Abstract
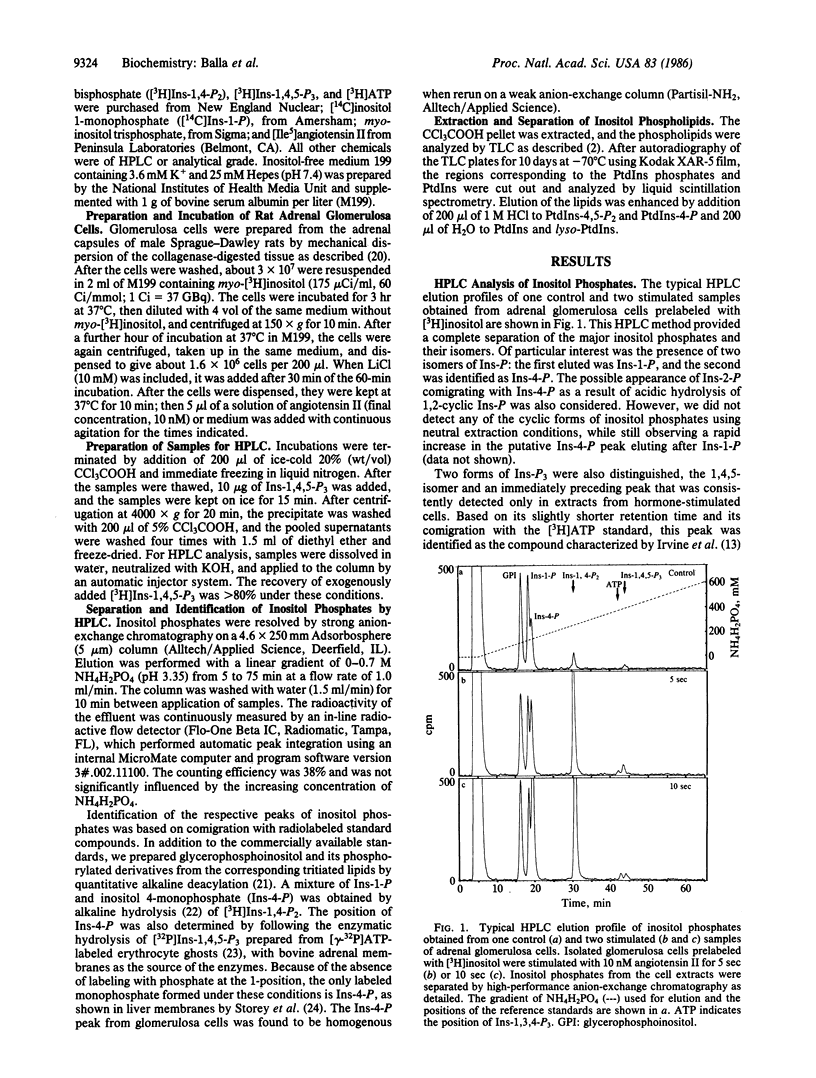
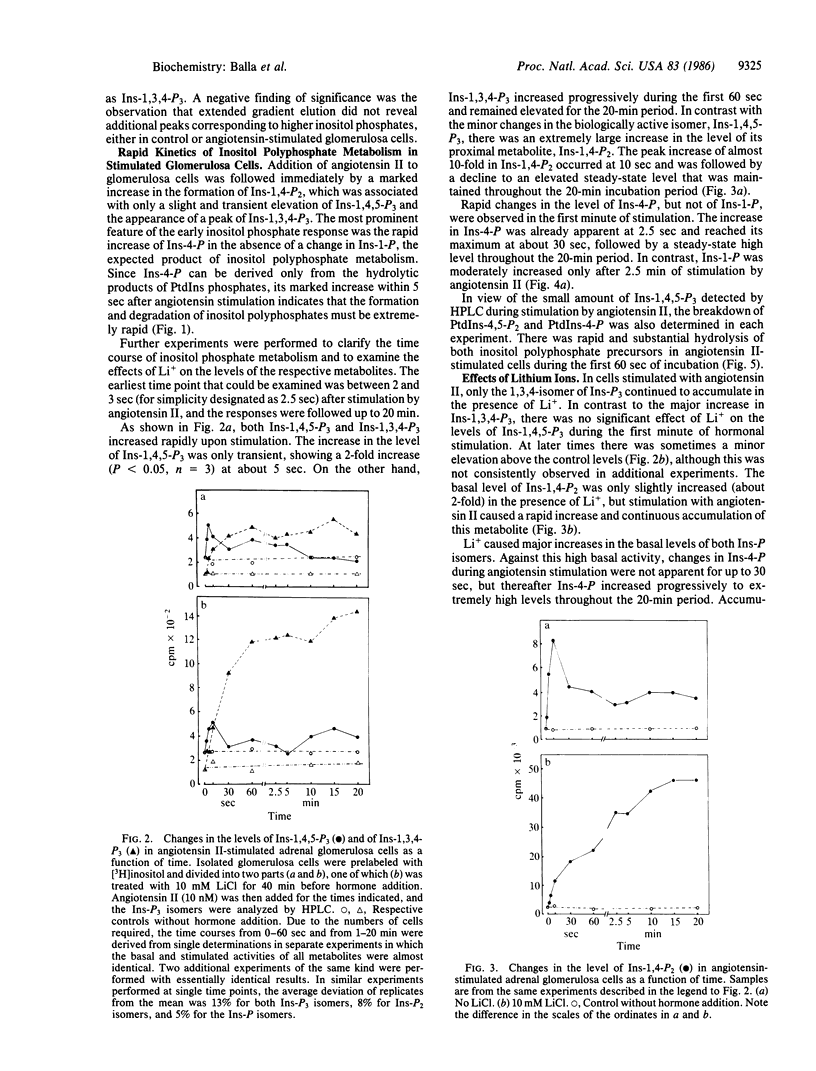
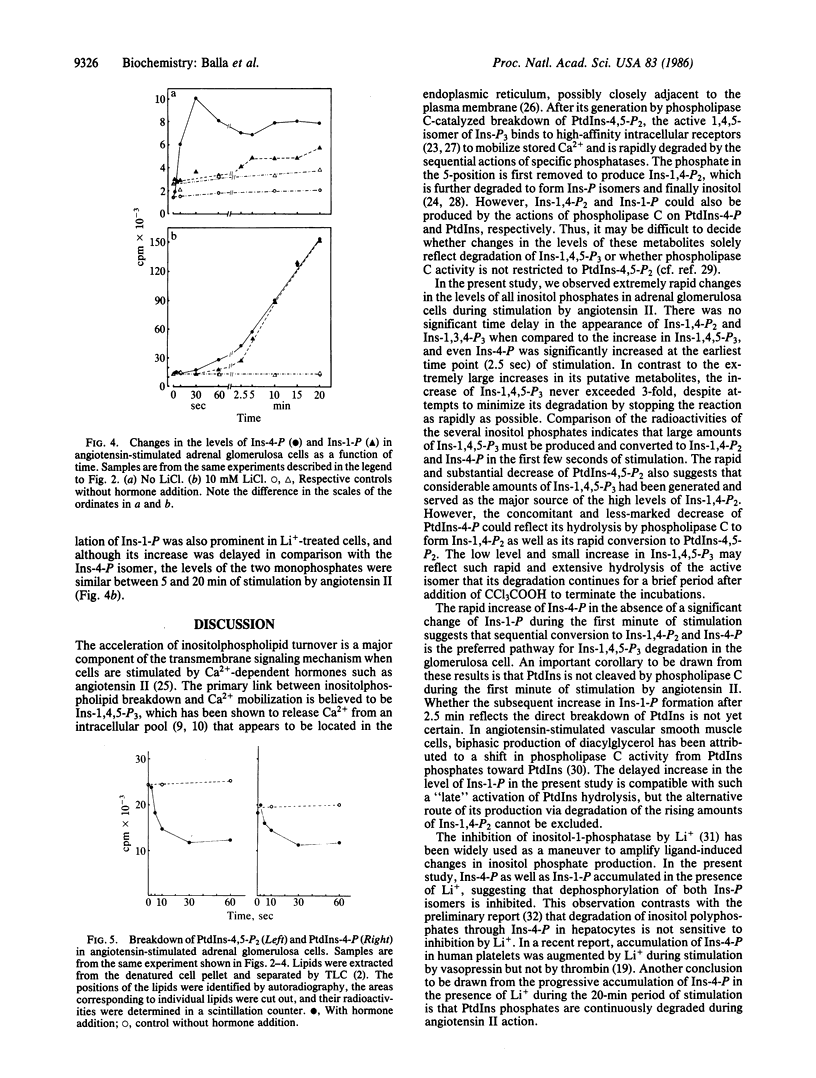
The production and metabolism of inositol phosphates in rat adrenal glomerulosa cells prelabeled with [3H]inositol and stimulated with angiotensin II were analyzed by high-performance anion-exchange chromatography. Exposure to angiotensin II was accompanied by a rapid and substantial decrease in the phospholipid precursor, phosphatidylinositol (PtdIns) 4,5-bisphosphate with only a slight and transient increase in the level of the biologically active product, inositol 1,4,5-trisphosphate (Ins-1,4,5-P3), to a peak at about 5 sec. Inositol 1,3,4-trisphosphate (Ins-1,3,4-P3), the putative metabolite of Ins-1,4,5-P3, was also formed rapidly and maintained an elevated steady-state level during stimulation by angiotensin II. Inositol 1,4-bisphosphate (Ins-1,4-P2) exhibited a simultaneous and prominent increase that could not be accounted for solely by direct breakdown of PtdIns 4-phosphate, indicating that large amounts of Ins-1,4,5-P3 must also have been produced and metabolized. The rapid formation of a substantial amount of inositol 4-monophosphate (Ins-4-P), with no significant change in the level of inositol 1-monophosphate (Ins-1-P) during the first minute of stimulation, was a notable feature of the glomerulosa cell response to angiotensin II. These observations indicate that PtdIns-4,5-P2 catabolism in the angiotensin-stimulated glomerulosa cell initially proceeds via Ins-1,4,5-P3 through Ins-1,3,4-P3 and Ins-1,4-P2 to form Ins-4-P rather than Ins-1-P and that direct hydrolysis of PtdIns by phospholipase C does not occur during the initial phase of angiotensin action. In glomerulosa cells stimulated by angiotensin II in the presence of Li+, the progressive accumulation of both Ins-4-P, and after a short lag period, Ins-1-P indicated that dephosphorylation of both isomers was inhibited by Li+. The increase of Ins-P isomers in the presence