Abstract
Sexual antagonism, whereby mutations are favourable in one sex and disfavourable in the other, is common in natural populations, yet the root causes of sexual antagonism are rarely considered in evolutionary theories of adaptation. Here, we explore the evolutionary consequences of sex-differential selection and genotype-by-sex interactions for adaptation in species with separate sexes. We show that sexual antagonism emerges naturally from sex differences in the direction of selection on phenotypes expressed by both sexes or from sex-by-genotype interactions affecting the expression of such phenotypes. Moreover, modest sex differences in selection or genotype-by-sex effects profoundly influence the long-term evolutionary trajectories of populations with separate sexes, as these conditions trigger the evolution of strong sexual antagonism as a by-product of adaptively driven evolutionary change. The theory demonstrates that sexual antagonism is an inescapable by-product of adaptation in species with separate sexes, whether or not selection favours evolutionary divergence between males and females.
Keywords: intralocus sexual conflict, Fisher's geometric model, sexual dimorphism, pleiotropy, evolutionary constraint, adaptation
1. Introduction
Species with separate sexes face two important evolutionary challenges that can limit their abilities to adapt to a changing environment. First, discrete sexes are common among complex organisms, in which pleiotropic effects can constrain adaptation [1–3]. Second, patterns of selection and the phenotypic effects of mutations each differ between the sexes [4–6], which can decouple the genetic basis of male versus female fitness. When male and female fitness is decoupled, mutations benefitting one sex will sometimes be deleterious to the other (i.e. their fitness effects are ‘sexually antagonistic’ [7]), which diminishes a population's ability to respond to selection through fixation of beneficial mutations.
An emerging body of data implies that sexual antagonism is an important feature of animal and plant populations [8–11]. Nevertheless, several fundamental questions regarding sexual antagonism remain unaddressed. For example, how often does sexual antagonism arise owing to sex differences in the direction of selection? How severely does sexual antagonism limit the rate of adaptation in each sex, and how do such constraints evolve over time? How does environmental change mediate opportunities for sexual antagonism, including differences between poorly versus well-adapted populations?
These questions can be addressed with theoretical models that effectively bridge the divide between empirically measurable properties of mutation and selection, and the evolutionary genetic patterns and processes that we seek to understand. Quantitative genetics theory has proved extremely useful for quantifying short-term constraints to sex-specific adaptive evolution [12], yet we currently lack clear theoretical predictions about the underlying population genetics of sexual antagonism, including the fraction of mutations that has sexually antagonistic fitness effects, and the role of sexual antagonism in shaping the long-term evolutionary trajectories of males and females in adaptively evolving populations. With these issues in mind, we developed a two-sex extension of Fisher's geometric model [13], and used it to characterize the sex-specific distribution of mutant fitness effects, and the population genetic dynamics of adaptation in species with separate sexes.
2. Fisher's geometric model in species with separate sexes
Fisher's original model provides a simplified mapping between genotype, phenotype and fitness that captures the basic biological details of adaptation within a complex organism: (i) mutations cause random changes within multi-dimensional trait space; (ii) selection favours those mutations that move the system of traits closer to an optimal phenotypic value that maximizes fitness; and (iii) a population approaches its fitness optimum by successively fixing beneficial mutations [14,15]. Although alternative modes of evolutionary change—e.g. polygenic adaptation involving modest allele frequency shifts at many loci—may also contribute to adaptation over short evolutionary time-scales, such short-term responses may be weak when pleiotropic constraints—including sexual antagonism—are severe [16–18]. In such cases, adaptation is likely to be dominated by the fixation of beneficial mutations, provided directional selection is sustained over time [19,20]. Fisher's model also makes simplifying assumptions about the mutational architecture of traits, yet its original assumptions can be relaxed without greatly altering the model's basic predictions [21–23] and these predictions are adept at explaining a variety of interesting empirical patterns that emerge from the study of real organisms (e.g. [24–28]).
In extending Fisher's model to a two-sex system, we characterize the evolution of n traits that are each expressed, but not necessarily equally, in males and females (see the electronic supplementary material). Sets of trait values are depicted using Cartesian coordinates, with each set of coordinates representing an individual's genetically determined position in n-dimensional phenotypic space. Following prior work, we assume that fitness depends on the Euclidean distance of each sex to its optimum, and mutational effects are unbiased in direction (uniformly oriented in n-dimensional space [13,29,30]). These assumptions are not restrictive as long as we interpret n as the ‘effective complexity’ of a species (i.e. the effective number of traits can be less than the actual number [21–23]). To simplify the presentation, we model mutation and evolution in a haploid population; our results also apply to diploids, with the caveat that mutations contributing to adaptation in diploids will sometimes involve a transient, balanced polymorphic state [30]. Finally, although we focus on evolution in dioecious (gonochoristic) species with distinct sexes, sexual antagonism may also manifest within hermaphrodites, by way of allocation trade-offs between male and female reproductive structures in simultaneous hermaphrodites, or antagonistic pleiotropy between male and female stages in sequential hermaphrodites [31]. Our results may imperfectly characterize adaptation in hermaphrodites, yet our underlying mathematical framework is sufficiently flexible to permit future theoretical extensions to mating systems that we do not specifically consider here.
