | .51⁎⁎⁎ | .54⁎⁎⁎ |
| OCI-R subscales | ||||
| Hoarding | .29⁎ | n.s | .29⁎ | .30⁎ |
| Checking | .30⁎ | .30⁎ | .33⁎ | .35⁎ |
| Ordering | .35⁎ | .35⁎ | .42⁎⁎ | .30⁎ |
| Neutralizing | .35⁎ | .37⁎ | .40⁎⁎ | .30⁎ |
| Cleaning | .40⁎⁎ | .37⁎ | .36⁎ | .34⁎ |
| Obsessing | .39⁎⁎ | .38⁎⁎ | .49⁎⁎ | .38⁎ |
| Total OCI-R | .47⁎⁎ | .45⁎⁎ | .51⁎⁎⁎ | .46⁎⁎ |
p < .05.
p < .01.
p < .001.
3.4. Comparison of separate experimental samples
In order to ensure that the use of two combined experimental samples did not influence our results, statistical analyses of differences between the two samples were run on baseline and TAF induced measures. A univariate ANOVA with Sample and Group as between-subjects factors showed no significant difference in initial anxiety between the two samples (F(1, 43) = 1.30, p = .26) and no interaction between Sample and Group (F(1, 43) = .003, p = .96). There were also no significant differences between the two samples in TAF-induced anxiety (F(1, 43) = 2.48, p = .12), guilt (F(1, 43) = .03, p = .86), likelihood (F(1, 43) = 1.57, p = .22) or urge to neutralize (F(1, 43) = .58, p = .45). Neither were there any significant interactions between Sample and Group in TAF-induced anxiety (F(1, 43) = .53, p = .70), guilt (F(1, 43) = .64, p = .43), likelihood (F(1, 43) = .25, p = .62), or urge to neutralize (F(1, 43) = .01, p = .94).
Analysis of EEG power also showed no significant interaction of Sample with Region in the beta-2 (F(2, 86) = .28, p = .76) or beta-3 (F(2, 86) = .83, p = .44) bands. Neither were there any significant interactions between Sample, Region and Group in the beta-2 (F(2, 86) = 1.17, p = .32) or beta-3 (F(2, 86) = 2.59, p = .09) bands.
Furthermore, analysis of LORETA current source density showed no significant differences between samples in precuneus beta-2 (F(1, 43) = 1.32, p = .26) or beta-3 (F(1, 43) = .69, p = .41), cingulate beta-2 (F(1, 43) = .12, p = .73) or cuneus beta-2 (F(1, 43) = 1.33, p = .25) activity. Further there were no significant interactions between Sample and Group in precuneus beta-2 (F(1, 43) = .05, p = .83) or beta-3 (F(1, 43) = .11, p = .74), cingulate beta-2 (F(1, 43) = .20, p = .65) or cuneus beta-2 (F(1, 43) = .05, p = .83) activity.
These altogether clearly justified the combining of two samples.
4. Discussion
The principle purpose of the current study was to investigate the neural correlates of thought–action fusion (TAF) by analysing EEG activity accompanying TAF-induction in individuals with high (High-OC) compared to low (Low-OC) levels of obsessive–compulsive symptoms. Significantly greater levels of TAF were induced in the High-OC compared to the Low-OC group, as shown by higher responses on Visual Analogue Scales (VAS) of anxiety, guilt, urge to neutralize, and estimations of likelihood of the negative events occurring. Analysis of EEG activity in the electrode space showed significant differences in the topographical distribution of beta-2 and beta-3 activity between the two groups during TAF-induction, with the High-OC group showing a greater concentration of this activity in posterior regions. Correlation analysis showed this posterior beta activity to be positively related with all VAS measures. Source analysis found group differences in posterior beta-2 and beta-3 activity, with significantly greater activity present in a large area of the precuneus in the High-OC group compared to the Low-OC group. Strong correlations were observed between beta activity in the precuneus and all VAS measures, particularly with self-reported likelihood, guilt and urge to neutralize; thus suggesting a specific role of the precuneus in thought–action fusion.
The precuneus resides in the medial posterior parietal cortex, and has rich anatomical and functional connections to widespread brain regions (Zhang and Li, 2012). There is an extreme diversity of function associated with this structure, including a wide-range of higher order cognitive functions (Cavanna and Trimble, 2006), and it is a major constituent of the brain's ‘default mode network’ or DMN (Gusnard and Raichle, 2001; Raichle et al., 2001). The reason for its vast plethora of functions has been accredited to its high centrality in the cortical network, in which it acts as a ‘hub’ between parietal and prefrontal regions (Bullmore and Sporns, 2009).
