Abstract
Human colonization of the New World is generally believed to have entailed migrations from Siberia across the Bering isthmus. However, the limited archaeological record of these migrations means that details of the timing, cause and rate remain cryptic. Here, we have used a combination of ancient DNA, 14C dating, hydrogen and oxygen isotopes, and collagen sequencing to explore the colonization history of one of the few other large mammals to have successfully migrated into the Americas at this time: the North American elk (Cervus elaphus canadensis), also known as wapiti. We identify a long-term occupation of northeast Siberia, far beyond the species’s current Old World distribution. Migration into North America occurred at the end of the last glaciation, while the northeast Siberian source population became extinct only within the last 500 years. This finding is congruent with a similar proposed delay in human colonization, inferred from modern human mitochondrial DNA, and suggestions that the Bering isthmus was not traversable during parts of the Late Pleistocene. Our data imply a fundamental constraint in crossing Beringia, placing limits on the age and mode of human settlement in the Americas, and further establish the utility of ancient DNA in palaeontological investigations of species histories.
Keywords: ancient DNA, Beringia, Bering isthmus, Pleistocene, wapiti
1. Introduction
During the Pleistocene epoch, global cooling led periodically to the expansion of glaciers and lowering of sea levels. This created land connections in various regions around the globe [1]. One such isthmus occurred in the Bering Strait, providing a link between northeast Siberia (western Beringia, up to the Verkhoyansk Mountains and Lena River Basin) and Alaska (Eastern Beringia, up to Northwest Territories of Canada) [2,3].
The biogeographic role of Beringia in allowing migration between Asia and North America is crucial to our understanding of the distribution of Holarctic fauna. Human migration across Beringia is a major focus of study, but the route taken, the timing of this event and its underlying causes, against a background of changing environments and resource availability, remain unclear [4–7].
Recently, evidence for early human activity in North America was reported from the Friedkin Site, TX, where artefact-bearing contexts have been dated by optically stimulated luminescence to as early as 15 500 years ago (15.5 ka) [8]. Elsewhere the first evidence of human presence is somewhat younger, with roughly synchronous deposits across the Americas (e.g. 14.6 ka at Monte Verde, Chile; 14.1 ka at Paisley Cave, OR; 13.8 ka at Manis, WA [9–11]). Surprisingly, the first evidence for human presence in Alaska (Swan Point), the most likely region for entry into the New World, has a similar mean date of 14.4 ka. Given its geographical distance from equivalently aged sites in the continental US and the distinctive microtools found there, Swan Point does not appear to have an ancestral relationship to sites further south. The situation for northeast Siberia extends the paradox, as almost all of the regional archaeology postdates 14 ka (with the Berelekh site dated to 14–11 ka [12]); the single exception is the much earlier (ca 32 ka) Yana RHS [13]. The relationship of Yana RHS to the earliest sites in North America is also uncertain, which is unsurprising given that they are separated by more than 15 ka.
A suite of much earlier sites, including Bluefish Cave, La Sena, Lovewell, Cactus Hill, Pendejo Cave and Pedro Furada [14], remain under varying degrees of contention, with problems of dating and the interpretation of artefact status. Critical to the validity of these early sites is a migration event across Beringia that significantly pre-dates the existing record for archaeology on both sides. The demands of reconstructing human migrations of this antiquity, with low population and site density, pose a major challenge.
An unexploited source of information, however, is available in other large mammal species that crossed, or failed to cross, Beringia at different times [15–18]. Recent reconstructions indicate why inferring colonization history is complicated, with regional sea-level history indicating a seaway continuously from ca 135 to 70 ka; a Siberian–New World land bridge between 70 and 60 ka; an intermittent connection from 60 to 30 ka; a land bridge again from ca 30 to 11 ka; and Holocene sea-level rise reopening the strait [3].
