Abstract
Our understanding of how anthropogenic habitat change shapes species interactions is in its infancy. This is in large part because analytical approaches such as network theory have only recently been applied to characterize complex community dynamics. Network models are a powerful tool for quantifying how ecological interactions are affected by habitat modification because they provide metrics that quantify community structure and function. Here, we examine how large-scale habitat alteration has affected ecological interactions among mixed-species flocking birds in Amazonian rainforest. These flocks provide a model system for investigating how habitat heterogeneity influences non-trophic interactions and the subsequent social structure of forest-dependent mixed-species bird flocks. We analyse 21 flock interaction networks throughout a mosaic of primary forest, fragments of varying sizes and secondary forest (SF) at the Biological Dynamics of Forest Fragments Project in central Amazonian Brazil. Habitat type had a strong effect on network structure at the levels of both species and flock. Frequency of associations among species, as summarized by weighted degree, declined with increasing levels of forest fragmentation and SF. At the flock level, clustering coefficients and overall attendance positively correlated with mean vegetation height, indicating a strong effect of habitat structure on flock cohesion and stability. Prior research has shown that trophic interactions are often resilient to large-scale changes in habitat structure because species are ecologically redundant. By contrast, our results suggest that behavioural interactions and the structure of non-trophic networks are highly sensitive to environmental change. Thus, a more nuanced, system-by-system approach may be needed when thinking about the resiliency of ecological networks.
Keywords: forest fragmentation, insectivorous birds, mixed-species flocks, network theory, Amazon, heterogeneous landscapes
1. Introduction
Biological systems are often organized as networks [1–3] and while these networks are nearly ubiquitous, analytical approaches have only recently been applied to identify common properties and understand system-level dynamics. In its simplest form, a biological network can be represented as a graph comprising nodes (individuals or species) and edges (biological interactions). Network theory offers the ideal conceptual framework to understand the structural complexity of biological systems, because it provides metrics to quantify and interpret interactions at the level of individuals or species, and documents the properties of the system as a whole [4]. Ultimately, these approaches have advanced our understanding of a variety of complex biological processes and types of interactions such as mutualisms [5], trophic interactions [6], fitness consequences of social behaviour [7,8], disease transmission [9] and robustness of communities to extinction [10].
Networks of trophic interactions have been the focus of a substantial body of research [6,11]. The structure of these networks can be influenced not only by intrinsic traits of participating organisms (e.g. phenotype) but also by extrinsic characteristics of the environment (e.g. habitat heterogeneity). For example, habitat modification affects trophic network structure via changes in species richness and frequency of interactions [12]. Previous work has shown that most ecological networks are resilient to environmental change, but that the threshold at which a community collapses is dependent upon the degree to which species are ecologically redundant and the responses of keystone species to habitat loss [5,10,13]. While trophic networks have been fairly well studied, non-trophic interactions, such as the social mutualisms observed in avian mixed-species flocks, have received considerably less attention. Ultimately, by characterizing the roles that species play within ecological networks we can begin to understand the assembly of ecological communities [14], the predisposition for species to engage in non-random spatio-temporal aggregations [15] and how those ecological and evolutionary processes are influenced by environmental change.
Mixed-species flocks are among the most complex multi-species aggregations found in terrestrial vertebrates [16]. Some forms of mixed-species flocking occurs throughout the world, but few reach the temporal stability and interdependency of understory mixed-species flocks in Amazonian rainforests. These flocks of insectivores have a year-round territory and consist of a core of eight to 10 obligate species, each represented by a breeding pair. The territories of these core species overlap in a flock territory of 8–10 ha [16–18]. Cinereous antshrikes (Thamnomanes caesius) play a nuclear role in these mixed-species flocks throughout the Amazon basin [19] by rallying individuals and giving alarm calls [20]. Up to 50 other species, mostly in breeding pairs, are known to join the core flock in varying periodicity [17]. Given that flocks are often species-rich and exhibit both spatial and temporal stability [21], they represent an important component of the Amazonian understory avifauna [22]. Flocks provide direct fitness benefits in participating birds, including improved predator detection and increased foraging efficiency [23], yet these benefits may vary by species and be strongly dependent upon habitat context and flock organization.
The Amazon provides an important setting to examine changes in interspecific avian interactions, because the area is subjected to substantial forest clearing which produces heterogeneous landscapes of primary forest (PF), secondary forest (SF), forest fragments, and interspersed roads [24]. These newly fragmented and regenerating Amazonian forests influence the dynamics [25–27] and diversity of avian communities [28]. Given that mixed-species flocks in the Amazon are largely forest dependent, they are highly susceptible to habitat disturbances. For example, most flock species avoid open areas, show reluctance to cross narrow roads [18], and often disappear in selectively logged forests [29] and small fragments [25,30]. Despite the detrimental effect of forest clearing on these species, depauperate flocks can still be detected in second-growth and small fragments [31]. To date, research on how mixed-species flocks change along disturbance gradients have largely focused on species richness and encounter rates (e.g. number of detections per unit time [32,33]), yet no studies, to our knowledge, have examined how habitat modifications influence interspecific interactions and the stability of flock structure.
Understanding how both species interactions and the subsequent structure of ecological networks change across landscape gradients are important because flocks can affect community dynamics and the fitness of participating species. To date, accurately characterizing interactions within mixed-species flocks has remained challenging because flock attendance is dynamic (i.e. many individuals join and leave a flock in both time and space). The analytical framework of network theory can advance our understanding of flock dynamics by characterizing changes at multiple levels of organization (species, i.e. node level and flock, i.e. network level). Here, we apply network theory to examine how individual species' interactions and flock-level structure change across a heterogeneous landscape mosaic in the Amazon. First, at the species level, we compare how the number (degree) and frequency (weighted degree) of interspecific interactions within mixed-species flocks varied among PF, 100 ha fragments (100 ha), 10 ha fragments (10 ha), a mix of primary and secondary forest (PSF) and SF. Second, we examine how environmentally induced changes at the species-level scale-up to influence flock-level network properties. In particular, we characterize how the connectedness (the distribution of interactions) and cohesion (clustering of species) of flocks change across a landscape gradient. Third, we examine the relationship between vegetation structure and network cohesion as one possible mechanism for changes in social structure among habitats. Given the changes in flock structure among habitats, we also compared species attendance among habitat types to determine whether network differences were causing species disappearance or reduction in flock attendance. This work builds a framework for understanding how environmental heterogeneity affects the resilience of complex ecological interactions by examining the integrity and stability of flock networks across a habitat mosaic.