Previous studies outlining its involvement in psychopathology as well as its various functions strongly support the results of the current experiment in ascribing the precuneus a pivotal role in thought–action fusion.
4.1. The precuneus in OCD, schizophrenia, anxiety and depression
While rarely mentioned in the models of OCD or discussed in detail in meta-analyses, a large proportion of neuroimaging studies have found abnormalities in precuneus structure and function in OCD populations. For example, reductions in precuneus grey matter have been found in OCD patients compared with controls in both adult (Soriano-Mas et al., 2007) and paediatric samples (Carmona et al., 2007), while Van den Heuvel et al. (2009) found positive correlations between the left precuneus grey matter and the harm/checking symptoms. Rotge et al.'s meta-analysis of 8 fMRI OCD symptom provocation studies found significant activation of the left precuneus (Rotge et al., 2008). Similarly, Menzies et al. (2008) found significant activation of the left precuneus in their meta-analysis of fMRI studies comparing OCD patients and controls during task performance (Menzies et al., 2008). It has also been found that regional cerebral blood flow (rCBF) to the precuneus is significantly reduced in OCD following successful cognitive-behavioural therapy, further supporting a relationship with symptoms (Berk et al., 2009). Moreover, evidence suggests that OCD patients have alterations in the functional relationship between the DMN and the more ‘task positive’ networks, such as the fronto-parietal network, so that the DMN does not ‘switch off’ during task processing as would normally be expected (Stern et al., 2011). Stern et al. (2012) found a reduction of negative correlations between the two networks in patients compared with controls, and found patients to have hyperconnectivity between the left anterior insula and the posterior cingulate/precuneus.
The precuneus has also been strongly implicated in schizophrenia. Structural studies have reported abnormal connectivity of the precuneus of patient groups (Cheung et al., 2008) and also cortical thinning of the precuneus (Narr et al., 2005; Schultz et al., 2010). Reduced grey matter in the left precuneus has been observed in a large voxel-based morphometry study of individuals at high genetic risk for psychosis compared with controls (Fusar-Poli et al., 2011), suggesting this to be a potential biomarker. Functional experiments, with particular relevance to TAF, have shown increased activity in the precuneus of schizophrenic patient groups during attribution tasks, suggested to reflect automatic self-reflection, and to accompany delusions of reference (Menon et al., 2011). Delusions of reference refers to the endorsement, normally found in schizophrenic patients, that generic stimuli are related to themselves. As previously mentioned, parallels have been drawn between TAF and delusions of reference (Starcevic and Berle, 2006), both of which involve misattributions of personal significance, so it is interesting to see that precuneus activity has been found to accompany this phenomenon.
In GAD, abnormal connectivity has been observed between the posterior cingulate and precuneus and prefrontal cortices (Strawn et al., 2012), and significantly greater right precuneus volume has been found in adolescents with GAD compared with healthy controls (Strawn et al., 2013). Further, in young adults a positive relationship has been found between cortical thickness of the precuneus/PCC and depression/anxiety symptoms (Ducharme et al., 2013).
Moreover, in addition to specific involvement of the precuneus in psychopathology, there is considerable evidence of a link between psychopathology and abnormal DMN function (see Broyd et al., 2009 for a review).
4.2. The precuneus in self-attribution, agency, responsibility, and causal reasoning
The functions which the precuneus appears to mediate in healthy participants provide further support to its role in TAF. Aside from the emotions that it elicits, we could speculate that TAF is composed of cognitive components including (i) (mis)-attribution of personal significance and reference, (ii) (mis)-identification of cause and effect, (iii) sense of agency as being the cause of a disastrous consequence, (iv) responsibility and obligation to rectify a disastrous consequence, and (v) in TAF-LO, the belief that harm will come to others rather than the self (Shafran et al., 1996).
If we first consider the attribution of personal significance, there is considerable evidence supporting precuneus involvement in self-referential processing (Cavanna and Trimble, 2006), and furthermore that the precuneus and the medial frontal cortex (including the ACC) are both involved in a self-referential network (Fossati et al., 2003; Johnson et al., 2006; Ochsner et al., 2005; Vogt and Laureys, 2005) allowing awareness of the self through the interlinking of personal identity and past experiences (Andreasen et al., 1995). For example, Johnson et al. (2002) found greater activation of these regions when participants answered questions relating to their personal traits and characteristics compared to semantic knowledge questions. In addition, applying TMS to the precuneus disrupts self-referent judgements, confirming the importance of the region in such tasks. The precuneus' involvement in the brain's DMN, particularly the ventral precuneus (Zhang and Li, 2012), is thought to be partially related to its role in self-referential processing, as self-reflection may be a common aspect of the DMN which occurs when participants are not otherwise engaged in a task (Gusnard and Raichle, 2001).