Patterns of selection depend on the distance and orientation of each sex to its phenotypic optimum. Let zm and zf represent the Euclidean distance between the optimal phenotype and the actual phenotype expressed by each sex (subscripts, m and f, hereafter refer to ‘male’ and ‘female’). Fitness functions for each sex are Gaussian, with wm = exp(–ωmzm2) and wf = exp(–ωfzf2), and ωm and ωf are positive constants specifying the strength of the fitness decline with increased distance from the optimum. The relative orientation of each sex to its optimum—the direction of selection—can be described by a pair of vectors that extend from the current locations of each sex within phenotypic space to the location of its optimum (figure 1). The strength of selection is defined as βm = |∂ln(wm)/∂zm| = 2ωmzm and βf = |∂ln(wf)/∂zf| = 2ωfzf, which parallel the definition of the selection gradient from quantitative genetics (see [32, pp. 122–123]). θsel represents the angle between male and female selection vectors, with ρsel = cos(θsel) representing the correlation between male and female orientations of directional selection (−1 < cos(θsel) < 1, with ρsel = 1 representing identical directions of selection; see [33]).
Figure 1.
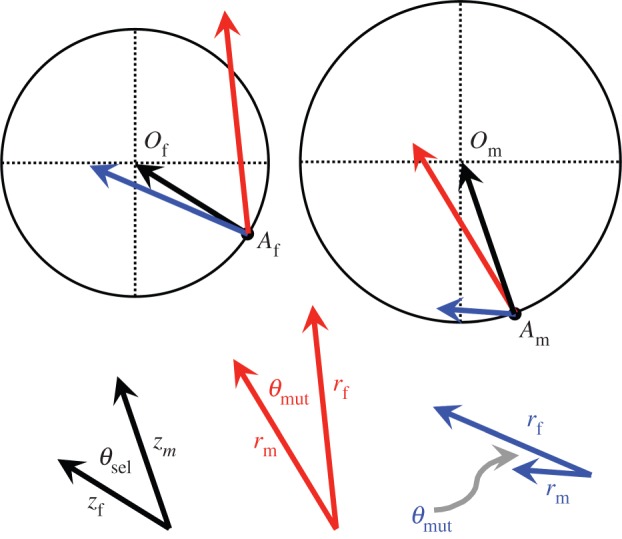
Fisher's geometric model in two dimensions and with two sexes. Trait values are represented by the x- and y-axes, with each of the traits expressed by both sexes. Af and Am depict the trait values expressed by females and males in the population, and Of and Om are the female and male trait optima. Black arrows represent the sex-specific selection vectors that reflect the distance of each sex to its optimum (vector lengths, zf and zm). Sex differences in directional selection are described by the angle between selection vectors (θsel). The fitness effects of each mutation depend on its phenotypic effect in each sex: its size (vector length, rm and rf) and orientation in phenotypic space. Sex differences in a mutation's orientation are described by the angle between mutation vectors (θmut). Mutations that bring a given sex closer to its optimum (i.e. within the relevant circle) are beneficial to that sex, whereas those causing movement away from the optimum will be deleterious (i.e. outside the relevant circle). Two mutations are shown: one that is beneficial to both sexes (blue), and the other that is sexually antagonistic (red).
Phenotypic effects of mutations are similarly described using paired vectors, with each mutation having a unique magnitude (rm and rf) and orientation within each sex (figure 1). For each mutation, θmut is a random variable, which represents the angle between male and female orientations of phenotypic change caused by the mutation. For mutations with specified magnitude rm and rf, the phenotypic correlation between the sexes for each trait axis is given by ρmut. In the two-sex extension of Fisher's model, there is a geometric relationship between ρmut and the angle between mutation vectors: ρmut = 〈cos(θmut)〉, where the angle brackets denote the mean among random mutations (see the electronic supplementary material).