2. Material and methods
(a). Study site and data collection
The study was conducted at the Biological Dynamics of Forest Fragments Project (BDFFP) in central Amazonia, Brazil. Fragments were isolated between 1980 and 1990 during the settlement of cattle ranches [34,35]. Following initial clear-cutting, some areas were burned to create pastures while others were abandoned. Most pastures were inactive by the 1990s, allowing the matrix around the fragments to regenerate. A buffer of 100 m was cleared around some fragments from the early 1990s to the early 2000s, but these areas have regenerated as well. Different management histories have created a structurally heterogeneous landscape [36], with fragments of different sizes surrounded by SFs varying in structure and age.
Understory mixed-species flocks gather in the same location every day at dawn and move through their territory until about 13 min prior to sunset [17]. Flock activity is conspicuous, allowing birds to be followed on foot from a distance of 10–20 m. Importantly, the mixed-species flocks described here should be differentiated from those observed at army-ant swarms, which comprise solitary species that become spatially aggregated around a resource. We followed 21 flocks for at least 17 h each (mean = 42.1, max. = 121.4, min. = 17.1), totalling 693 h between March and November of 2010 and 2011. We recorded flock composition in 30 min time blocks, generating a total of 12 414 species entries. A species was noted as participating with a flock if it was seen within 15 m of core flock activity for more than 30 min. To assess that we had adequately sampled flocks in each habitat, we constructed ‘sample-based’ species accumulation rarefaction curves using the program EstimateS [37] (see the electronic supplementary material, figure S1). Based on flock sampling criteria, we also used the program EstimateS and the frequencies of species in the original sampling data to generate a non-parametric estimator of species richness (Chao II) [37,38]. Likewise, we estimated encounter rate as the number of times a species was detected corrected for total sampling time.
(b). Habitat and vegetation characterization
To measure flock territories, flock positions were recorded at 30 s intervals with a Garmin eTrex Vista HCx unit (approx. 10 m resolution). A quadratic kernel was generated using Geospatial Modeling Environment software [39], 99% isopleth was generated at 1 m resolution, 275 bandwidth at default scaling factor. Vegetation was measured using LIDAR (Light Detection and Ranging) canopy height models provided by Scott Saleska (University of Arizona) and Michael Lefsky (Colorado State University). We generated the zonal statistics for the vegetation located inside the isopleth (see the electronic supplementary material, table S1).
Flock territories were categorized in five habitat types: PF, if flocks used more than half of the territory in continuous PF; 100 ha, if flocks inhabited a 100 ha fragment; 10 ha, these fragments are only large enough for one flock territory; PSF mix, if flocks used more than half of their territory in degraded SF and patches of semi-isolated PF; and SFs, if a territory was exclusively in SF. Mean vegetation height was used as an indication of habitat structure and quality.
(c). Network and statistical analyses
We constructed networks for mixed-species flocks in all five habitat types. Based on species co-occurrences in each time block, we used the cumulative frequency of associations to construct weighted networks for each flock. Specifically, network edges were defined using species co-occurences within sampling time blocks. As such, any species associated with the flock aggregation is by default associating with all species present in that sampling time block. Hereafter, we use the term ‘interspecific association’ to describe these interactions. Using spatial proximity to define network associations in this manner is termed the ‘gambit of the group’ such that all individuals within a spatial and temporal range will have reciprocal ties in the network [40,41]. Although, many species will appear accidentally in flocks, our threshold (30 min sampling time blocks) removes accidental species, which do not accompany the flock for more than a few metres. We chose not to apply filtering techniques to remove low-frequency co-occurrences, because we used weighted network metrics and were interested in how common and rare species influenced network structure across habitat types. Moreover, data from replicates of independent flocks within habitat types should produce more precise measures of network co-occurrence.
At the species level, for each flock, we calculated unweighted and weighted degree metrics using UCINET [42] and the R package tnet [43]. Degree is the number of edges (co-occurrences) one given species (network node) maintains with other species in a flock (i.e. species connectedness). Weighted degree is the sum of the frequency of interspecific associations for each node. Networks were visualized using R package ‘network’ [44]. We calculated the average network degree following [45], the degree distribution skewness using R package moments [46] and global weighted clustering coefficients following [47] using the tnet package in R. To ensure that interspecific associations and network structure could be differentiated from random, we used iterative permutation procedures (for methods and results, see the electronic supplementary material). Network metrics for replicate flocks were grouped within habitat type for subsequent analyses (see above).
We used a suite of analyses to examine the effect of habitat on species- and flock-level network properties. First, we looked at the response of species richness and encounter rate to habitat type. To examine how species richness (Chao II estimator) varied by habitat type, we used a GLMM (generalized linear mixed model) with flock as a random effect and habitat as a fixed effect. All GLMM models used Poisson error distributions and log-link functions unless otherwise noted (see below). To examine how encounter rate (detections per unit time) varied by habitat, we used a zero-inflated negative binomial mixed model to account for over-dispersion in the data. The residuals of all models were normally distributed and we compared the effects of habitat type with null models using likelihood ratio tests.
Second, we used GLMMs to examine how habitat influenced species-level network metrics (i.e. degree and weighted degree). Given that network data are not independent [48], we used flock replicates within each habitat type and included flock identity as a random effect and habitat as a fixed effect to explain variation in degree and weighted degree. To compare across networks in different habitats, we accounted for the number of possible species interactions and sampling time using a log (n or t) offset [49] where n represents the number of possible interspecific associations within the network (n − 1, number of nodes) and t represents sampling time. These corrections enabled us to compare networks with different number of species (nodes) and sampling effort, which is a common problem in network analyses [40]. Hereafter, we report the corrected values of normalized degree [50] and weighted degree (i.e. frequency of associations are corrected by sampling time). Maximum-likelihood estimates of β coefficients and p-values for fixed effects from each model are reported.