There is also some evidence to suggest that the precuneus plays a role in cause and effect judgements, supporting its involvement in this aspect of TAF. Fugelsang and Dunbar (2005) reported that precuneus activation accompanies the evaluation of causal hypotheses, Elliott and Dolan (1998) found increased rCBF in the right precuneus, dorsal ACC, VLPFC and thalamus during hypothesis testing of a complex, insoluble task, and Satpute et al. (2005) found right precuneus activity to accompany causal, as opposed to associate, reasoning. Den Ouden et al. (2005) found significant activation of the precuneus when participants answered questions about hypothetical scenarios relating to the causal link between their own intentions and actions or physical events and their consequences. It was suggested that the precuneus/PCC is specifically involved in processing intentions related to the self.
Additionally, there is a reason to believe that the precuneus may underlie cause and effect judgements which are not entirely rational. In a study by Paulus et al. (2001) a two-choice task was used in which participants were required to either predict the location in which a stimulus would appear, in circumstances where no correct answer existed and reinforcement was random. Significant activation of the precuneus was seen during this task, which was directly related to the prediction strategy utilised by participants, so that activation was seen when response selection was not based on previous correct responses rather than when correct responses were repeated. This then provides evidence for the precuneus' involvement in processing cause–effect relationships, particularly those which are not entirely consistent with previous experience. In this way we can see that there may be a role for the precuneus in thought–action fusion, which involves making causal inferences which are not based on previous prediction success, i.e. in the absence of previous experience of thoughts causing events. Similarly, there is evidence to suggest that the precuneus is involved in ‘free’ decision making, that is, based on entirely internal reasoning or feeling rather than perceptually or evidence driven (Bode et al., 2012; Pesaran et al., 2008).
In terms of agency, increased activation of the precuneus has been reported when participants take first-person perspective in a story (participant as agent) compared to third-person (other as agent) perspective (Vogeley et al., 2001). However, there have been inconsistent results in terms of agency, with other studies reporting greater precuneus activation with third-person than first-person perspective taking (Farrer and Frith, 2002; Ruby and Decety, 2001; Vogeley et al., 2004), although this has been suggested to be due to the more vivid representation of the self that may be required in order to imagine another person in detail (Ruby and Decety, 2001) or mental imagery.
The final factor, a sense of responsibility and obligation to negate catastrophe, also has evidence of precuneus involvement. The precuneus has been implicated in risk avoidance (Roy et al., 2011) – although see Paulus et al. (2003) – and has been found to activate when participants focus on their duties and obligations rather than hopes and aspirations (Johnson et al., 2006). Further, Johnson et al. (2006) found that participants' duties and obligations had a greater number of references to other people than their hopes and aspirations, supporting a link responsibility to prevent harm coming to others rather than oneself. Perhaps the most compelling support for the role of the precuneus in TAF comes from the recent studies of Morey et al. (2012) and Cabanis et al. (2012). Morey et al. (2012) presented participants with scenarios describing events in which an action by the participant caused a negative consequence to either themselves or others. They found that in an other-vs-self comparison, greater precuneus and posterior cingulate activity was present when participants imagined being the cause of harm to another person. Similarly, Cabanis et al. (2012) found precuneus activation related to the process of attributing causes to the self. Cabanis et al. presented subjects with sentences describing positive or negative social situations, and required participants to decide whether the situation had been caused by themselves or by the other person involved in the scenario. Their results showed the posterior precuneus (particularly right-sided) to be preferentially activated during self- compared with other- attribution. Furthermore, they found activation of the bilateral insular cortex during the self-attribution of negative vs. positive situations. This is similar to our previous findings of left insula activation during visualisation of scenarios following TAF-induction in a negative vs. a positive TAF condition (Jones and Bhattacharya, 2012).
4.3. The role of upper beta activity in TAF
All between-group differences in the current analysis were found in the beta-2 and beta-3 frequency bands. In contrast to the lower frequency bands, comparatively little is known about the functional correlates of upper beta activity. However, it has been linked to both clinical and non-clinical anxieties, which support a role in TAF: Alterations in the upper beta frequencies have been found in comparisons between non-clinical controls and OCD patients (Maihöfner et al., 2007; Velikova et al., 2010) and schizophrenic patients (Yeragani et al., 2006). Furthermore, synchronized activity in the upper beta band has been found during modulations of a sense of agency (Kang et al., 2011).