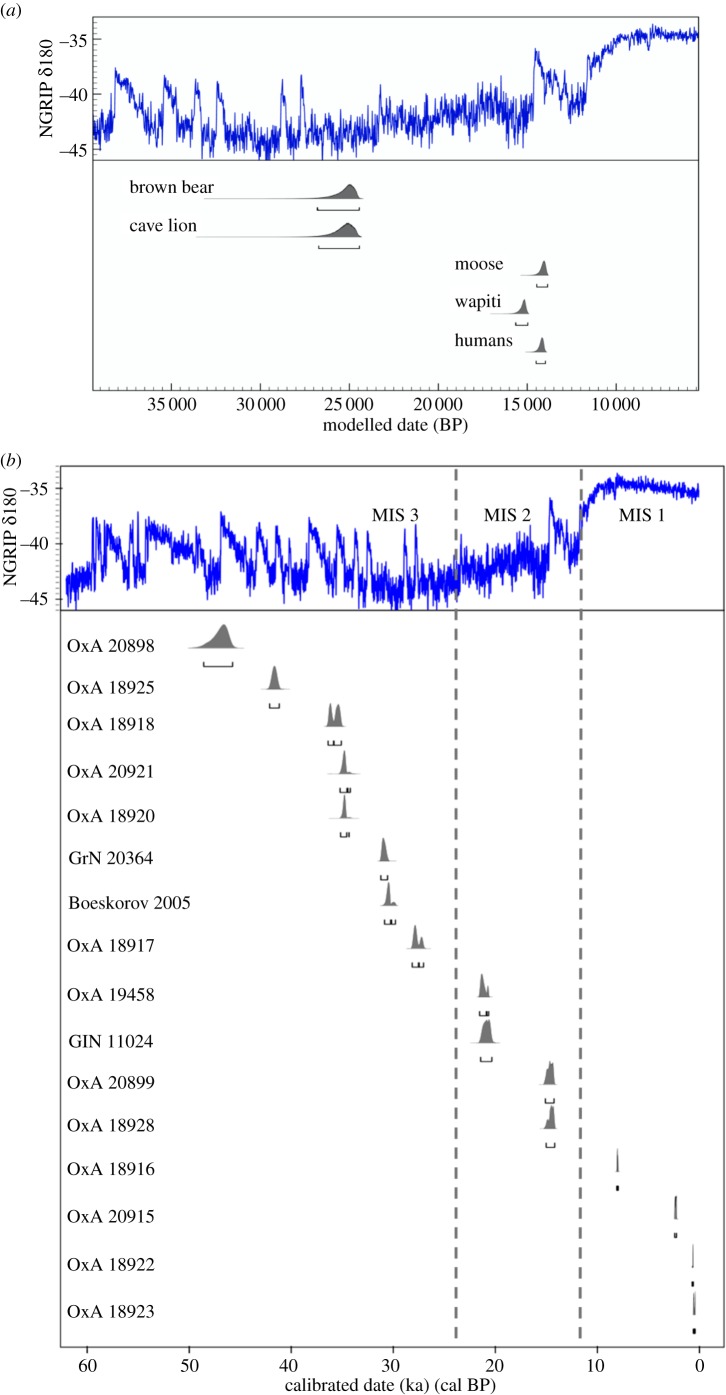
In order to establish the pattern of faunal migration through the last glaciation, we first collated radiocarbon dates (figure 1a) for mammal taxa that have been proposed to undergo range expansion into Alaska during this period: brown bear (Ursus arctos), wapiti (Cervus elaphus), moose (Alces alces) and the Pleistocene lion (Panthera spelaea) [15,17,18]. Previous ancient DNA studies on brown bears identified a regional extinction in Alaska during Marine Isotope Stage 3 (MIS 3; 60–24 ka), with a subsequent recolonization from Siberia at 25 ka [15,19]. A similar high concentration of radiocarbon-dated lion bones from Alaska around 25 ka suggests either an expansion of a pre-existing population or further dispersal across Beringia. For wapiti and moose, by contrast, there are no unambiguous fossil records in Alaska until some 10 kyr later at 15 ka, contemporaneous with the earliest uncontested evidence of humans in eastern Beringia (Alaska) [18]. Wapiti and moose therefore have the potential to illuminate the timing and mode of faunal and human expansion into the New World, especially the problem of migration during MIS 3.
Figure 1.
(a) Bayesian phase-modelled timing of the late-glacial colonization of Alaska and Yukon by brown bears, cave lions, moose, wapiti and humans. The distributions are start boundaries. (b) Finite radiocarbon dates of wapiti occupying northeast Siberia plotted against NorthGRIP δ18O data. (Online version in colour.)
Here, we have analysed wapiti from Late Pleistocene to modern specimens across Siberia, Alaska and the rest of North America. The taxonomy of wapiti (or North American elk) has been the subject of debate; here, we follow current IUCN definitions by placing wapiti as a subspecies (Cervus elaphus canadensis), while recognizing the morphological and ecological differences from its European counterpart (red deer; C. e. elaphus). The migration of this subspecies across Beringia is intriguing, as wapiti is currently a temperate to boreal species with a northern limit around 60° N [20–24]. We hypothesized that wapiti dispersed rapidly to North America, during a narrow window of relatively mild climate and low sea level, with only a short residence time in northeast Siberia. We used radiocarbon dating, coupled with ancient DNA, to test that proposal and to evaluate the potential for migration across Beringia during this time period.
2. Material and methods
(a). Sample collection
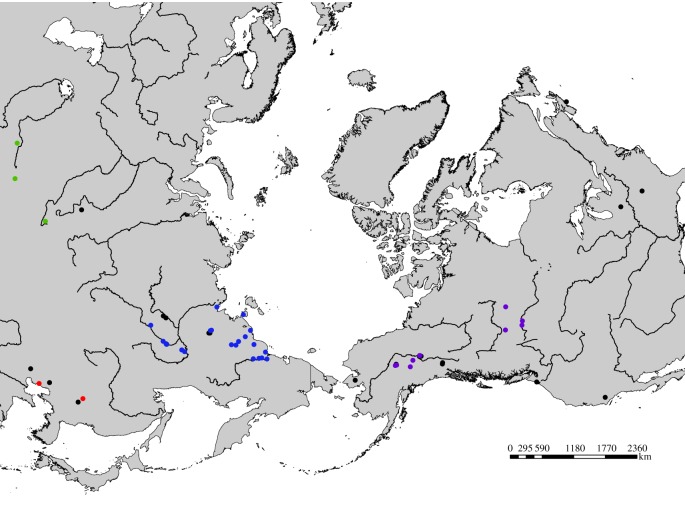
A total of 113 samples from ancient antlers, teeth and bones of Asian and North American wapiti were collected from museums across Eurasia and North America (figure 2; see also the electronic supplementary material, table S2). Seventy-four modern wapiti specimens from across Asia and North America were also collected, including the extinct subspecies Cervus elaphus merriami (see the electronic supplementary material, table S2). Sika deer (Cervus nippon), Bukhara deer (Cervus elaphus bactrianus) and European red deer (Cervus elaphus atlanticus) were used as out-groups, as in other studies [25,26].
Figure 2.
Map showing approximate locations of ancient Cervus remains sampled in this study. Colours correspond to geographical locations: purple, North America; blue, northeast Siberia; green, central Asia; red, east China; black, samples that did not yield DNA.
(b). ZooMS analyses
Five specimens that were candidates for pre-13 ka migration into North America were chosen for the analysis. The bones were extracted in various ways. For samples MM069, MM070 and MM230, bone powder (less than 1 mg) was incubated for 1 h at 65°C in 50 mM of ammonium bicarbonate (pH 8.0). For sample MM281, 10 mg of bone powder was decalcified for 24 h in 0.6 M HCl and incubated in ammonium bicarbonate for 3 h at 65°C. For sample MM403, 10 mg of bone powder was sonicated three times in 2 : 1 dichloromethane and methanol (v : v). The sample was then rinsed in methanol and ultrapure water and incubated in ammonium bicarbonate buffer for 1 h at 65°C.
All the extracts were then incubated overnight (less than 18 h) with 1 mg ml−1 sequencing-grade-modified porcine trypsin at 37°C, purified over a C18 column (ZipTip, Millipore, Durham, UK) and were analysed by MALDI-TOF mass spectrometry (Bruker Daltonics Ultraflex III, Bremen, Germany). The spectra obtained were then compared with a database, which is mostly based on translated sequences from cDNA libraries, and uses a MASCOT-based search.