of Li+ was associated with increased and progressive accumulation of Ins-1,4-P2 and Ins-1,3,4-P3 but not of Ins-1,4,5-P3. These data demonstrate that sustained and massive breakdown of PtdIns phosphates begins within seconds during cell activation by angiotensin II. The Ca2+-mobilizing metabolite, Ins-1,4,5-P3, is rapidly converted to Ins-1,3,4-P3 and degraded through Ins-1,4-P2 and Ins-4-P, in contrast to the previous view that conversion to Ins-1-P is the major route of PtdIns 4,5-bisphosphate metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baukal A. J., Guillemette G., Rubin R., Spät A., Catt K. J. Binding sites for inositol trisphosphate in the bovine adrenal cortex. Biochem Biophys Res Commun. 1985 Dec 17;133(2):532–538. doi: 10.1016/0006-291x(85)90939-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Braley L., Menachery A., Brown E., Williams G. The effects of extracellular K+ and angiotensin II on cytosolic Ca++ and steroidogenesis in adrenal glomerulosa cells. Biochem Biophys Res Commun. 1984 Sep 17;123(2):810–815. doi: 10.1016/0006-291x(84)90302-4. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Jornot L., Vallotton M. B. Correlation between cytosolic free Ca2+ and aldosterone production in bovine adrenal glomerulosa cells. Evidence for a difference in the mode of action of angiotensin II and potassium. J Biol Chem. 1984 Jul 25;259(14):8863–8869. [PubMed] [Google Scholar]
- Clarke N. G., Dawson R. M. Alkaline O leads to N-transacylation. A new method for the quantitative deacylation of phospholipids. Biochem J. 1981 Apr 1;195(1):301–306. doi: 10.1042/bj1950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. The formation of inositol 1,2-cyclic phosphate on agonist stimulation of phosphoinositide breakdown in mouse pancreatic minilobules. Evidence for direct phosphodiesteratic cleavage of phosphatidylinositol. J Biol Chem. 1985 Dec 25;260(30):16068–16071. [PubMed] [Google Scholar]