In terms of DMN processing, the frequency in which our results were found appears to be supportive of activation in this network. While the relationship between DMN processing and EEG oscillations is complex, it appears that higher frequencies (in this case alpha and above) are positively correlated with DMN activation, while lower frequencies show a negative relationship (Knyazev, 2013). In a study of DMN processing using simultaneous fMRI and EEG, Laufs et al. (2003) observed significant positive correlations specifically between beta-2 band and BOLD signal in DMN regions including the precuneus, PCC, and retrosplenial cortex. The authors stated that the activity in the 17–23 Hz beta band is a “signature of activity in the brain network that underlies this default mode of brain function” (pp.11057).
4.4. Limitations
The results of the current study showed a significant positive relationship between induced TAF and accompanying brain activity and measures of OCD symptoms and magical ideation. However, as participants' anxiety and depression were not measured we cannot infer a direct relationship. Indeed, it is very likely that anxiety and depressive symptoms would also correlate, and that the relationship between OC symptoms and TAF would be mediated by this negative affect (Abramowitz et al., 2003). However, as the aim of this study was to examine the neural correlates of TAF as viewed as a pathognomonic phenomenon, rather to identify the personality or symptom dimensions most strongly associated with TAF, we do not consider this limitation to reduce the validity of the current findings — particularly as no evidence to our knowledge has found qualitative differences between the experience of TAF-LO found in different patient groups. There are, on the other hand, likely to be differences between the neural correlates of TAF-LO and TAF-LS, as well as with TAF-M.
A second limitation is that while the source localisation used in the current study has high validity of localising cortical sources (Pascual-Marqui et al., 2002), it is unable to localise activity to deep brain regions; hence differences in thalamic activity, for example, cannot be ruled out. An additional limitation of the present study involves the use of two combined experimental samples: While ‘sample’ is used as a covariate in all analyses, and there appear to be no significant baseline or TAF-induced differences between samples, this combination may still have consequences for the statistical analysis and significance.
Furthermore, it should be noted that although the High-OC group scored significantly higher than the Low-OC group on TAF-induced anxiety, guilt, likelihood and urge to neutralize, their mean scores for all of these measures was still rather low (< 5). This appears to be lower than those reported by Rachman et al. (1996) who asked participants to use a verbal scale from 0 to 100, and Bocci and Gordon (2007) who used a paper VAS with anchors between 0 and 100%. It is possible that the method of supplying responses in the current experiment (via a keypad) led to this reduction, as a result of increased time and effort required to indicate a higher score. Alternatively the use of multiple trials may have had an effect, either through sensitization over the course of the experiment, or through a difference in the absolute likelihood of the negative events used. If possible, future studies could use a single-trial design but with a much larger sample size to avoid these difficulties.
5. Conclusions
The current study provides a novel insight into the neural mechanisms of thought–action fusion. In suggesting high frequency activity in the precuneus to mediate this phenomenon, the current results increase understanding of the link between brain activity and specific symptoms, with potential implications for a plethora of psychological disorders. Further neuroscientific research is needed to replicate the current findings, and explore the possibility of using this knowledge to modify the bias.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Abramowitz J.S., Whiteside S., Lynam D., Kalsy S. Is thought–action fusion specific to obsessive–compulsive disorder? A mediating role of negative affect. Behav. Res. Ther. 2003;41:1069–1079. doi: 10.1016/s0005-7967(02)00243-7. [DOI] [PubMed] [Google Scholar]