(c). Radiocarbon dating
Out of the 113 ancient specimens used in this study, 32 were submitted by us for radiocarbon dating at the Oxford Radiocarbon Accelerator Unit (ORAU). Another 22 specimens were previously dated (see the electronic supplementary material, tables S1 and S2).
We created a dataset with only directly radiocarbon-dated wapiti from our database and from the literature (see the electronic supplementary material, table S1). Radiocarbon dates of brown bear, cave lion and moose were collected from the literature (see the electronic supplementary material, table S1). All ages cited in the text were calibrated using the IntCal-09 curve [27]; medians are quoted unless otherwise stated. To calculate the most likely age of the occupation events, we applied Bayesian phase modelling, using the OxCal calibration and age modelling software [28].
(d). Hydrogen and oxygen isotopic analyses
Bone or antler samples from 18 specimens were used in the study (see the electronic supplementary material, table S3). Decalcification followed the method described by Tuross et al. [29]. Bone or antler pieces were decalcified in 0.5 M EDTA, pH 7.8 for up to one week. The collagen pseudomorph was washed 15 times with deionized water, freeze-dried and allowed to equilibrate at room temperature for a minimum of one week. Mass spectrometry was carried out in a thermal combustion/elemental analyser unit as described by Tuross et al. [29]. For more details, see the electronic supplementary material.
(e). DNA amplification and sequencing
Ancient DNA extraction was carried out in a dedicated laboratory. DNA of historical museum specimens was extracted in a different laboratory from the modern specimens, to reduce the possibility of contamination. Two elements of the mtDNA genome were analysed: 423 bp of the 5′ end of the cytochrome b (cyt b) gene, and 408–412 bp at the 3′ end of tRNA-Pro and 5′ end of the control region (CR). For the ancient samples, five and four primer pairs were designed to amplify sections of cyt b and CR, respectively, consisting of overlapping fragments of around 150 bp (see the electronic supplementary material, table S4). DNA was successfully amplified and sequenced from 44 of the 113 ancient samples and from 49 of the 74 modern samples used. For full details, see the electronic supplementary material.
(f). Data analyses
Cyt b and CR fragments were concatenated to provide a higher number of potentially informative sites for phylogenetic analyses [30,31]. The sequences were visually inspected and corrected using Sequencher v. 4.7, and manually aligned. Owing to the existence of tandem repeats in the CR [32], one amplified fragment (primers: CR4) did not overlap the previous one (primers: CR3), and this 20 bp gap region was excluded from the analyses. Phylogenetic relationships were estimated using maximum likelihood (ML) [33], Bayesian inference [34,35] and minimum spanning network (MSN) [36] (with no out-groups). The ML analyses were conducted using Raxml v. 7.0.3 [37], as implemented in raxmlGUI v. 1.1 [37,38], and MrBayes v. 3.1.2 [34,35] was used to conduct the Bayesian Markov chain Monte Carlo phylogenetic inference using best-fit model indicated by jMODELTEST v. 2.1 [39,40]. We used Bayesian skyline plots (BSP) [41] implemented in BEAST v. 1.7 [42] to estimate changes in wapiti effective population size from the Late Pleistocene to present. Thirty finite radiocarbon-dated specimens were used for tip calibration. Markov chains were run for 100 million generations and sampled every 1000 generations, discarding the first 10% as burn-in. The results were analysed using Tracer v. 1.5 to produce the BSP. The MSN was based only on wapitoid haplotypes (out-groups were not included) and produced using Arlequin v. 3.5 [36], and the output was uploaded to HapStar to visualize the network [43]. Sequence diversity summary statistics were generated based on geographical location (North America, central and east Asia and northeast Asia) using Arlequin v. 3.5 [36]. For full details, see the electronic supplementary material.
3. Results
(a). Analysis of putative early wapiti from North America
In order to establish that the Late Pleistocene migration into Alaska represents the first colonization of the New World by wapiti, we reviewed the palaeontological record for this species. While several specimens have been proposed to represent Late Pliocene or Pleistocene wapiti [44], there are no unequivocal records of wapiti until the terminal Pleistocene [45,46]. None of the earlier remains are directly dated and they are frequently highly fragmented, with questionable identifications.
We were able to sample five wapiti specimens that are candidates for earlier (i.e. Late Pleistocene but pre-13 ka) migration into North America. None yielded amplifiable DNA, but four gave diagnostic fingerprints with ZooMS (zooarchaeology by mass spectrometry), a method that identifies taxonomically diagnostic collagen markers. Of those four, only the most recent one (MM403; McKittrick, CA) gave a probable cervid sequence (see the electronic supplementary material, figure S1a). This sample is dated to 13.1 ka, consistent with a late-glacial trans-Beringian migration. Two early dating samples from Alaska (MM069, 21.8 ka; MM070, 29.2 ka) were reidentified by ZooMS as bovid (see the electronic supplementary material, figure S1b) and a fourth sample, from Tennessee (MM281, undated but considered Late Pleistocene), was identified as a horse (see the electronic supplementary material, figure S1c).
(b). Radiocarbon dating of Beringian wapiti
The radiocarbon record indicates that wapiti were present in northeast Siberia since at least 50 ka (with several dates beyond the calibration range; see the electronic supplementary material, table S2). Radiocarbon dates range through MIS 3 (ca 57–24 ka), MIS 2 (ca 24–11 ka) and into the late Holocene (figure 1b; see also the electronic supplementary material, table S1). The two most recent dates have calibrated median values of 446 and 531 years, and indicate that wapiti were present in northeast Siberia up to latitude 71° N in recent history. By contrast, the oldest of the 89 North American dates has a median value of 15.2 ka (MM057), with the most recent wapiti in Alaska—excluding historical reintroductions—dating to 5.1 ka [18].