Third, to examine how flock-level network properties varied by habitat type, we compare the distribution of species interspecific associations (degree distributions) using Wilcox sign-rank tests (see the electronic supplementary material). In addition, given that there was substantial variation in habitat structure among replicates (see the electronic supplementary material, table S1) we used least-squares regression to examine how a continuous measure of habitat, vegetation height, influenced flock attendance and cohesion as measured by weighted clustering coefficients. We used the base package of program R for regressions and lme4 [51] and glmmADMB [52] for GLMMs. Graphs were generated using gpplot2 [53].
Finally, we estimated changes in species participation by using detections within each flock. We used PF as the template for comparison under the assumption that it represents baseline flock species composition and attendance rates. To differentiate between species disappearance and decreased attendance, we compared presence/absence and attendance data from altered habitat (100 ha, 10 ha, PSF and SF) to those observed in PF. We used all possible pairwise combinations of PF flocks and those in other habitats. For example, the comparison of PF (nine flocks) and SF (three flocks) would generate 27 values. We report percentages of species that disappeared (if only one species was present in a determined habitat type) or decreased attendance (if both species were present in both habitat types) relative to PF. Because some novel species appear in certain habitats and other increase in attendance, reported values do not sum to 100%. The magnitude of change in attendance is reported as averages across flocks sampled in each habitat type where negative values represent decreases.
3. Results
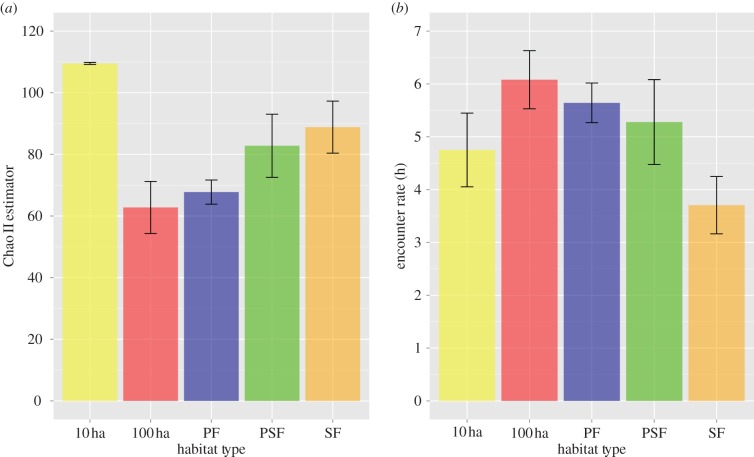
We sampled 21 flocks across five habitat types with each habitat type receiving a minimum of 86 h (table 1 and electronic supplementary material, S1). Habitat was a good predictor of both species richness (χ2 = 15.85, p = 0.003) and encounter rate (χ2 = 11.92, p = 0.017) as evidenced by those models being better fit than null models (see the electronic supplementary material, table S2). Species richness was significantly higher in 10 ha fragments than in intact habitats (β100-ha = −0.56, p = 0.0003; βPF = −0.48, p = 0.0001; figure 1a). By contrast, more intact forest environments (e.g. PF and 100 ha) had higher encounter rates than 10 ha fragments and SF after controlling for sampling effort (β100-ha = 0.88, p = 0.003; βPF = 1.01, p = 0.0002; figure 1b).
Table 1.
Summary of sampling for mixed-species flocks in five habitat types at the BDFFP in central Amazonian Brazil.
| canopy |
||||||
|---|---|---|---|---|---|---|
| habitat type | n flocks | max. ht. (m) | avg. ht. (m) | sampling time (h)a | attendance | no. spp.b |
| primary forest | 9 | 45.7 ± 1.5 | 23.8 ± 0.35 | 304.0 | 851.9 ± 51.1 | 109 |
| 100 ha fragment | 5 | 46.3 ± 1.3 | 21.4 ± 1.2 | 151.5 | 918.0 ± 60.5 | 88 |
| 10 ha fragment | 2 | 45.0 ± 5.6 | 16.4 ± 1.8 | 134.5 | 717.3 ± 22.2 | 103 |
| primary-secondary | 2 | 40.4 ± 3.7 | 16.8 ± 0.90 | 86.0 | 797.0 ± 19.9 | 79 |
| secondary forest | 3 | 39.1 ± 5.5 | 14.4 ± 1.97 | 88.0 | 559.5 ± 121.4 | 82 |
aTotal sampling time.
bCumulative number of species observed during sampling.
Figure 1.
Mixed-species flocks showed substantial variation in both (a) species richness and (b) encounter rate across a habitat gradient in the Brazilian Amazon. Bars represent mean±s.e. (Online version in colour.)
Models constructed to test for the effect of habitat type on interspecific associations showed that environmental heterogeneity influenced species-level network metrics. Specifically, models that included habitat were a significantly better fit than null models for both normalized degree (χ2 = 22.28, p = 0.0002) and weighted degree (χ2 = 15.69, p = 0.003; see the electronic supplementary material, table S3). Flocking species in PF and 100 ha fragments associated with a proportionately greater number of other species (normalized degree) than did species in degraded habitats (table 2 and figure 2a). Likewise, flocks in less disturbed areas also had a higher frequency of interspecific associations (weighted degree) than did flocks in degraded habitats (table 2 and figure 2b). Flock identity (random effect) explained only a small portion of the variance in our models, suggesting that results are consistent across habitat-type replicates.
Table 2.
Parameters estimates and p-values from models examining the effect of habitat type on mixed-species flock network structure at the BDFFP in central Amazonian Brazil.
| habitat | normalized degree |
weighted degree |
||
|---|---|---|---|---|
| β | p-value | β | p-value | |
| 100 ha | 0.57 | 0.0001 | 0.88 | 0.003 |
| primary forest | 0.45 | 0.0002 | 1.01 | 0.002 |
| primary-secondary | 0.25 | 0.11 | 0.70 | 0.05 |
| secondary forest | −0.004 | 0.98 | −0.06 | 0.84 |
Figure 2.