Unique mutations arise randomly within a population that is initially fixed at each locus for a resident (wild-type) allele. For a given mutation, its sex-specific fitness effect in the jth sex is sj = (wj(zj*)/wj(zj+) – 1), where zj+ and zj* represent distances to the optimum for wild-type and mutant individuals, respectively. Letting tj = ln(1+sj) represent the logarithm of relative fitness, f(tm, tf ; rm, rf) is the bivariate distribution of tm and tf among mutations with arbitrary magnitude, rm and rf(rm, rf > 0). In many dimensions (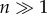 ), this distribution converges to a bivariate normal distribution, with marginal mean and variance 〈tj〉 = –ωjrj2 and var(tj) = (2ωjzjrj)2/n, respectively, and between-sex correlation coefficient of ρmf = cos(θsel)〈cos(θmut)〉 = ρselρmut (see the electronic supplementary material, noting that sm, sf will also be bivariate normal in the limit: sm, sf → 0). This simple identity for ρmf implies that genotype-by-sex interactions and sex-differential selection contribute multiplicatively to the fitness effect correlation between the sexes. Moreover, either factor is sufficient to generate an imperfect genetic correlation between male and female fitness.
), this distribution converges to a bivariate normal distribution, with marginal mean and variance 〈tj〉 = –ωjrj2 and var(tj) = (2ωjzjrj)2/n, respectively, and between-sex correlation coefficient of ρmf = cos(θsel)〈cos(θmut)〉 = ρselρmut (see the electronic supplementary material, noting that sm, sf will also be bivariate normal in the limit: sm, sf → 0). This simple identity for ρmf implies that genotype-by-sex interactions and sex-differential selection contribute multiplicatively to the fitness effect correlation between the sexes. Moreover, either factor is sufficient to generate an imperfect genetic correlation between male and female fitness.
Adaptive evolutionary change involves the sequential fixation of mutations with sex-averaged beneficial fitness effects (savg = sm/2 + sf/2 > 0). Similar to previous work in Fisher's geometric model (e.g. [29]), we characterize evolutionary change in a large population where beneficial mutations are rare, selection coefficients of beneficial alleles are small (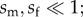 see [34]), and therefore each mutation's probability of fixation depends only on its net fitness effect. Provided the sex-averaged fitness effect is positive (savg > 0), a mutation's probability of fixation is 1 – exp(– 2savg), and otherwise its fixation probability is zero. Mutations that are successfully fixed trigger a change in the location of males and females within phenotypic space. Following each substitution, new distances and relative orientations of males and females to their optima may be calculated (i.e. new values of zm, zf, θsel), and the next step of adaptation can proceed from this new population state (see the electronic supplementary material).
see [34]), and therefore each mutation's probability of fixation depends only on its net fitness effect. Provided the sex-averaged fitness effect is positive (savg > 0), a mutation's probability of fixation is 1 – exp(– 2savg), and otherwise its fixation probability is zero. Mutations that are successfully fixed trigger a change in the location of males and females within phenotypic space. Following each substitution, new distances and relative orientations of males and females to their optima may be calculated (i.e. new values of zm, zf, θsel), and the next step of adaptation can proceed from this new population state (see the electronic supplementary material).
Because substitutions in Fisher's model are discrete (see above and [29]), each time point during evolution is associated with specific values for zm, zf, and θsel, and these terms may therefore be treated as population parameters appropriate for a given time point. We assume throughout that the phenotypic distributions of mutations remain constant during evolution (distributions of rm, rf, and θmut remain constant). Therefore, ρmf can be treated as a population parameter, albeit one that can evolve over the course of an adaptive walk. Accordingly, we first consider the sex-specific fitness effects of new mutations and the rate of adaptation, for an arbitrary population with parameters zm, zf, and θsel. We then characterize the long-term evolution of such populations as they adapt, with each sex approaching its fitness optimum.