- Amir N., Freshman M., Ramsey B., Neary E., Brigidi B. Thought–action fusion in individuals with OCD symptoms. Behav. Res. Ther. 2001;39:765–776. doi: 10.1016/s0005-7967(00)00056-5. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., O'Leary D.S., Cizadio T., Arndt S., Rezai K., Watkins G.L., Ponto L.L., Hichwa R.D. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatr. 1995;152:1576–1587. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Berk G., Ergun B.M., Narin Y., Basoglu C., Gonul A.S., Ebrinc S., Cetin M., Sungar M.Z. The effects of cognitive-behavioural therapy on brain regional cerebral blood flow in obsessive–compulsive disorder. Basic Clin. Neurosci. Brain Imaging. 2009:S312. [Google Scholar]
- Bocci L., Gordon P.K. Does magical thinking produce neutralising behaviour? An experimental investigation. Behav. Res. Ther. 2007;45:1823–1833. doi: 10.1016/j.brat.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Bode S., Bogler C., Soon C.S., Haynes J.D. The neural encoding of guesses in the human brain. Neuroimage. 2012;16:1924–1931. doi: 10.1016/j.neuroimage.2011.08.106. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Burke E.J.S. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cabanis M., Pyka M., Mehl S., Müller B.W., Loos-Jankowiak S., Winterer G., Wölwer W., Musso F., Klingberg S., Rapp A.M., Langohr K., Wiedermann G., Herrlich J., Walter H., Wagner M., Schnell K., Vogely K., Kockler H., Shah N.J., Stöcker T., Thienel R., Pauly K., Krug A., Kircher T. The precuneus and the insula in self-attributional processes. Cogn. Affect. Behav. Neurosci. 2012;13:330–345. doi: 10.3758/s13415-012-0143-5. [DOI] [PubMed] [Google Scholar]
- Carmona S., Bassas N., Rovira M., Gispert J.-D., Soliva J.-C., Prado M., Tomas J., Bulbena A., Vilarroya O. Pediatric OCD structural brain deficits in conflict monitoring circuits: a voxel-based morphometry study. Neurosci. Lett. 2007;421:218–223. doi: 10.1016/j.neulet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.F., Gründler T.O.J., Frank M.J., Allen J.J.B. Altered cingulate sub-region activation accounts for task-related dissociation in ERN amplitude as a function of obsessive–compulsive symptoms. Neuropsychologia. 2010;48:2089–2109. doi: 10.1016/j.neuropsychologia.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cheung V., Cheung C., McAlonan G.M., Deng Y., Wong J.G., Yip L., Tai K.S., Khong P.L., Sham P., Chua S.E. A diffusion tensor imaging study of structural connectivity in never-medicated, first-episode schizophrenia. Psychol. Med. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Den Ouden H.E.M., Frith U., Frith C., Blakemore S.-J. Thinking about intentions. Neuroimage. 2005;28:787–796. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ducharme S., Albaugh M.D., Hudziak J.J., Botteron K.N., Nguyen T.-V., Truong C., Evans A.C., Karama S. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Urso G., Mantovani A., Micillo M., Priori A., Muscettola G. Transcranial direct current stimulation and cognitive-behavioural therapy: evidence of a synergistic effect in treatment-resistant depression. Brain Stimul. 2013;6:465–467. doi: 10.1016/j.brs.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Eckblad M., Chapman L.J. Magical ideation as an indicator of schizotypy. J. Consult. Clin. Psychol. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Elliott R., Dolan R.J. Activation of different anterior cingulate foci in association with hypothesis testing and response selection. Neuroimage. 1998;8:17–29. doi: 10.1006/nimg.1998.0344. [DOI] [PubMed] [Google Scholar]
- Evans D.W., Hersperger C., Capaldi P.A. Thought–action fusion in childhood: measurement, development, and association with anxiety, rituals and other compulsive-like behaviors. Child Psychiatry Hum. Dev. 2011;42:12–23. doi: 10.1007/s10578-010-0198-x. [DOI] [PubMed] [Google Scholar]
- Farrer C., Frith C.D. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Huppert J.D., Leiberg S., Langner R., Kichic R. The obsessive–compulsive inventory: development and validation of a short version. Psychol. Assess. 2002;14:485–496. [PubMed] [Google Scholar]
- Fossati P., Hevenor S.J., Graham S.J., Grady C., Keightley M.L., Craik F., Mayberg H. In search of the emotional self: an fMRI study using positive and negative emotional words. Am. J. Psychiatr. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Frei E., Gamma A., Pascual-Marqui R., Lehmann D., Hell D., Vollenweider F.X. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA) Hum. Brain Mapp. 2001;14:152–165. doi: 10.1002/hbm.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugelsang J.A., Dunbar K.N. Brain-based mechanisms underlying complex causal thinking. Neuropsychologia. 2005;43:1204–1213. doi: 10.1016/j.neuropsychologia.