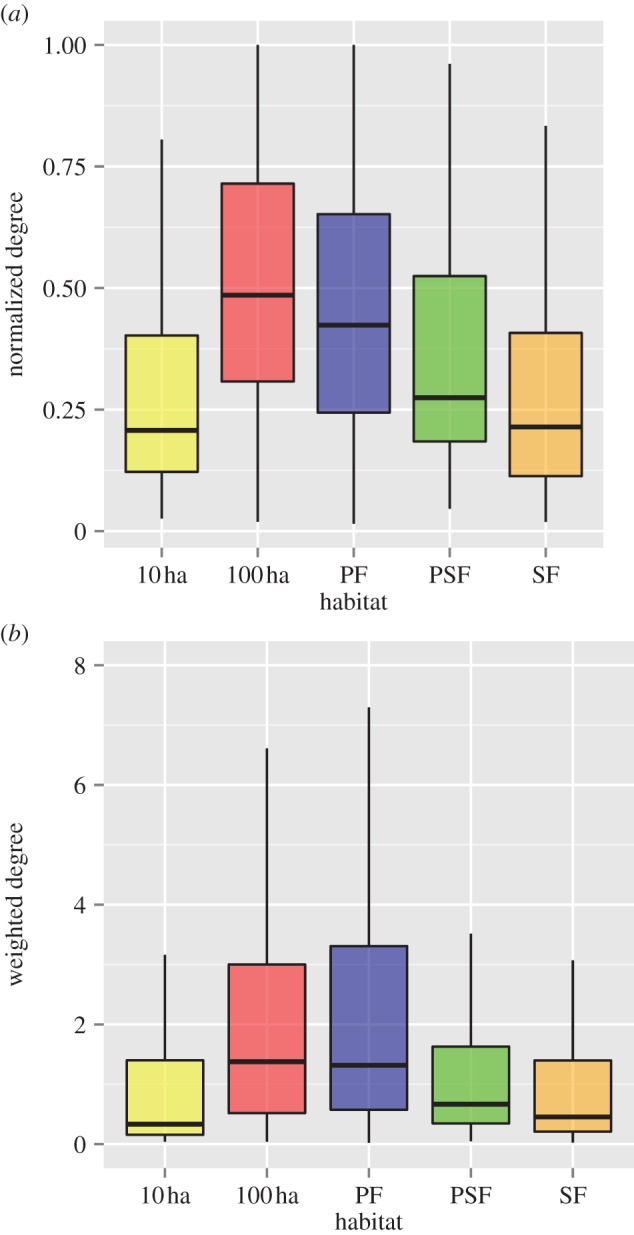
Box plots show that species in mixed-species flocks in more preserved habitats (PF and 100 ha) (a) had a greater number of interspecific interactions (normalized degree) and (b) a higher frequency of interactions (weighted degree) than in degraded forest habitats (SF, PSF and 10 ha). (Online version in colour.)
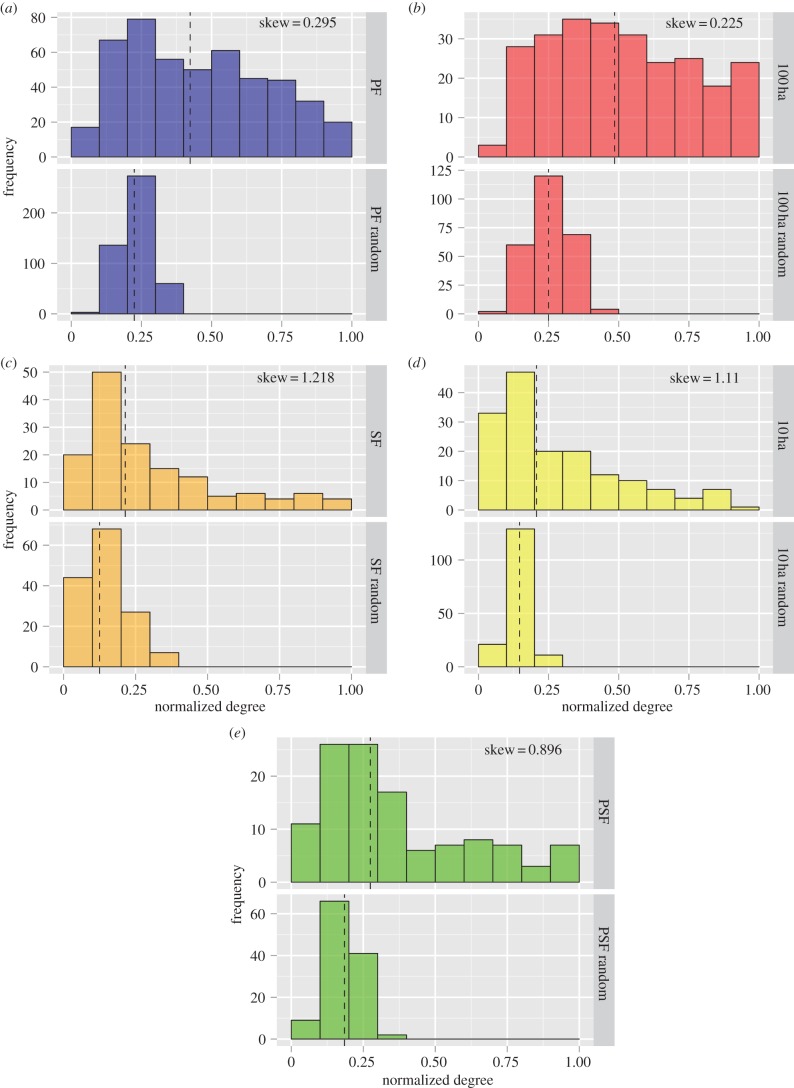
Organization of flock networks differed across different habitat types (figure 3). A consistent group of core species, particularly Thamnomanes caesius, Xyphorhynchus pardalotus and Myrmotherula axillaris, was present across all habitat types, but the complexity of the network was far greater in PF than in 10 ha fragments and SF. An examination of cumulative degree distributions revealed habitat-specific differences in global network structure (figure 4). Networks in degraded forest habitats were composed of many weakly associated birds (low degree) with few well-connected species (high degree), which resulted in flocks with low median normalized degree and degree distributions with strong positive skew (i.e. long-tail to the distribution; figure 4c–e; electronic supplementary material, table S4). In comparison, networks in PFs and 100 ha fragments had a higher median normalized degree and weaker positive skew (figure 4a,b; electronic supplementary material, table S4). Differences in social structure at the level of the flock appear to be in part, driven by vegetation characteristics. Vegetation height was positively correlated with flock cohesion as measured by species attendance patterns (r² = 0.37, F1,19 = 11.08, p = 0.003; figure 5a) and global clustering coefficients (r2 = 0.50, F1,19 = 19.07, p = 0.0003; figure 5b).