(c). Confirming region of origin for the Siberian wapiti samples
The unexpectedly broad time range of the northeast Siberian material—and in particular the identification of Late Holocene individuals—raises the possibility that some of the material was incorrectly provenanced during curation or redeposited from a southern population by human activity. To establish that these samples represent individuals that were native to northeast Siberia, we analysed hydrogen (δD) and oxygen (δ18O) isotopes (see the electronic supplementary material, table S3). Previous studies have shown that δD and δ18O are indicative of latitude, and these isotopes have been used to study migrations [47–49]. All of the putative northeast Siberian samples tested fall within a single cluster, with δD values between −132‰ and −185‰, and δ18O values between −3.1‰ and −0.7‰, supporting a single, northern geographical origin of these individuals (see the electronic supplementary material, figure S2). Two specimens (MM005 and MM055) from the Altai and Tian Shan have isotopic values typical of their lower latitudes (less than 50° N). Thus, while previous work had suggested that wapiti survived historically to only 61° N in northeast Siberia [50], our findings indicate that wapiti is an even more flexible species than the modern range suggests, with a long Holocene and Late Pleistocene Arctic history.
(d). DNA from Beringian wapiti
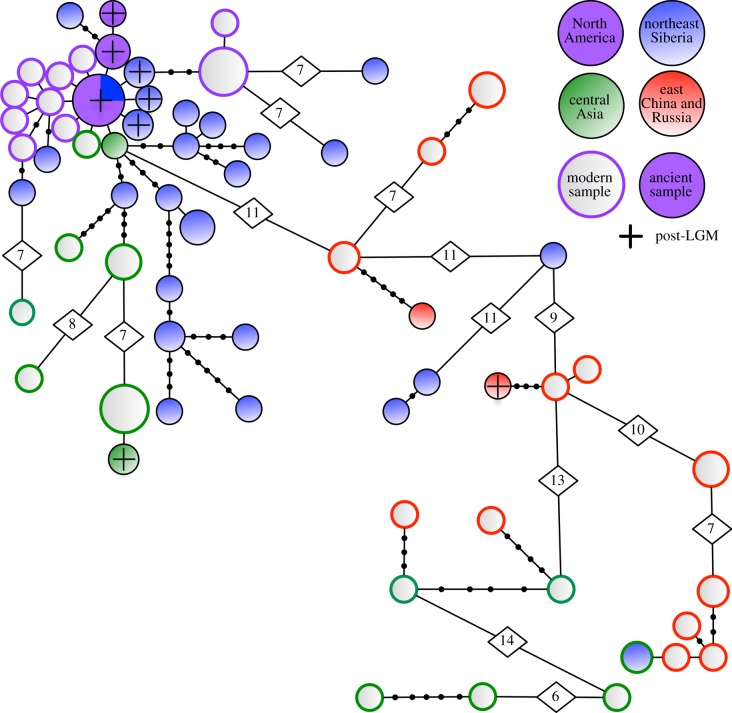
Mitochondrial cyt b and CR DNA were successfully amplified and sequenced from 44 of the 113 ancient samples, and from 49 of the 74 modern or archival samples (see the electronic supplementary material, table S2), using a combination of nine primer pairs depending on preservation (see the electronic supplementary material, table S4). The overlapping fragments were consistent between fragments and all the amplifications from the re-extractions gave the same results. No stop codons or indels were found in cyt b, but four indels were found in the CR and these positions were excluded from subsequent analyses. These sequences cover the range of individuals not only from northeast Siberia and Alaska, but also from central and east Asia (figure 2; see also the electronic supplementary material, table S2). The MSN (figure 3) and the phylogenetic tree (see the electronic supplementary material, figure S3) identify two main groups. The first is a very diverse group of modern wapiti from central Asia (Altai and Tian Shan), northeastern China (Inner Mongolia) and eastern Russia (Primorsky Krai), and ancient specimens from northeastern China and northeast Siberia. The Siberian specimens in this clade are relatively early (see the electronic supplementary material, figure S3) and suggest a low level of migration from central–eastern Asia into Beringia during MIS 3 (ca 57–24 ka).
Figure 3.
MSN representing 65 haplotypes of wapiti. Lines represent a single mutation step. The black circles and numbers (higher than 10) represent missing haplotypes.
The second major group includes modern American wapiti, further central Asian individuals, and ancient samples from Beringia (both northeast Siberia and Alaska) and Alberta. While the central Asian–Chinese clade is genetically diverse (π = 22.5 × 10–3 ± 11.3 × 10–3; table 1), all modern North American haplotypes are genetically very similar to one another (π = 3.3 × 10–3 ± 2.1 × 10–3), to ancient haplotypes from Alaska (π = 2.9 × 10–3 ± 1.8 × 10–3) and to a subset of those from northeast Siberia, with one or two mutations between them. These data strongly support an expansion of a subset of the northeast Siberia population into North America via Beringia, with all modern North American wapiti being the result of that colonization. Conversely, we find no support for earlier proposals that fossil Beringian wapiti constitute a distinct species or subspecies [24,51]; the larger body size and complex antler type of these individuals reflect morphological variation within C. e. canadensis.
Table 1.
Summary statistics of molecular diversity within the different regions. Molecular diversity indices: n, number of individuals; H, number of haplotypes; h, haplotype diversity; π, nucleotide diversity.