3. Results and discussion
Adaptation requires beneficial genetic variation, which serves as the fuel for evolutionary change. In populations with separate sexes, random mutations can be deleterious to both sexes, beneficial to both, or beneficial to one sex and deleterious to the other (‘sexually antagonistic’). Among mutations that are beneficial to at least one sex (sm > 0 and/or sf > 0), the fraction that is sexually antagonistic is:
| 3.1a |
which, in the limit of small mutation size, reduces to a simple function of the fitness effect correlation (ρmf):
| 3.1b |
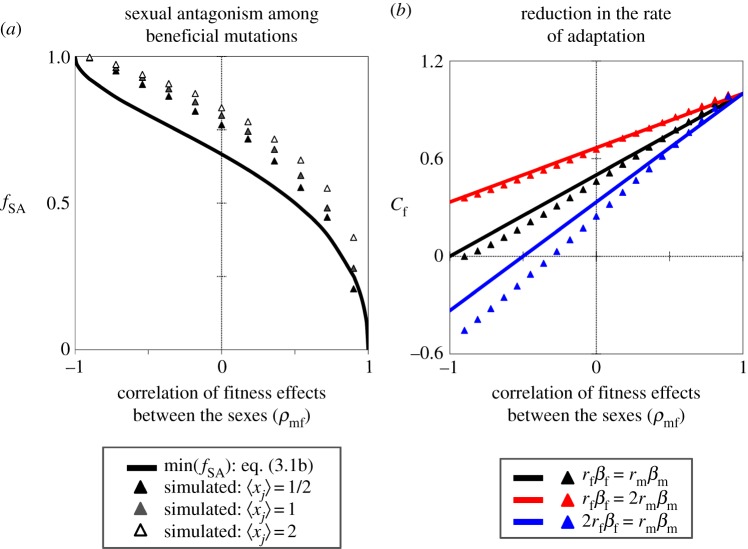
Equation (3.1b) approximates the minimum proportion of beneficial mutations that are sexually antagonistic (see the electronic supplementary material). Numerical evaluation of equation (3.1), along with exact computer simulations across a distribution of rm and rf values, shows that fSA increases with dimensionality and mutation size—factors that also increase the ‘scaled size’ of mutations in Fisher's geometric model (i.e. the scaled mutation size in sex j is defined as xj = rj√(n)/(2zj); see [13,29]). Overall, sexual antagonism is pervasive, and is a dominant feature of beneficial mutations, across the entire range of ρmf (figure 2).
Figure 2.
The fitness effect correlation between sexes (ρmf) predicts the fraction of sexually antagonistic mutations, and the severity of constraints to sex-specific adaptation. The minimum fraction of beneficial mutations that are sexually antagonistic (fSA; (a)) is based on the approximation in equation (3.1b) (solid curve), and from exact simulation results, using three different mutation size distributions (triangles). Shown are results using a bivariate exponential distribution for rm and rf, with corr(rm, rf) = 0.9 and equal marginal distributions for rm and rf [35]. The mean scaled mutation size is 〈xj〉 = 〈rj〉√(n)/(2zj), with n = 25 dimensions, and zj = 1/2; j = {m, f}. Each simulation result is based on 10 000 randomly generated beneficial mutations (e.g. beneficial to at least one sex). The relative reduction in the rate of adaptation in females (Cf; (b)) is based on the small mutation approximation in equation (3.2) (solid lines). Exact simulation results are depicted by triangles, based on the parameters: rm = rf = 0.05, zm = zf = 1/2, n = 25 (xj = 0.25) and βm/βf = ωm/ωf. Additional details of the simulation procedure can be found in the electronic supplementary material.
Adaptation represents a compromise between fitness benefits and costs to males and females, with selection favouring mutations with positive, sex-averaged fitness effects (i.e. savg = sm/2 + sf/2 > 0). Rates of adaptation in each sex can be approximated by taking into account both the mutation rate to positively selected alleles and the fixation probabilities of individual mutations [1]. To quantify the effect of an imperfect fitness correlation on the rate of adaptation, constraint is expressed as the ratio of the expected rate of adaptation in a population with imperfectly correlated male and female fitness effects (ρmf < 1) to the rate of adaptation in an ideal population with perfectly correlated fitness effects (ρmf = 1). We formally define this constraint as Cj = (dzj/dt | ρmf < 1)/(dzj/dt | ρmf = 1), where dzj/dt is the rate of evolutionary change of sex j towards its optimum (see the electronic supplementary material; Cj = 1 in the absence of sexually antagonistic constraints, and otherwise, Cj < 1). In the limit of small mutation size (rf, rm → 0), these ratios in males and females are approximately:
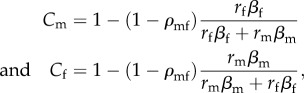 |
3.2 |
which compares well with exact computer simulations of small to moderately sized mutations, and it underestimates the true magnitude of constraint, particularly when scaled mutation sizes are large (figure 2, where xj = rj√(n)/(2zj) is the scaled size). An imperfect fitness correlation between the sexes significantly decreases the rate of adaptation (Cm and Cf can be substantially lower than one), with the relative magnitude of constraint dependent upon mutation and selection asymmetries between the sexes. With equally strong directional selection in each sex and similar-sized mutations (rmβm = rfβf), males and females experience equal degrees of constraint: Cm = Cf = 1 – (1 − ρmf)/2. Asymmetries between the sexes (rmβm ≠ rfβf) shift the burden of constraint to the sex that experiences weaker selection or mutation (figure 2).