- Douglas J., Aguilera G., Kondo T., Catt K. Angiotensin II receptors and aldosterone production in rat adrenal glomerulosa cells. Endocrinology. 1978 Mar;102(3):685–696. doi: 10.1210/endo-102-3-685. [DOI] [PubMed] [Google Scholar]
- Enyedi P., Büki B., Muscsi I., Spät A. Polyphosphoinositide metabolism in adrenal glomerulosa cells. Mol Cell Endocrinol. 1985 Jun;41(1):105–112. doi: 10.1016/0303-7207(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Larson R. E., Davis J. S. Rapid effects of angiotensin-II on polyphosphoinositide metabolism in the rat adrenal glomerulosa. Endocrinology. 1984 Jan;114(1):302–304. doi: 10.1210/endo-114-1-302. [DOI] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Sano K., Hoshijima M., Takai Y., Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982 Oct;3(4-5):323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Michell B. Inositol phosphates. Profusion and confusion. Nature. 1986 Jan 16;319(6050):176–177. doi: 10.1038/319176a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Munsell L. Y., Gish B. G., Honchar M. P. Effects of systemically administered lithium on phosphoinositide metabolism in rat brain, kidney, and testis. J Neurochem. 1985 Mar;44(3):798–807. doi: 10.1111/j.1471-4159.1985.tb12886.x. [DOI] [PubMed] [Google Scholar]
- Siess W. Evidence for the formation of inositol 4-monophosphate in stimulated human platelets. FEBS Lett. 1985 Jun 3;185(1):151–156. doi: 10.1016/0014-5793(85)80760-2. [DOI] [PubMed] [Google Scholar]
- Spät A., Fabiato A., Rubin R. P. Binding of inositol trisphosphate by a liver microsomal fraction. Biochem J. 1986 Feb 1;233(3):929–932. doi: 10.1042/bj2330929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Sherman W. R., Berger R. A., Majerus P. W. Inositol cyclic phosphates are produced by cleavage of phosphatidylphosphoinositols (polyphosphoinositides) with purified sheep seminal vesicle phospholipase C enzymes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4013–4017. doi: 10.1073/pnas.82.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]