| region | n | H | h | π (×10–3) |
|---|---|---|---|---|
| North America | ||||
| modern samples | 14 | 10 | 0.92 ± 0.06 | 3.3 ± 2.1 |
| ancient samples | 9 | 3 | 0.56 ± 0.17 | 0.9 ± 0.8 |
| ancient and modern samples | 23 | 13 | 0.91 ± 0.04 | 2.9 ± 1.8 |
| Asia | ||||
| modern samples | 34 | 24 | 0.97 ± 0.02 | 22.5 ± 11.3 |
| northeast Siberia | 30 | 25 | 0.99 ± 0.01 | 13.3 ± 6.9 |
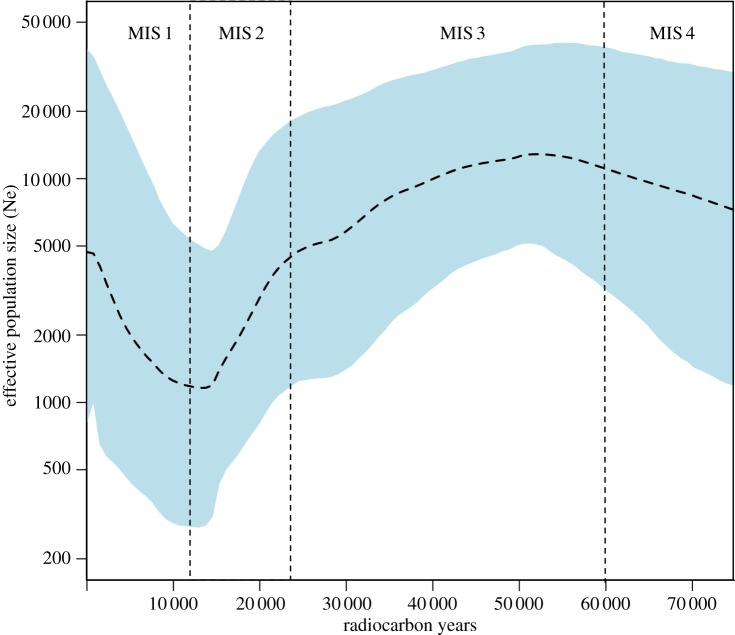
To reconstruct the demographic history of the Beringian wapiti population and its North American descendants, we generated a BSP [41] using a dataset comprising finite-dated and modern northeast Siberian and North American individuals (n = 30 ancient and 14 modern). The resulting plot (figure 4) demonstrates a slow increase in effective population size up to around 50 ka, followed by a decrease, which accelerated around 22 ka, around the Last Glacial Maximum. Taking median values, the overall decrease in effective population size is around tenfold over a 35 kyr period. This decline was followed by continuous population growth to the present, starting at around 15 ka, shortly after the radiocarbon record indicates initial colonization of North America.
Figure 4.
Bayesian skyline plot derived from the analysis of modern and ancient wapiti sequences from northeast Siberia and North America. Only sequences with finite radiocarbon dates were included in the analysis, using a fixed clock and partitioned by gene region. The x-axis is in calibrated radiocarbon years BP, and the y-axis is the calculated effective population size using a generation time of 7 years. The dashed line represents median values, and the shaded area represents the 95% highest posterior density (HPD) limits.
4. Discussion
(a). Proposed model of new world colonization for wapiti
Based on the combined radiocarbon and genetic records, we propose a three-stage model for the colonization of North America by wapiti. (i) Initial colonization of northeast Siberia occurred prior to the MIS 3—beyond the range of radiocarbon dating. (ii) This population remained in the region during MIS 3, with some migration to or from areas further south and west. Population size began to sharply decline during MIS 2, but with no obvious genetic discontinuity to indicate a local extinction. (iii) Migration across the Bering isthmus enabled colonization of Alaska around 15 ka and rapid expansion southward to the rest of North America. A temporal gradient in wapiti radiocarbon dates from Alaska southwards supports this scenario [23,46], supporting the proposal that this wave of migration is the source for present North American populations.
This model raises two important questions about the timing of faunal dispersal into North America. First, given a long history in northeast Siberia, why didn't this species migrate eastward earlier? Second, what conditions allowed migration to occur at this time?
Previous work has invoked competitive exclusion by two abundant large herbivores, mammoth and horse, as a possible answer to the first question [18]. We can now reject this proposal, given the coexistence of all three species—mammoth, horse and wapiti—in northeast Siberia during MIS 3, and their repeated co-occurrence in numerous Siberian Upper Palaeolithic archaeological sites (see the electronic supplementary material, table S5). Recent studies have highlighted that the Late Pleistocene palaeoenvironment of Beringia was not as uniform as previously thought, but rather a mosaic of biological communities [2,16,52]. Steppe–tundra vegetation dominated during large parts of MIS 3 with high diversity of grasses and herbs [2,16,53]. However, alongside the steppe–tundra, there were patches of shrub tundra, as well as isolated refugia of larch (Larix) and spruce (Picea) forest with birch (Betula) and alder (Alnus) trees [2,52–54]. Guthrie [55] proposed that a large and diverse megafaunal community, including a generalist herbivores such as wapiti, could only have been sustained at high latitudes during the Late Pleistocene in a highly diverse and productive environment. It is clear that ecological conditions for wapiti were present in northeast Siberia throughout this time.
Our data strongly suggest that central Beringia (the Bering isthmus proper) was a barrier that prevented wapiti (and moose) from crossing to Alaska for much of MIS 3–2. The nature of this barrier is less clear, but palaeoecological reconstructions suggest a relatively mesic climate and shrub–tundra-dominated habitat on the land bridge, very different from the environments on either side [16,56,57]. Recent analysis of MIS 3–2 beetle faunas from Chukotka, at the Siberian edge of the land bridge, indicates an environment colder and wetter than the steppe–tundra to the east and west [58]. Changes to this environment provide the most likely explanation for migrations after 15 ka. Based on pollen and beetle data [56,59], a significant environmental shift occurred in the central Bering Strait region around 17–18 ka, from birch graminoid to birch–heath–graminoid tundra. This precedes the colonization of humans, wapiti and moose by ca 2 kyr, so cannot alone account for their spread across the land bridge. However, at around 15 ka there is evidence of warming in various areas of Beringia [58], corresponding to the start of Greenland Interstadial 1. The increased forage productivity for browsers and mixed feeders expected as a result of warming apparently provided the conditions suitable for these taxa.
(b). Human colonization of the New World
Our three-stage model of trans-Beringian migration for wapiti resembles a model derived from genetic data for human colonization of North America [4,7,60]. The human colonization model identifies a population with diverged Asian genetic history that expanded to northeast Siberia late in MIS 3, remained during MIS 2 with little change in population size, and then rapidly expanded to North America around 15 ka. The presence of wapiti, a currently temperate–boreal taxon, in northeast Siberia through periods of environmental change during MIS 3 and MIS 2, supports the notion that conditions were amenable for other species of flexible adaptation but not generally considered to be Arctic-adapted, such as humans.