The results above describe features of selection and adaptation at single time points during evolution, and consequently they treat fitness effect distributions and sex-specific rates of adaptation and constraint as static rather than evolutionarily labile population properties. However, each mutation that is fixed during adaptation will carry the population to a new location in phenotypic space and will alter the distance and orientation of each sex to its optimum. Fitness effect distributions and subsequent opportunities for adaptation will therefore change following each genetic substitution. To characterize such changes during the course of adaptive evolution, we analysed adaptive walks of populations towards stationary male and female fitness optima (see the electronic supplementary material; figure 3). We focused on the strength and orientation of sex-specific directional selection (βm, βf and ρsel)—quantities that directly impact the distribution of mutant fitness effects.
Figure 3.
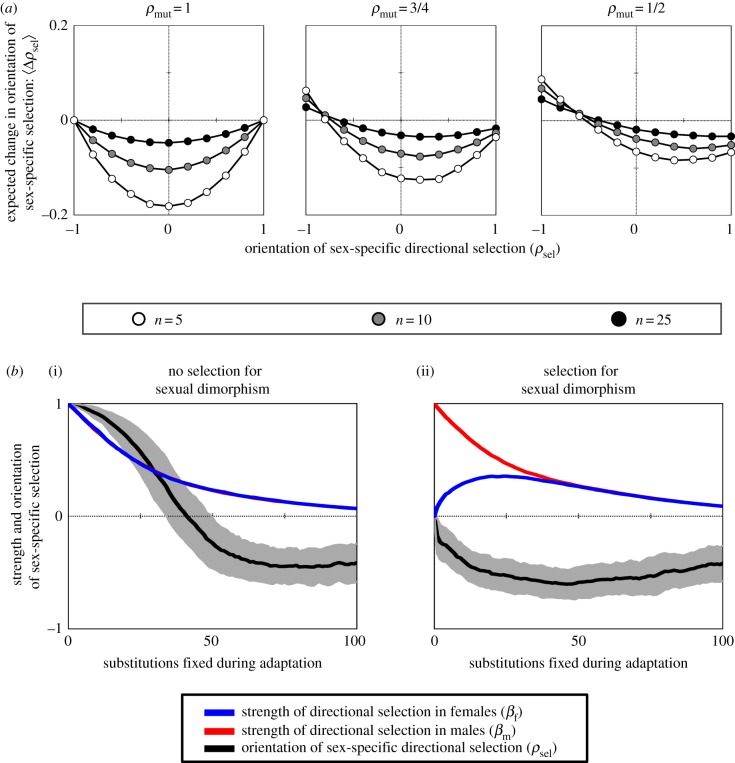
Adaptation generates opposing directional selection between the sexes. (a) The average change in the orientation of directional selection in males and females during adaptation: 〈Δρsel〉. Each data point represents the average change for 10 000 simulated substitutions, with simulation parameters zm = zf = 1/2, ωm = ωf = 1, and mutation sizes drawn from a bivariate exponential distribution with marginal mean of 〈rm〉 = 〈rf〉 = 0.1 and between-sex correlation of corr(rm, rf) = 〈cos(θmut)〉 = ρmut. (b) The strength of directional selection in males and females (βm and βf) and the relative orientation of directional selection between the sexes (represented by ρsel), during adaptive walks. Each step of adaptation coincides with fixation of a positively selected mutation (with savg > 0), and the solid lines show averages over 100 simulated adaptive walks (shaded area shows ± one standard deviation for ρsel). Two extreme initial conditions of the population are shown: (b(i)) directional selection is initially identical in both sexes and phenotypic divergence between the sexes is not favoured (initial conditions: zm = zf = 1/2; θsel = 0; ρsel = 1); and (b(ii)) selection favours phenotypic divergence between males and females (males are initially displaced from their optimum but females are not; initial conditions: zm = 1/2; zf = 0). Mutation sizes were drawn from a bivariate exponential distribution with marginal means 〈rm〉 = 〈rf〉 = 0.05, and corr(rm, rf) = 〈cos(θmut)〉 = ρmut = 0.75. Additional parameters include ωm = ωf = 1. Additional results, and details of the simulation procedure, can be found in the electronic supplementary material.