Figure 3.
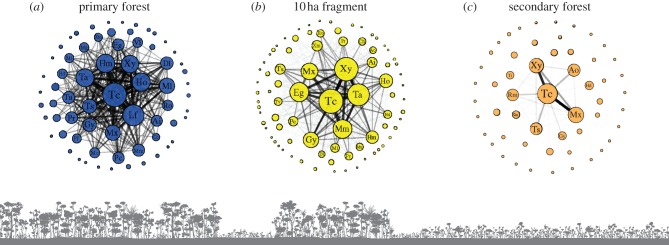
Example of networks and habitat configurations for three flocks found in PF, 10 ha fragment and SF habitat types in the Brazilian Amazon. Differences in network structure reflect the decay of interspecific interactions in mixed-species flocks across a disturbance gradient. Edge thickness and transparency in each network are proportional to numbers of interactions. Interaction values at the lowest 10% are set to transparent. Nodes sizes are proportional to flock attendance. Legends for species with participation above 6% are given. Ai, Automolus infuscatus; Ao, Automolus ochrolaemus; Cc, Cariothraustes canadensis; Ch, Cacicus haemorrhous; Dl, Deconychura longicauda; Eg, Epinecrophylla gutturalis; Gy, Glyphorhynchus spirurus; ; Hd, Herpsilochmus dorsimaculatus; Hm, Hylophilus muscicapinus; Ho, Hylophilus ochraceiceps; Lf, Lanio fulvus; Mg, Myiopagis gaimardii; Mi, Mionectes sp.; Ml, Myrmotherula longipennis; Mm, Myrmotherula menetriesii; Mu, Myrmotherula brachyura; Mx, Myrmotherula axillaris; Pc, Piprites chloris; Pf, Piculus flavigula; Pr, Philydor erythrocercum; Rm, Ramphocaenus melanurus; Ro, Rhynchocyclus olivaceus; Ta, Thamnomanes ardesiacus; Tc, Thamnomanes caesius; Ts, Tolmomyias assimilis; Tu, Tachyphonus surinamus; Tv, Trogon viridis; Vl, Vireolanus leucotis; Xm, Xenops minutus; Xy, Xiphorhynchus pardalotus. (Online version in colour.)
Figure 4.
Cumulative degree distributions highlight differences in mixed-species flock network structure across a habitat gradient in the Brazilian Amazon. Pairs of histograms for observed and randomized networks show that degraded habitats are characterized by low medians and high skew while more intact habitats have higher medians and lower skew. Dashed lines represent the median normalized degree. (Online version in colour.)
Figure 5.
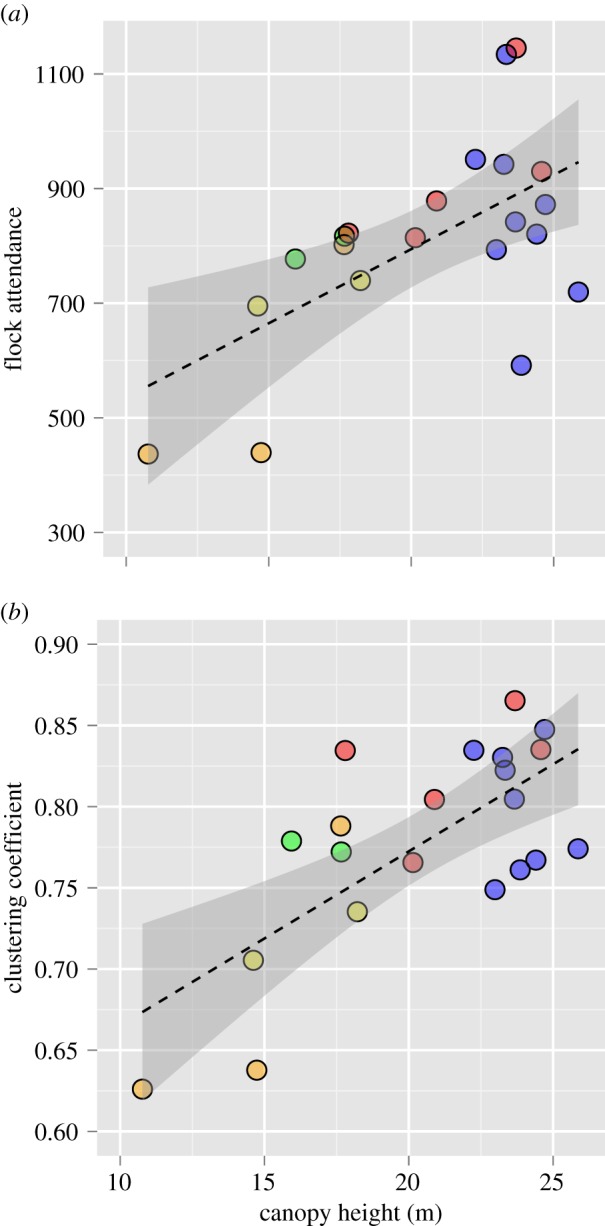
Mixed-species flock cohesiveness (clustering) and attendance show strong positive correlation with vegetation structure. (a) Relationship between flock global clustering coefficient and mean vegetation height. (b) Relationship between species attendance and mean vegetation height. The y-axis represents the cumulative amount of time all species in a given habitat participated in a flock. Flocks (points in the graph) are shaded for habitat type (medium grey, PF; light grey, 100 ha; white, 10 ha; dark grey, PSF; black, SF). Dashed line represents the β coefficient from the model and the grey shaded area is the 95% confidence interval. (Online version in colour.)