Significantly, our data suggest that the timing of human migration across the land bridge was subject to the same conditions that determined that timing in other species. Wapiti provide the clearest example so far of a species with a long history in northeast Siberia, which first colonized Alaska in late MIS 2, and then expanded southwards. One possible interpretation could be that human migration was an outcome of hunting specialization on wapiti, tracking the migrating population. However, it is unlikely that human populations in a marginal environment, for example Beringia, would have specialized on a relatively rare component of the mammal fauna, when other more common large game were available. In southern Siberia, wapiti remains occur in Upper Palaeolithic archaeological sites, but are typically represented by few specimens. Their increasing importance as a prey item after 14 ka may relate to the extinction of horse and mammoth (see the electronic supplementary material, table S5).
More persuasively, a model emerges from our data that the migration was blocked by a sea barrier until 70 ka and inappropriate conditions on the intermittent isthmus during 70–15 ka. Conditions suitable for dispersal were driven by the warming at 15 ka, until the last flooding of the Strait ca 11 ka. The subsequent expansion of both humans and wapiti through the lower 48 states also appears to have occurred as part of a common ecological event. This expansion scenario accords with the temporal gradient of wapiti radiocarbon ages, with the oldest definite radiocarbon-dated specimens found in Alaska and younger dates encountered as one proceeds southwards [23,46]. Some of the oldest tools from North America were produced of wapiti antler, and chronicle the spread of Clovis culture in the northern Great Plains [61]. The synchronicity in timing of both colonization and expansion implies that the ecology of wapiti (and moose) includes key limiting factors that can enhance our understanding of human dispersal.
5. Conclusion
We demonstrate that faunal remains from ecologically sensitive taxa have significant potential to identify barriers and corridors to migration, and are especially important in cases where human remains are scarce. Although additional data on archaeological sites on either side of the Bering Strait will continue to be difficult to obtain, end-Pleistocene faunal migration histories should be more straightforward to infer. In this case, we show that the pattern of dispersal of wapiti has marked similarities to that of humans, implying that dispersal in both cases may have resulted from exploitation of similar ecological conditions. The extent to which additional taxa follow the pattern proposed here will provide an important test of this proposal. We also identify the first indication of the long-term presence in Beringia of a species typically associated with more temperate environments, a point that has potential implications for the future management of this species, particularly at a time when changes to the environment are occurring rapidly across the Arctic.
Acknowledgements
We thank the following people and museum curators for providing modern and ancient samples: Kim Aaris-Sørensen, Michael Brett-Surman, Judith Chupasko, Andy Currant, Wei Dong, Robert G. Dundas, Alfred Gardner, Christopher Jass, Dale McCullough, Ben Potter, Richard Sabin and Eileen Westwig. We also thank the following museums for providing samples: American Natural History, New York; Geological Museum, Yakutsk; Ice-Age Museum, Moscow; Institute of Vertebrate Palaeontology and Palaeoanthropology, Beijing; Museum of Comparative Zoology, Harvard University, Cambridge; Mammoth Museum, Yakutsk; Natural History Museum, London; Paleontological Institute, Russian Academy of Science, Moscow; Royal Alberta Museum, Edmonton; Smithsonian Institution, National Museum of Natural History, Washington, DC; University of California Museum of Palaeontology, Berkeley; Zoological Institute, Russian Academy of Sciences, St Petersburg; Natural History Museum of Denmark Zoological Museum, University of Copenhagen, Copenhagen. We warmly thank Selina Brace, Paula Campos and Shai Meiri for technical assistance and useful discussion.
Data accessibility
DNA sequences have been deposited in NCBI (http://www.ncbi.nlm.nih.gov/) with accession numbers KF879622–KF879805.
Funding statement
This research was supported by NERC grant no. NE/G00269X/1 through the European Union FP7 ERA-NET programme, BiodivERsA. Funding for accelerator mass spectrometry dating was provided by Natural Environment Research Council Arts and Humanities Research Council Oxford Radiocarbon Accelerator Dating Service grant no. NF/2007/2/5. The ZooMS analyses were financially supported by Marie Curie FP7 Framework (MC-ITN 215362 LeCHE). The Ultraflex III was used courtesy of the York Centre of Excellence in Mass Spectrometry.
References
- 1.Lowe JJ, Walker M. 1997. Reconstructing quaternary environments, 2nd edn Harlow, UK: Prentice Hall [Google Scholar]
- 2.Hoffecker JF, Elias SA. 2007. Human ecology of Beringia. New York, NY: Columbia University Press [Google Scholar]
- 3.Hu AX, Meehl GA, Otto-Bliesner BL, Waelbroeck C, Han WQ, Loutre MF, Lambeck K, Mitrovica JX, Rosenbloom N. 2010. Influence of Bering Strait flow and North Atlantic circulation on glacial sea-level changes. Nat. Geosci. 3, 118–121 (doi:10.1038/Ngeo729) [Google Scholar]