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S., Crescini A., Deste G., Kempton M.J., Lawrie S., McGuire P., Schetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Grieve S.M., Korgaonkar M.S., Koslow S.H., Gordon E., Williams L.M. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründler T.O.J., Cavanagh J.F., Figueroa C.M., Frank M.J., Allen J.J.B. Task-related dissociation in ERN amplitude as a function of obsessive–compulsive symptoms. Neuropsychologia. 2009;47:1978–1987. doi: 10.1016/j.neuropsychologia.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hazlett-Stevens H., Zucker B.G., Craske M.G. The relationship of thought–action fusion to pathological worry and generalized anxiety disorder. Behav. Res. Ther. 2002;40:1199–1204. doi: 10.1016/s0005-7967(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Hossein G.K., Ne'mat M., Niloofar M. Comparison of thought–action fusion in peoples with obsessive–compulsive disorder and major depression disorder. Int. J. Appl. Psychol. 2012;2:77–82. [Google Scholar]
- Jasper H.H. The ten-twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Johnson M.K., Baxter L.C., Wilder L.S., Pipe J.G., Heiserman J.E., Prigatano G.P. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Raye C.L., Mitchell K.J., Touryan S.R., Greene E.J., Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. SCAN. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Bhattacharya J. Alpha activity in the insula accompanies the urge to neutralize in sub-clinical obsessive–compulsive participants. J. Behav. Addict. 2012;1:96–105. doi: 10.1556/JBA.1.2012.005. [DOI] [PubMed] [Google Scholar]
- Kabakci E., Demir B., Demirel H., Şevik A.E. Thought–action fusion: Is it present in schizophrenia? Behav. Chang. 2008;25:169–177. [Google Scholar]
- Kang S.Y., Sohn Y.H., Park J., Nahab F.B., Kakareka J., Miletta N., Im C.-H., Hallett M. Brain networks responsible for sense of agency: an EEG study. Clin. Neurophysiol. 2011;122, S1:9. [Google Scholar]
- Knyazev G.G. EEG correlates of self-referential processing. Front. Hum. Neurosci. 2013;7:264. doi: 10.3389/fnhum.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M.-F., Paulus W., Nitsche M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- Laufs H., Krakow K., Sterzer P., Eger E., Beyerle A., Salek-Haddadi A., Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. PNAS. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-J., Cougle J.R., Telch M.J. Thought–action fusion and its relationship to schizotypy and OCD symptoms. Behav. Res. Ther. 2005;43:29–41. doi: 10.1016/j.brat.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Maihöfner C., Sperling W., Kaltenhäuser M., Bleich S., de Zwaan M., Wiltfang J., Thürauf N., Elstner S., Reulbach U., Lewzuk P., Kornhuber J., Ropohl A. Spontaneous megnetoencephalographic activity in patients with obsessive–compulsive disorder. Brain Res. 2007;1129:200–205. doi: 10.1016/j.brainres.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Marcks B.A., Woods D.W. Role of thought-related beliefs and coping strategies in the escalation of intrusive thoughts: An analog to obsessive–compulsive disorder. Behav. Res. Ther. 2007;45:2640–2651. doi: 10.1016/j.brat.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Menon M., Schmitz T.W., Anderson A.K., Graff A., Korostil M., Mamo D., Gerretsen P., Addington J., Remington G., Shitij K. Exploring the neural correlates of delusions of reference. Biol. Psychiatry. 2011;70:1127–1133. doi: 10.1016/j.biopsych.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Menzies L., Chamberlain S.R., Laird A.R., Thelen S.M., Sahakian B.J., Bullmore E.T. Integrating evidence from neuroimaging and neuropsychological studies of obsessive–compulsive disorder: the orbitofronto-striatal model revisited. Neurosci. Biobehav. Rev. 2008;32:525–549. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.F., Brown T.A. Psychometric evaluation of the thought-action fusion scale in a large clinical sample. Assessment. 2012 doi: 10.1177/1073191112436670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., McCarthy G., Selgrade E.S., Seth S., Nasser J.D., LaBar K.S. Neural systems for guilt from actions affecting self versus others. Neuroimage. 2012;60(1):683–692. doi: 10.1016/j.neuroimage.2011.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P., Merckelbach H. Thought–action fusion and schizotypy in undergraduate students. Br. J. Clin. Psychol. 2003;42:211–216. doi: 10.1348/014466503321903616. [DOI] [PubMed] [Google Scholar]