A comparison of presence/absence data and species attendance between disturbed and PF habitats shows that decreased attendance rather than disappearance is the primary driver of differences in network structure (table 3). Specifically, a relatively small proportion of species were absent in disturbed habitats (2–12%), whereas nearly half of species detected in both habitats decreased attendance rates (47–56%) relative to PFs. The magnitude of change was largest in the two most degraded habitat types (e.g. 10 ha and SF; table 3).
Table 3.
The relative changes in the presence/absence and attendance from PF to degraded forest for mixed-species flocks. (Species were recorded as absent when detected in PF but not degraded habitats and decreasing attendance when detected in both habitats but in a lower relative proportion. Magnitude of change describes the average differences in encounter rate between primary and degraded habitats.)
| habitat type | % spp. absent | % spp. decreasing attendance | mean magnitude of change |
|---|---|---|---|
| 10 ha | 3.0 | 47.7 | −3.79 ± 2.50 |
| secondary forest | 12.3 | 47.7 | −5.75 ± 3.44 |
| primary-secondary | 3.3 | 56.7 | −2.62 ± 4.37 |
| 100 ha | 2.4 | 55.1 | −1.94 ± 2.50 |
4. Discussion
Interspecific interactions in communities are an essential component of ecosystem function and have important implications for the ecological and evolutionary dynamics of species [54]. To date, research on trophic interaction networks has shown that habitat changes can affect interspecific networks, yet their structure is often resilient to habitat change because species are ecologically redundant [10,55,56]. Our results corroborate that the interspecific associations which comprise flock networks are also affected by habitat degradation. While little is known about the functional roles of species within flocks, our results suggest that flock social structure may be comparatively more sensitive than other ecological networks studied to date. Here, we document changes in species richness, encounter rates, species connectedness and the frequency of interspecific associations within mixed-species flocks along a habitat mosaic. Our results suggest that habitat modification and changes in vegetation structure alter flock attendance and subsequent interspecific associations resulting in reduced flock cohesion and stability. Given that mixed-species flocks host a diversity of understory insectivorous birds, these results highlight the potential negative impact of habitat alteration on the dynamics of species interactions.
(a). Species- and flock-level changes in network structure
Multi-species interactions form the basis of ecological networks, and changes in species presence or behaviour can have profound impacts on network structure and ecosystem function [57]. Research on food webs suggests that habitat degradation tends to promote homogenization (loss of species) resulting in reduced network complexity and stability [12,58,59]. Our results, from a non-trophic flock network, corroborate the idea that extrinsic environmental features, like habitat, can affect interspecific social structure. At the species level, our results indicate that flocking birds in small fragments and degraded SFs associated with fewer species (degree) and did so less frequently (weighted degree) than individuals in intact habitats. Structural differences among networks at the level of the flock are best illustrated by habitat-specific degree distributions, which show the cumulative effect of changes in species associations across habitat types. In particular, the majority of associations in 10 ha fragments and SFs were driven by a few remaining core species, while associations were more evenly distributed across species in intact forest environments.
Our results also highlight that habitat configuration can influence patterns of species richness. Specifically, species richness was higher in some of the more degraded habitats than in large intact forests tracts. These results differ from previous studies in the old world tropics, which found decreases [32] or no changes in species richness [33] in mixed-species flocks along disturbance gradients. Increases in species richness in 10 ha fragments were not altogether surprising given the available habitat matrix and community composition. In particular, the proximity of fragment borders and SF probably enabled canopy and edge specialists to interact with understory flocks, thereby increasing diversity. Despite high richness and the addition of novel species in degraded habitats, species were encountered less frequently and had reduced flock attendance in 10 ha fragments and SFs. These results are consistent with the idea that flocking species are disproportionately affected by habitat disturbance when compared with other guilds [29,33]. Ultimately, increases in species richness do not appear to have meaningfully modified flock social dynamics.
Our results suggest that changes in flock attendance rather than species loss across the habitat gradient is probably driving changes in flock social structure. Differences in attendance may result from either reduced bird density in fragmented and sub-optimal habitats [29,60] and/or changes in propensity to join flocks [20,60–62]. Previous work in this system and our data suggest that these flocking species may have lower densities but are not completely absent in degraded habitats [25,63]. Comparisons of flock participation show that a large proportion of species appear to alter their behaviour by reducing flock attendance. Moreover, such changes in behaviour also suggest that the costs and benefits of flocking behaviour may vary with environmental context [32]. While we have no data on the mechanisms for decreases in flock attendance, we believe that arthropod prey abundance declines in more degraded environments [25,64,65]. Many permanent flock species are known to have specialized foraging niches, and a reduction in their food resource could increase space use, which might decrease flock attendance. Regardless of the mechanism, reduced attendance and subsequent changes in flock composition will influence the stability, cohesion and integrity of these complex multi-species interactions [66].
(b). Habitat and the consequences of changes in social structure
Flock cohesiveness within the network, as measured by weighted clustering coefficients, was positively correlated with mean vegetation height (figure 5b). Importantly, vegetation height is a good proxy for structural habitat complexity [67,68] as PFs and 100 ha fragments also tended to have greater vertical forest stratification than small fragments and SF. The positive relationship between vegetation height and clustering coefficients is best explained by two possible mechanisms. First, vegetation height could have directly influenced network structure if the number of interspecific associations within flocks is driven, in part, by structural components of the habitat. The documentation of strong vertical stratification within Amazonian bird communities partially support this idea [69,70]. Second, vegetation height could indirectly affect network structure if predation pressure covaries with habitat type. For example, predator communities have been shown to drive flocking propensity and may vary across degraded tropical forests [71,72].