- 4.Kitchen A, Miyamoto MM, Mulligan CJ. 2008. A three-stage colonization model for the peopling of the Americas. PLoS ONE 3, e1596 (doi:10.1371/journal.pone.0001596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffecker JF, Powers WR, Goebel T. 1993. The colonization of Beringia and the peopling of the New World. Science 259, 46–53 (doi:10.1126/science.259.5091.46) [DOI] [PubMed] [Google Scholar]
- 6.Goebel T. 1999. Pleistocene human colonization of Siberia and peopling of the Americas: an ecological approach. Evol. Anthropol. 8, 208–227 (doi:10.1002/(SICI)1520-6505(1999)8:6<208::AID-EVAN2>3.0.CO;2-M) [Google Scholar]
- 7.Mulligan CJ, Kitchen A, Miyamoto MM. 2008. Updated three-stage model for the peopling of the Americas. PLoS ONE 3, e3199 (doi:10.1371/journal.pone.0003199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters MR, et al. 2011. The Buttermilk Creek complex and the origins of Clovis at the Debra L. Friedkin site, Texas. Science 331, 1599–1603 (doi:10.1126/science.1201855) [DOI] [PubMed] [Google Scholar]
- 9.Jenkins DL, et al. 2012. Clovis age Western Stemmed projectile points and human coprolites at the Paisley Caves. Science 337, 223–228 (doi:10.1126/science.1218443) [DOI] [PubMed] [Google Scholar]
- 10.Dillehay TD. 1997. Monte Verde: a Late Pleistocene settlement in Chile, vol.2. The archaeological context and interpretation. Washington, DC: Smithsonian Institution Press; [DOI] [PubMed] [Google Scholar]
- 11.Waters MR, et al. 2011. Pre-Clovis mastodon hunting 13,800 years ago at the Manis site, Washington. Science 334, 351–353 (doi:10.1126/science.1207663) [DOI] [PubMed] [Google Scholar]
- 12.Pitulko VV. 2011. The Berelekh quest: a review of forty years of research in the mammoth graveyard in northeast Siberia. Geoarchaeol. 26, 5–32 (doi:10.1002/gea.20342) [Google Scholar]
- 13.Pitulko VV, Nikolsky PA, Girya EY, Basilyan AE, Tumskoy VE, Koulakov SA, Astakhov SN, Pavlova EY, Anisimov MA. 2004. The Yana RHS site: humans in the Arctic before the Last Glacial Maximum. Science 303, 52–56 (doi:10.1126/science.1085219) [DOI] [PubMed] [Google Scholar]
- 14.Meltzer DJ. 2009. First peoples in a New World: colonizing Ice Age America. Berkeley, CA: University of California Press [Google Scholar]
- 15.Barnes I, Matheus P, Shapiro B, Jensen D, Cooper A. 2002. Dynamics of Pleistocene population extinctions in Beringian brown bears. Science 295, 2267–2270 (doi:10.1126/science.1067814) [DOI] [PubMed] [Google Scholar]
- 16.Elias SA, Crocker B. 2008. The Bering land bridge: a moisture barrier to the dispersal of steppe-tundra biota? Q. Sci. Rev. 27, 2473–2483 (doi:10.1016/JQuascirev.2008.09.011) [Google Scholar]
- 17.Barnett B, et al. 2009. Phylogeography of lions (Panthera leo ssp.) reveals three distinct taxa and a late Pleistocene reduction in genetic diversity. Mol. Ecol. 18, 1668–1677 (doi:10.1111/j.1365-294X.2009.04134) [DOI] [PubMed] [Google Scholar]
- 18.Guthrie RD. 2006. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441, 207–209 (doi:10.1038/nature04604) [DOI] [PubMed] [Google Scholar]
- 19.Leonard JA, Wayne RK, Cooper A. 2000. Population genetics of Ice Age brown bears. Proc. Natl Acad. Sci. USA 97, 1651–1654 (doi:10.1073/pnas.040453097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guthrie RD. 1996. Four late Pleistocene large-mammal localities in interior Alaska. In American beginnings: the prehistory and palaeoecology of Beringia (ed. West F.), pp. 119–128 Chicago, IL: University of Chicago Press [Google Scholar]
- 21.Dolan JM. 1988. A deer of many lands: a guide to subspecies of the red deer Cervus elaphus L. Zoonooz 62, 4–34 [Google Scholar]
- 22.Geist V. 1999. Deer of the world: their evolution, behaviour, and ecology. Shrewsbury, UK: Swan Hill Press [Google Scholar]
- 23.O'Gara BW, Dundas RG. 2002. Distribution: past and present. In North American elk: ecology and management (eds Toweill DE, Thomas JW.), pp. 67–119 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 24.Boeskorov GG. 2005. Taxonomic poistion of red deer Cervus elaphus L. (Cervidae, Artiodactyla, Mammalia) from the Neopleistocene of northeastern Asia. Paleontol. J. 39, 535–547 [Google Scholar]
- 25.Mahmut H, Masuda R, Onuma M, Takahashi M, Nagata J, Suzuki M, Ohtaishi N. 2002. Molecular phylogeography of the red deer (Cervus elaphus) populations in Xinjiang of China: comparison with other Asian, European, and North American populations. Zool. Sci. 19, 485–495 (doi:10.2108/zsj.19.485) [DOI] [PubMed] [Google Scholar]
- 26.Ludt CJ, Schroeder W, Rottmann O, Kuehn R. 2004. Mitochondrial DNA phylogeography of red deer (Cervus elaphus) Mol. Phylogenet. Evol. 31, 1064–1083 (doi:10.1016/j.ympev.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 27.Reimer P, et al. 2009. INTCAL09 and MARINE09 radiocarbon age calibration curves, 0–50,000 years cal BP. Radiocarbon 51, 1111–1150 [Google Scholar]
- 28.Bronk Ramsey C. 2009. Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337–360 [Google Scholar]
- 29.Tuross N, Kirsanow K, Barnes I. 2009. Limits and possibilities for subsistence and climate reconstruction based on organic and inorganic oxygen isotopes in vertebrate calcified tissues. Geochim. Cosmochim. Acta 73, A1355–A1355 [Google Scholar]
- 30.Chippindale PT, Wiens JJ. 1994. Weighting, partitioning, and combining characters in phylogenetic analysis. Syst. Biol. 43, 278–287 [Google Scholar]
- 31.Huelsenbeck JP, Bull JJ, Cunningham CW. 1996. Combining data in phylogenetic analysis: reply. Trends Ecol. Evol. 11, 335–335 (doi:10.1016/S0169-5347(96)91643-2) [DOI] [PubMed] [Google Scholar]
- 32.Cook CE, Wang Y, Sensabaugh G. 1999. A mitochondrial control region and cytochrome b phylogeny of sika deer (Cervus nippon) and report of tandem repeats in the control region. Mol. Phylogenet. Evol. 12, 47–56 (doi:10.1006/mpev.1998.0593) [DOI] [PubMed] [Google Scholar]