- Muris P., Meesters C., Rassin E., Merckelbach H., Campbell J. Thought–action fusion and anxiety disorders symptoms in normal adolescents. Behav. Res. Ther. 2001;39:843–852. doi: 10.1016/s0005-7967(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Narr K.L., Toga A.W., Szeszko P., Thompson P.M., Woods R.P., Robinson D., Sevy S., Wang Y., Schrock K., Bilder R.M. Cortical thinning in the cingulate and orbital cortices in first episode schizophrenia. Biol. Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Nicols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Beer J.S., Robertson E.R., Cooper J.C., Gabrieli J.D., Kihsltrom J.F., D'Esposito M. Te neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Review of methods for solving the EEG inverse problem. Int. J. Biomagn. 1999;1:75–86. [Google Scholar]
- Pascual-Marqui R.D., Michel C.M., Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D., Esslen M., Kochi K., Lehmann D. Functional imaging with low resolution brain electromagnetic tomography (LORETA): a review. Methods Find. Exp. Clin. Pharmacol. 2002;24C:91–95. [PubMed] [Google Scholar]
- Paulus M.P., Hozack N., Zauscher B., McDowell J.E., Frank L., Brown G.G., Braff D.L. Prefrontal, parietal, and temporal cortex networks underlie decision-making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Rogalsky C., Simmons A., Feinstein J.S., Stein M.B. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Pesaran B., Nelson M.J., Anderson R.A. Free choice activates a decision circuit between frontal and parietal. Nature. 2008;15:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F., Piras F., Chiapponi C., Girardi P., Caltagirone C., Spalletta G. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2013 doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Rachman S. Obsessions, responsibility and guilt. Behav. Res. Ther. 1993;31:149–154. doi: 10.1016/0005-7967(93)90066-4. [DOI] [PubMed] [Google Scholar]
- Rachman S., Thordarson D.S., Shafran R., Woody S.R. Perceived responsibility: structure and significance. Behav. Res. Ther. 1995;33:779–784. doi: 10.1016/0005-7967(95)00016-q. [DOI] [PubMed] [Google Scholar]
- Rachman S., Shafran R., Mitchell D., Trant J., Teachman B. How to remain neutral: an experimental analysis of neutralization. Behav. Res. Ther. 1996;34:889–898. doi: 10.1016/s0005-7967(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Radomsky A.S., Shafran R., Coughtrey A.E., Rachman S. Cognitive-behavior therapy for compulsive checking in OCD. Cogn. Behav. Pract. 2010;17:119–131. [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassin E. The contribution of thought-action fusion and thought suppression in the development of obsession-like intrusions in normal participants. Behav. Res. Ther. 2001;39:1023–1032. doi: 10.1016/s0005-7967(00)00075-9. [DOI] [PubMed] [Google Scholar]
- Rassin E., Muris P., Schmidt H., Merckelbach H. Relationships between thought-action fusion, thought suppression and obsessive–compulsive symptoms: a structural equation modelling approach. Behav. Res. Ther. 2000;38:889–897. doi: 10.1016/s0005-7967(99)00104-7. [DOI] [PubMed] [Google Scholar]
- Rassin E., Diepstraten P., Merckelbach H., Muris P. Thought–action fusion and thought suppression in obsessive–compulsive disorder. Behav. Res. Ther. 2000;39:757–764. doi: 10.1016/s0005-7967(00)00051-6. [DOI] [PubMed] [Google Scholar]
- Rassin E., Merckelbach H., Muris P., Schmidt H. The thought–action fusion scale: further evidence for its reliability and validity. Behav. Res. Ther. 2001;39:537–544. doi: 10.1016/s0005-7967(00)00031-0. [DOI] [PubMed] [Google Scholar]
- Rotge J.-Y., Dominique G., Dilharreguy B., Cuny E., Tognol J., Bioulac B., Allard M., Burbaud P., Aouzerate B. Provocation on obsessive–compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J. Psychiatry Neurosci. 2008;33:405–412. [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Gotimer K., Kelly A.M.C., Castellanos F.X., Milham M.P., Ernst M. Uncovering putative neural markers of risk avoidance. Neuropsychologia. 2011;49:937–944. doi: 10.1016/j.neuropsychologia.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P., Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Salkovskis P.M. Obsessional-compulsive problems: a cognitive-behavioural analysis. Behav. Res. Ther. 1985;25:571–583. doi: 10.1016/0005-7967(85)90105-6. [DOI] [PubMed] [Google Scholar]
- Satpute A.B., Fenker D.B., Waldmann M.R., Tabibnia G., Holyoak K.J., Lieberman M.D. An fMRI study of causal judgements. Eur. J. Neurosci. 2005;22:1233–1238. doi: 10.1111/j.1460-9568.2005.04292.x. [DOI] [PubMed] [Google Scholar]