Despite an enormous range of possible initial conditions and sex-specific mutation and selection parametrizations, two general patterns emerge from the analysis. First, as long as mutant phenotypic effects are positively but imperfectly correlated between the sexes, as appears likely [6,36,37] (see below), the genetically coupled evolution of male and female phenotypes will inevitably generate sex-specific selection in opposing directions in phenotypic space (figure 3; i.e. adaptation eventually causes ρsel < 0, and thus, ρmf < 0 and fSA > 2/3; see the electronic supplementary material). In other words, adaptation generates opposing selection on traits expressed by males and females, and widespread sexual antagonism among mutations that are individually beneficial to males or females. This outcome includes cases where directional selection is initially identical between the sexes (figure 3 and electronic supplementary material, figure S2). Second, initial differences in the strength of directional selection decrease during adaptation (βm/βf → 1), which causes the severity of sex-specific adaptive constraints to converge over time (figure 3 and electronic supplementary material, figure S3). Given that ρmf eventually evolves to be negative, sexual antagonism is expected to ultimately reduce the subsequent rate of adaptation in each sex by at least twofold (Cf, Cm < 1/2), with a strong majority of beneficial mutations having sexually antagonistic fitness effects (fSA > 2/3).
Our model yields two general insights into the evolution of species with separate sexes. First, conditions for sexual antagonism among beneficial mutations are extremely permissive and readily emerge when directional selection or the phenotypic effects of mutations differ between the sexes. Indeed, the correlation of mutant fitness effects is equally sensitive to both factors, as reflected in the identity: ρmf = ρselρmut = cos(θsel)〈cos(θmut)〉. Second, decoupling the male and female fitness effects of random mutations significantly reduces the rate of adaptation of both sexes, with sexual antagonism and the magnitude of adaptive constraints increasing during the process of adaptation. Evolution of sexual antagonism occurs whether or not selection favours phenotypic divergence between the sexes (figure 3 and electronic supplementary material, figure S2). Opposing selection between the sexes—for homologous traits expressed by both sexes and mutations affecting these traits—is therefore an inescapable by-product of adaptation in populations with separate sexes.
The model is parametrized using measurable properties of mutation and selection and should therefore be useful for inferring the extent of sexual antagonism in populations conducive to such measures. Although sex-specific phenotypic properties of spontaneous mutations have yet to receive much attention, current data on genetic correlations between the sexes (based on quantitative genetic data [36,37], and mutation-accumulation experiments in Drosophila [6]) suggest that mutations are likely to have strong, positively correlated effects between the sexes. Estimates of selection on single traits suggest that opposing directional selection is common, and often of similar magnitude between the sexes [5,38]. Of the few studies estimating the angle between male and female directional selection on multiple traits, two support the general prediction that opposing selection readily evolves (ρsel ≈ −0.61 and −0.73 in a moth and fly population, respectively [39,40]), whereas a third reveals a positive correlation between male and female directional selection, albeit in a modern human population that has had little time to adapt to its current environment (ρsel ≈ 0.22 [41]). To systematically test whether opposing selection between the sexes evolves during adaptation, future studies could contrast patterns of sex-specific directional selection in poorly versus well-adapted populations, with the latter expected to exhibit more strongly opposing selection between males and females (for a similar prediction, see [42]).
We have identified general conditions yielding sexually antagonistic selection, and although such antagonism does not require that selection favour evolutionary divergence between the sexes, the pervasiveness and intensity of antagonism is expected to increase when selection does favour sexual dimorphism. Discordance between the fitness landscapes of males and females—the root cause of selection for dimorphism—emerges from the unique interactions between each sex and its environment [8–11], which can lead to sex-differential opportunities for niche partitioning, reproductive investment, competition for mates or their susceptibilities to predation and parasitism. We emphasize the population genetic consequences of distinct male and female fitness landscapes, while placing no special emphasis on the specific ecological causes for divergent male and female optima. The ubiquity of sexually dimorphic phenotypes [43], including conspicuous differences between closely related species [44], attests to the biological fact of fitness landscape dimorphism and implies a central role for antagonistic selection during the process of adaptation within lineages and speciation between them.
Acknowledgements
We thank Deborah Charlesworth, Angela Early, Ted Morrow, Chris Stieha, Akane Uesugi, Rob Unckless, Cris van Hout, and members of the Clark laboratory for valuable discussion and comments on an early version of the manuscript, Allen Orr for valuable correspondence during the initial stages of the project, and two anonymous referees for thoughtful comments and suggestions that substantially improved the paper.
Funding statement
This work was funded by NIH grant GM64590 to A.G.C. and A. B. Carvalho.