- 33.Felsenstein J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17, 368–376 (doi:10.1007/BF01734359) [DOI] [PubMed] [Google Scholar]
- 34.Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (doi:10.1093/bioinformatics/17.8.754) [DOI] [PubMed] [Google Scholar]
- 35.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (doi:10.1093/Bioinformatics/Btg180) [DOI] [PubMed] [Google Scholar]
- 36.Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567 (doi:10.1111/j.1755-0998.2010.02847) [DOI] [PubMed] [Google Scholar]
- 37.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690 (doi:10.1093/bioinformatics/btl446) [DOI] [PubMed] [Google Scholar]
- 38.Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Organ. Divers. Evol. 12, 335–337 (doi:10.1007/s13127-011-0056-0) [Google Scholar]
- 39.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (doi:10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guindon S, Gascuel O. 2003. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst. Biol. 52, 696–704 (doi:10.1080/10635150390235520) [DOI] [PubMed] [Google Scholar]
- 41.Drummond AJ, Rambaut A, Shapiro B, Pybus OG. 2005. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 22, 1185–1192 (doi:10.1093/Molbev/Msi103) [DOI] [PubMed] [Google Scholar]
- 42.Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (doi:10.1093/molbev/mss075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teacher AGF, Griffiths DJ. 2011. HapStar: automated haplotype network layout and visualisation. Mol. Ecol. Resour. 11, 151–153 (doi:10.1111/j.1755-0998.2010.02890) [DOI] [PubMed] [Google Scholar]
- 44.Kurten B, Anderson E. 1980. Pleistocene mammals of North America. New York, NY: Columbia University Press [Google Scholar]
- 45.FAUNAMAP 1994. A database documenting late Quaternary distributions of mammal species in the United States. Springfield, IL: Illinois State Museum Scientific Papers [Google Scholar]
- 46.Burns JA. 2009. Mammalian faunal dynamics in Late Pleistocene Alberta, Canada. Q. Int. 217, 37–42 (doi:10.1016/j.quaint.2009.08.003) [Google Scholar]
- 47.Rubenstein D, Chamberlain C, Holmes R, Ayres M, Waldbauer J, Graves G, Tuross N. 2002. Linking breeding and wintering ranges of a migratory songbird using stable isotopes. Science 295, 1062–1065 (doi:10.1126/science.1067124) [DOI] [PubMed] [Google Scholar]
- 48.Rubenstein D, Hobson K. 2004. From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol. Evol. 19, 256–263 (doi:10.1016/j.tree.2004.03.017) [DOI] [PubMed] [Google Scholar]
- 49.Leyden J, Wassenaar L, Hobson K, Walker E. 2006. Stable hydrogen isotopes of bison bone collagen as a proxy for Holocene climate on the Northern Great Plains. Palaeogeogr. Palaeoecol. 239, 87–99 (doi:10.1016/j.palaeo.2006.01.009) [Google Scholar]
- 50.Heptner V, Nasimovich A, Bannikov A. 1988. Mammals of the Soviet Union. New Delhi, India: Amerind Publishing [Google Scholar]
- 51.Guthrie RD. 1966. The extinct wapiti of Alaska and Yukon territory. Can. J. Zool. 44, 47–57 (doi:10.1139/z66-004) [Google Scholar]
- 52.Brigham-Grette J, Lozhkin AV, Anderson PM, Glushkova OY. 2004. Paleoenvironmental conditions in Western Beringia before and during the Last Glacial Maximim. In Entering America, northeast Asia and Beringia before the last glacial maximum (ed. Madsen DB.), pp. 29–61 Salt Lake City, UT: University of Utah Press [Google Scholar]
- 53.Sher AV, Kuzmina SA, Kuznetsova TV, Sulerzhitsky LD. 2005. New insights into the Weichselian environment and climate of the East Siberian Arctic, derived from fossil insects, plants, and mammals. Q. Sci. Rev. 24, 533–569 (doi:10.1016/JQuascirev.2004.09.007) [Google Scholar]
- 54.Anderson PH, Lozhkin AV. 2001. The Stage 3 interstadial complex (Karginskii/middle Wisconsinan interval) of Beringia: variations in paleoenvironments and implications for paleoclimatic interpretations. Q. Sci. Rev. 20, 93–125 (doi:10.1016/S0277-3791(00)00129-3) [Google Scholar]
- 55.Guthrie RD. 1982. Mammals of the mammoth steppe as paleoenvironmental indicators. In Paleoecology of Beringia (eds Hopkins DM, Matthews JV, Schweger CE, Young SB.), pp. 307–324 New York, NY: Academic Press [Google Scholar]
- 56.Elias SA, Short SK, Birks HH. 1997. Late Wisconsin environments of the Bering land bridge. Palaeogeogr. Palaeoecol. 136, 293–308 (doi:10.1016/S0031-0182(97)00038-2) [Google Scholar]
- 57.Guthrie RD. 2001. Origin and causes of the mammoth steppe: a story of cloud cover, woolly mammal tooth pits, buckles, and inside-out Beringia. Q. Sci. Rev. 20, 549–574 (doi:10.1016/S0277-3791(00)00099-8) [Google Scholar]
- 58.Kuzmina SA, Sher AV, Edwards ME, Haile J, Yan EV, Kotov AV, Willerslev E. 2011. The late Pleistocene environment of the Eastern West Beringia based on the principal section at the Main River, Chukotka. Q. Sci. Rev. 30, 2091–2106 (doi:10.1016/j.quascirev.2010.03.019) [Google Scholar]
- 59.Elias SA, Short SK, Nelson CH, Birks HH. 1996. Life and times of the Bering land bridge. Nature 382, 60–63 (doi:10.1038/382060a0) [Google Scholar]
- 60.Reich D, et al. 2012. Reconstructing native American population history (vol. 488, 370, 2012) Nature 491, 288 (doi:10.1038/Nature11667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morrow JE, Fiedel SJ. 2006. New radiocarbon dates for the Clovis component of the Anzick site, Park County, Montana. In Paleoindian archaeology: a hemispheric perspective (eds Morrow JE, Gnecco C.), pp. 123–138 Gainesville, FL: University Press of Florida [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
DNA sequences have been deposited in NCBI (http://www.ncbi.nlm.nih.gov/) with accession numbers KF879622–KF879805.