- Schultz C.C., Koch K., Wagner G., Roebel M., Nenedic I., Schachtzabel C., Reichenbach J.R., Sauer H., Schlösser R.G.M. Complex pattern of cortical thinning in schizophrenia: results from an automated surface based analysis of cortical thickness. Psychiatry Res. Neuroimaging. 2010;182:134–140. doi: 10.1016/j.pscychresns.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Shafran R., Rachman S. Thought–action fusion: a review. J. Behav. Ther. Exp. Psychiatry. 2004;35:87–107. doi: 10.1016/j.jbtep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Shafran R., Thordarson D.S., Rachman S. Thought–action fusion in obsessive–compulsive disorder. J. Anxiety Disord. 1996;10(5):379–391. [Google Scholar]
- Soriano-Mas C., Pujol J., Alonso P., Cardoner M., Menchón J.M., Harrison B.J., Deus J., Vallejo J., Gaser C. Identifying patients with obsessive–compulsive disorder using whole-brain anatomy. Neuroimage. 2007;35:1028–1037. doi: 10.1016/j.neuroimage.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Starcevic V., Berle D. Cognitive specificity of anxiety disorders: a review of selected key constructs. Depress. Anxiety. 2006;23:51–61. doi: 10.1002/da.20145. [DOI] [PubMed] [Google Scholar]
- Stern E.R., Welsh R.C., Fitzgerald K.D., Gehring W.J., Liste J.J., Himle J.A., Abelson J.L., Taylor S.F. Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive–compulsive disorder. Biol. Psychiatry. 2011;69:583–591. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern E.R., Fitzgerald K.D., Welsh R.C., Abelson J.L., Taylor S.F. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive–compulsive disorder. PLoS One. 2012;7:e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn J.R., Bitter S.M., Weber W.A., Chu W.-J., Whitsel R.M., Adler C., Cerullo M.A., Eliassen J., Strakowski S.M., DelBello M.P. Neurocircuitry of generalized anxiety disorder in adolescents: a pilot functional neuroimaging and functional connectivity study. Depress. Anxiety. 2012;29:939–947. doi: 10.1002/da.21961. [DOI] [PubMed] [Google Scholar]
- Strawn J.R., Wehry A.M., Chu W.J., Adler C.M., Eliassen J.C., Cerullo M.A., Strakowski S.M., Delbello M.P. Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: a voxel-based morphometry study. Depress. Anxiety. 2013 doi: 10.1002/da.22089. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tourboux P. Thieme; Stuttgart: 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Thompson-Hollands J., Farchione T.J., Barlow D.H. Thought–action fusion across anxiety disorder diagnoses: specificity and treatment effects. J. Nerv. Ment. Disord. 2013;201(5):407–413. doi: 10.1097/NMD.0b013e31828e102c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel O.A., Remijnse P.L., Mataix-Cols D., Vrenken H., Groenewegen H.J., Uylings H.B.M., van Balkom A.J.L.M., Veltman D.J. The major symptom dimensions of obsessive–compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- Van den Hout M., Van Pol M., Peters M. On becoming neutral: effects of experimental neutralizing reconsidered. Behav. Res. Ther. 2001;39:1439–1448. doi: 10.1016/s0005-7967(00)00109-1. [DOI] [PubMed] [Google Scholar]
- Van den Hout M., Kindt M., Weiland T., Peters M. Instructed neutralization, spontaneous neutralization and prevented neutralization after an obsession-like thought. J. Behav. Ther. Exp. Psychiatry. 2002;33:177–189. doi: 10.1016/s0005-7916(02)00048-4. [DOI] [PubMed] [Google Scholar]
- Velikova S., Locatelli M., Insacco C., Smeraldi E., Comi G., Leocani L. Dysfunctional brain circuitry in obsessive–compulsive disorder: source and coherence analysis of EEG rhythms. Neuroimage. 2010;49:977–983. doi: 10.1016/j.neuroimage.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Vogeley K., Bussfeld P., Newen A., Herrman S., Happé F., Falkai P., Maier W., Shah N.J., Fink G.R., Zilles K. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vogeley K., May M., Ritzl A., Falkai P., Zilles K., Fink G.R. Neural correlates of first-person perspective as one constituent of human self-consciousness. J. Cogn. Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P.D. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans. Audio Electroacoust. 1967;AU-15:70–73. [Google Scholar]
- Yeragani V.K., Cashmere D., Miewald J., Tancer M., Keshavan M.S. Decreased coherence in higher frequency ranges (beta and gamma) between central and frontal EEG in patients with schizophrenia: a preliminary report. Psychiatry Res. 2006;141:53–60. doi: 10.1016/j.psychres.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang S., Li C.S. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012;59(4):3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker B.G., Craske M.G., Barrios V., Holguin M. Thought–action fusion: can it be corrected? Behav. Res. Ther. 2002;40:653–664. doi: 10.1016/s0005-7967(01)00054-7. [DOI] [PubMed] [Google Scholar]