References
- 1.Orr HA. 2000. Adaptation and the cost of complexity. Evolution 54, 13–20 (doi:10.1111/j.0014-3820.2000.tb00002.x) [DOI] [PubMed] [Google Scholar]
- 2.Welch JJ, Waxman D. 2003. Modularity and the cost of complexity. Evolution 57, 1723–1734 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Liao BY, Zhang J. 2010. Genomic patterns of pleiotropy and the evolution of complexity. Proc. Natl Acad. Sci. USA 107, 18 034–18 039 (doi:10.1073/pnas.0914711107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackay TAF. 2001. The genetic architecture of quantitative traits. Ann. Rev. Genet. 35, 303–339 (doi:10.1146/annurev.genet.35.102401.090633) [DOI] [PubMed] [Google Scholar]
- 5.Cox RM, Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. Am. Nat. 173, 176–187 (doi:10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 6.Houle D, Fierst J. 2012. Properties of spontaneous mutational variance and covariance for wing size and shape in Drosophila melanogaster . Evolution 67, 1116–1130 (doi:10.1111/j.1558-5646.2012.01838.x) [DOI] [PubMed] [Google Scholar]
- 7.Rice WR. 1992. Sexually antagonistic genes: experimental evidence. Science 256, 1436–1439 (doi:10.1126/science.1604317) [DOI] [PubMed] [Google Scholar]
- 8.Bonduriansky R, Chenoweth SF. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288 (doi:10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 9.van Doorn GS. 2009. Intralocus sexual conflict. Ann. N. Y. Acad. Sci. 1168, 52–71 (doi:10.1111/j.1749-6632.2009.04573.x) [DOI] [PubMed] [Google Scholar]
- 10.Pennel TM, Morrow EH. 2013. Two sexes, one genome: the evolutionary dynamics of intralocus sexual conflict. Ecol. Evol. 3, 1819–1834 (doi:10.1002/ece3.540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maklakov AA, Lummaa V. 2013. Evolution of sex differences in lifespan and aging: causes and constraints. BioEssays 35, 717–724 (doi:10.1002/bies.201300021) [DOI] [PubMed] [Google Scholar]
- 12.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- 13.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: The Clarendon Press [Google Scholar]
- 14.Orr HA. 2005. The genetic theory of adaptation: a brief history. Nat. Rev. Genet. 6, 119–127 (doi:10.1038/nrg1523) [DOI] [PubMed] [Google Scholar]
- 15.Orr HA. 2005. Theories of adaptation: what they do and don't say. Genetica 123, 3–13 (doi:10.1007/s10709-004-2702-3) [DOI] [PubMed] [Google Scholar]
- 16.Blows M, Walsh B. 2009. Spherical cows grazing on flatland: constraints to selection and adaptation. In Adaptation and fitness in animal populations (eds J van der Werf, Graser HU, Frankham R, Gondro C.), pp. 83–101 Dordrecht, The Netherlands: Springer Science+Business Media B.V [Google Scholar]
- 17.Walsh B, Blows M. 2009. Abundant genetic variation+strong selection=multivariate genetic constraints: a geometric view of adaptation. Annu. Rev. Ecol. Evol. Syst. 40, 41–59 (doi:10.1146/annurev.ecolsys.110308.120232) [Google Scholar]
- 18.Kirkpatrick M. 2009. Patterns of quantitative genetic variation in multiple dimensions. Genetica 136, 271–284 (doi:10.1007/s10709-008-9302-6) [DOI] [PubMed] [Google Scholar]
- 19.Hill WG. 1982. Rates of change in quantitative traits from fixation of new mutations. Proc. Natl Acad. Sci. USA 79, 142–145 (doi:10.1073/pnas.79.1.142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill WG. 1982. Predictions of response to artificial selection from new mutations. Genet. Res. Camb. 40, 255–278 (doi:10.1017/S0016672300019145) [DOI] [PubMed] [Google Scholar]
- 21.Rice SH. 1990. A geometric model for the evolution of development. J. Theor. Biol. 143, 319–342 (doi:10.1016/S0022-5193(05)80033-5) [Google Scholar]
- 22.Waxman D, Welch JJ. 2005. Fisher's microscope and Haldane's ellipse. Am. Nat. 166, 447–457 (doi:10.1086/444404) [DOI] [PubMed] [Google Scholar]
- 23.Martin G, Lenormand T. 2006. A general multivariate extension of Fisher's geometrical model and the distribution of mutation fitness effects across species. Evolution 60, 893–907 [PubMed] [Google Scholar]