Changes in network properties may influence individual performance of species whose natural history revolves around joining flocks. For example, reduced flock attendance may reduce predation avoidance and foraging optimality [60,73]. Flocks in highly disturbed areas were unstable, not lasting more than a few weeks, where pairs of Thamnomanes caesius were inconsistently present. By contrast, flocks in continuous forest are known for their long-term stability, even as individual participants disappear and are replaced [17,74]. Assuming that changes in flock network structure influence individual fitness, future work should focus on measures of fitness by gathering species-level data on foraging efficiency and space use, as well as community-level data on predator communities and resource distribution.
5. Conclusion
Forest clearing is one of the largest threats to biodiversity today [75]. In the Amazon, the impact of forest fragmentation on avian species is well documented [25,63,76], yet changes in behaviour, interspecific interactions and community dynamics are less well known. Identifying the mechanisms that disrupt ecological processes in human-modified habitats is an essential step in mitigating and conserving diverse tropical communities. Network analyses are a powerful tool for quantifying how trophic and non-trophic interactions and subsequent ecological networks change across landscape gradients, because they enable us to quantify the role that species play in community structure and function. Moreover, this approach is likely to be especially useful in the tropics because of the high diversity and subsequent complexity of interspecific interactions. Future research must move beyond simply tallying species lists and towards identifying mechanisms that alter species interactions and community function [12]. The results presented here advance our understanding of how non-trophic interspecific interactions and subsequent community structure change along a disturbance gradient. Ultimately, if behavioural interactions and the structure of non-trophic networks tend to be highly sensitive to environmental change, as shown here, a more nuanced approach may be needed when thinking about the resiliency of ecology networks.
Acknowledgements
We are thankful for the logistical support from the BDFFP crew. Field support was provided by J. Lopes, J. de Deus F. Faria, C. L. da Silva, A. M. dos Reis, A. Nunes, P. Hendrigo, E. L. Retroz, B. Souza, A. C. Vilela and M. Campos. M. Cohn-Haft, P. Guimarães, E. I. Johnson and L. L. Powell provided valuable discussion and comments. This article represents publication no. 629 in the BDFFP Technical Series. This is contribution no. 29 in the Amazonian Ornithology Technical Series of the INPA Zoological Collections Program. This manuscript was approved for publication by the Director of the Louisiana Agricultural Experiment Station as manuscript 2013-241-11819.
Animal care protocols were approved by CEMAVE and IBAMA in Brazil (CNPq Processo EXC 021/06-C) and Louisiana State University Agriculture Center (IACUC A2006-02).
Funding statement
Funding for the research was provided by US National Science Foundation grant LTREB-0545491 and by an AOU 2010 research award.
References
- 1.May RM. 2006. Network structure and the biology of populations. Trends Ecol. Evol. 21, 394–399 (doi:10.1016/j.tree.2006.03.013) [DOI] [PubMed] [Google Scholar]
- 2.Girvan M, Newman MEJ. 2002. Community structure in social and biological networks. Proc. Natl Acad. Sci. USA 99, 7821–7826 (doi:10.1073/pnas.122653799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proulx SR, Promislow DEL, Phillips PC. 2005. Network thinking in ecology and evolution. Trends Ecol. Evol. 20, 345–353 (doi:10.1016/j.tree.2005.04.004) [DOI] [PubMed] [Google Scholar]
- 4.Bascompte J, Jordano P, Olesen JM. 2006. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 312, 431–433 (doi:10.1126/science.1123412) [DOI] [PubMed] [Google Scholar]
- 5.Guimarães PR, Jordano P, Thompson JN. 2011. Evolution and coevolution in mutualistic networks. Ecol. Lett. 14, 877–885 (doi:10.1111/j.1461-0248.2011.01649.x) [DOI] [PubMed] [Google Scholar]
- 6.Stouffer DB, Camacho J, Guimera R, Ng CA, Amaral LAN. 2005. Quantitative patterns in the structure of model and empirical food webs. Ecology 86, 1301–1311 (doi:10.1890/04-0957) [Google Scholar]
- 7.Ryder TB, McDonald DB, Blake JG, Parker PG, Loiselle BA. 2008. Social networks in the lek-mating wire-tailed manakin (Pipra filicauda). Proc. R. Soc. B 275, 1367–1374 (doi:10.1098/rspb.2008.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryder TB, Parker PG, Blake JG, Loiselle BA. 2009. It takes two to tango: reproductive skew and social correlates of male mating success in a lek-breeding bird. Proc. R. Soc. B 276, 2377–2384 (doi:10.1098/rspb.2009.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naug D. 2008. Structure of the social network and its influence on transmission dynamics in a honeybee colony. Behav. Ecol. Sociobiol. 62, 1719–1725 (doi:10.1007/s00265-008-0600-x) [Google Scholar]
- 10.Kaiser-Bunbury CN, Muff S, Memmott J, Muller CB, Caflisch A. 2010. The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behaviour. Ecol. Lett. 13, 442–452 (doi:10.1111/j.1461-0248.2009.01437.x) [DOI] [PubMed] [Google Scholar]
- 11.Dunne JA, Williams RJ, Martinez ND. 2002. Food-web structure and network theory: the role of connectance and size. Proc. Natl Acad. Sci. USA 99, 12 917–12 922 (doi:10.1073/pnas.192407699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tylianakis JM, Tscharntke T, Lewis OT. 2007. Habitat modification alters the structure of tropical host-parasitoid food webs. Nature 445, 202–205 (doi:10.1038/nature05429) [DOI] [PubMed] [Google Scholar]