- 24.Martin G, Elena SF, Lenormand T. 2007. Distributions of epistasis in microbes fit predictions from a fitness landscape model. Nat. Genet. 39, 555–560 (doi:10.1038/ng1998) [DOI] [PubMed] [Google Scholar]
- 25.Gordo I, Campos PRA. 2013. Evolution of clonal populations approaching a fitness peak. Biol. Lett. 9, 20120239 (doi:10.1098/rsbl.2012.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hietpas RT, Bank C, Jensen JD, Bolon DNA. In press. Shifting fitness landscapes in response to altered environments. Evolution. (doi:10.1111/evo.12207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perfeito L, Sousa A, Bataillon T, Gordo I. In press. Rates of fitness decline and rebound suggest pervasive epistasis. Evolution. (doi:10.1111/evo.12234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caudle SB, Miller CR, Rokyta DR. In press. Environment determines epistatic patterns for a ssDNA virus. Genetics. (doi:10.1534/genetics.113.158154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orr HA. 1998. The population genetics of adaptation; the distribution of factors fixed during adaptive evolution. Evolution 52, 935–949 (doi:10.2307/2411226) [DOI] [PubMed] [Google Scholar]
- 30.Sellis D, Callahan BJ, Petrov DA, Messer PW. 2011. Heterozygote advantage as a natural consequence of adaptation in diploids. Proc. Natl Acad. Sci. USA 108, 20 666–20 671 (doi:10.1073/pnas.1114573108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott JK. 2011. Intra-locus sexual conflict and sexually antagonistic genetic variation in hermaphroditic animals. Proc. R. Soc. B 178, 161–169 (doi:10.1098/rspb.2010.1401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlesworth B, Charlesworth D. 2010. Elements of evolutionary genetics. Greenwood Village: Roberts and Company Publishers [Google Scholar]
- 33.Rodgers JL, Nicewander WA. 1988. Thirteen ways to look at the correlation coefficient. Am. Stat. 42, 59–66 (doi:10.2307/2685263) [Google Scholar]
- 34.Nagylaki T. 1979. Selection in dioecious populations. Ann. Hum. Genet. 14, 143–150 (doi:10.1111/j.1469-1809.1979.tb02007.x) [DOI] [PubMed] [Google Scholar]
- 35.Michael JR, Schucany WR. 2002. The mixture approach for simulating bivariate distributions with specified correlations. Am. Stat. 56, 48–54 (doi:10.1198/000313002753631367) [Google Scholar]
- 36.Poissant J, Wilson AJ, Coltman DW. 2010. Sex-specific genetic variance and the evolution of sexual dimorphism: a systematic review of cross-sex genetic correlations. Evolution 64, 97–107 (doi:10.1111/j.1558-5646.2009.00793.x) [DOI] [PubMed] [Google Scholar]
- 37.Griffin RM, Dean R, Grace JL, Rydén P, Friberg U. 2013. The shared genome is a pervasive constraint on the evolution of sex-biased gene expression. Mol. Biol. Evol. 30, 2168–2176 (doi:10.1093/molbev/mst121) [DOI] [PubMed] [Google Scholar]
- 38.Connallon T, Cox RM, Calsbeek R. 2010. Fitness consequences of sex-specific selection. Evolution 64, 1671–1682 (doi:10.1111/j.1558-5646.2009.00934.x) [DOI] [PubMed] [Google Scholar]
- 39.Lewis Z, Wedell N, Hunt J. 2011. Evidence for strong intralocus sexual conflict in the Indian meal moth, Plodia interpunctella. Evolution 65, 2085–2097 (doi:10.1111/j.1558-5646.2011.01267.x) [DOI] [PubMed] [Google Scholar]
- 40.Gosden TP, Shastri KL, Innocenti P, Chenoweth SF. 2012. The B-matrix harbors significant and sex-specific constraints on the evolution of multicharacter sexual dimorphism. Evolution 66, 2106–2116 (doi:10.1111/j.1558-5646.2012.01579.x) [DOI] [PubMed] [Google Scholar]
- 41.Stearns SC, Govindaraju DR, Ewbank D, Byars SG. 2012. Constraints on the coevolution of contemporary human males and females. Proc. R. Soc. B 279, 4836–4844 (doi:10.1098/rspb.2012.2024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long TAF, Agrawal AF, Rowe L. 2012. The effect of sexual selection on offspring fitness depends on the nature of genetic variation. Curr Biol. 22, 204–208 (doi:10.1016/j.cub.2011.12.020) [DOI] [PubMed] [Google Scholar]
- 43.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 44.Panhuis TM, Butlin R, Zuk M, Tregenza T. 2001. Sexual selection and speciation. Trends Ecol. Evol. 16, 364–371 (doi:10.1016/S0169-5347(01)02160-7) [DOI] [PubMed] [Google Scholar]