- 13.Fortuna MKA, Bascompte J. 2012. Habitat loss and the disassembly of mutualistic networks. Oikos 122, 938–942 (doi:10.1111/j.1600-0706.2012.00042.x) [Google Scholar]
- 14.Wey T, Blumstein DT, Shen W, Jordan F. 2008. Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav. 75, 333–344 (doi:10.1016/j.anbehav.2007.06.020) [Google Scholar]
- 15.Farine DR, Garroway CJ, Sheldon BC. 2012. Social network analysis of mixed-species flocks: exploring the structure and evolution of interspecific social behaviour. Anim. Behav. 84, 1271–1277 (doi:10.1016/j.anbehav.2012.08.008) [Google Scholar]
- 16.Munn C. 1985. Permanent canopy and understory flocks in Amazonia: species composition and population density. In Ornithological monographs (eds Buckley P, Foster MS, Morton ES, Ridgely RS, Buckley FG.), pp. 683–712 Washington, DC: American Onithologist's Union [Google Scholar]
- 17.Jullien M, Thiollay JM. 1998. Multi-species territoriality and dynamic of neotropical forest understorey bird flocks. J. Anim. Ecol. 67, 227–252 (doi:10.1046/j.1365-2656.1998.00171.x) [Google Scholar]
- 18.Develey PF, Stouffer PC. 2001. Effects of roads on movements by understory birds in mixed-species flocks in central Amazonian Brazil. Conserv. Biol. 15, 1416–1422 (doi:10.1046/j.1523-1739.2001.00170.x) [Google Scholar]
- 19.Powell GVN. 1985. Sociobiology and adaptive significance of interspecific foraging flocks in the neotropics. Ornithol. Monogr. 36, 713–732 (doi:10.2307/40168313) [Google Scholar]
- 20.Martinez AE, Zenil RT. 2012. Foraging guild influences dependence on heterospecific alarm calls in Amazonian bird flocks. Behav. Ecol. 23, 544–550 (doi:10.1093/beheco/arr222) [Google Scholar]
- 21.Martinez AE, Gomez JP. 2013. Are mixed-species bird flocks stable through two decades? Am. Nat. 181, E53–E59 (doi:10.1086/669152) [DOI] [PubMed] [Google Scholar]
- 22.English PE. 1998. Ecology of mixed-species understory flocks in Amazonian Ecuador. Austin, TX: University of Texas [Google Scholar]
- 23.Beauchamp G. 2004. Reduced flocking by birds on islands with relaxed predation. Proc. R. Soc. B 271, 1039–1042 (doi:10.1098/rspb.2004.2703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler RA, Laurance WF. 2008. New strategies for conserving tropical forests. Trends Ecol. Evol. 23, 469–472 (doi:10.1016/j.tree.2008.05.006) [DOI] [PubMed] [Google Scholar]
- 25.Stouffer PC, Bierregaard RO. 1995. Use of Amazonian forest fragments by understory insectivorous birds. Ecology 76, 2429–2445 (doi:10.2307/2265818) [Google Scholar]
- 26.Stouffer PC, Bierregaard RO. 2007. Recovery potential of understory bird communities in Amazonian rainforest fragments. Rev. Bras. Ornitol. 15, 219–229 [Google Scholar]
- 27.Barlow J, et al. 2007. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl Acad. Sci. USA 104, 18 555–18 560 (doi:10.1073/pnas.0703333104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodhi NS, Posa MRC, Lee TM, Warkentin IG. 2008. Effects of disturbance or loss of tropical rainforest on birds. Auk 125, 511–519 (doi:10.1525/auk.2008.1708) [Google Scholar]
- 29.Thiollay JM. 1997. Disturbance, selective logging and bird diversity: a neotropical forest study. Biodivers. Conserv. 6, 1155–1173 (doi:10.1023/A:1018388202698) [Google Scholar]
- 30.Maldonado-Coelho M, Marini MA. 2000. Effects of forest fragment size and successional stage on mixed-species bird flocks in southeastern Brazil. Condor 102, 585–594 (doi:10.1650/0010-5422(2000)102[0585:eoffsa]2.0.co;2) [Google Scholar]
- 31.Stotz DF. 1993. Geographic variation in species composition of mixed species flocks in lowland humid forests in Brazil. Papéis Avulsos de Zoologia 38, 15 [Google Scholar]
- 32.Lee TM, Soh MCK, Sodhi N, Koh LP, Lim SLH. 2005. Effects of habitat disturbance on mixed species bird flocks in a tropical sub-Montane rainforest. Biol. Conserv. 122, 193–204 (doi:10.1016/j.biocon.2004.07.005) [Google Scholar]
- 33.Sridhar H, Sankar K. 2008. Effects of habitat degradation on mixed-species bird flocks in Indian rain forests. J. Trop. Ecol. 24, 135–147 (doi:10.1017/s0266467408004823) [Google Scholar]
- 34.Lovejoy TE, et al. 1986. Edge and other effects of isolations on Amazon forest fragments. In Conservation biology: the science of scarcity and diversity (ed. Soulé ME.), p. 584 Sunderland, MA: Sinauer [Google Scholar]
- 35.Bierregaard RO, et al. 2001. Principles of forest fragmentation and conservation in the Amazon. In Lessons from Amazonia: the ecology and conservation of a fragmented forest (eds Bierregaard RO, Gascon C, Lovejoy TE, Mesquita R.), pp. 371–385 New Haven, CT: Yale University Press [Google Scholar]
- 36.Mesquita RCG, Ickes K, Ganade G, Williamson GB. 2001. Alternative successional pathways in the Amazon Basin. J. Ecol. 89, 528–537 (doi:10.1046/j.1365-2745.2001.00583.x) [Google Scholar]
- 37.Colwell RK.2013. EstimateS: Statistical estimation of species richness and shared species from samples (9th edn). See http://viceroy.eeb.uconn.edu/estimates/ .
- 38.Chao A, Chazdon RL, Colwell RK, Shen TJ. 2006. Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 62, 361–371 (doi:10.1111/j.1541-0420.2005.00489.x) [DOI] [PubMed] [Google Scholar]
- 39.Beyer HL.2012. Geospatial modelling environment. (0.7.2.0) edn. See http://www.spatialecology.com/gme/
- 40.Croft D, James R, Krause J. 2008. Exploring social animal networks, p. 192 Princeton, NJ: Princeton University Press [Google Scholar]
- 41.Franks DW, Ruxton GD, James R. 2010. Sampling animal association networks with the gambit of the group. Behav. Ecol. Sociobiol. 64, 493–503 (doi:10.1007/s00265-009-0865-8) [Google Scholar]
- 42.Borgatti SP, Everett MG, Freeman LC. 2002. Ucinet for windows: software for social network analysis. (6.456 edn). Harvard, MA: Analytic Technologies [Google Scholar]
- 43.Opshal T. 2009. Structure and evolution of weighted networks. London, UK: University of London [Google Scholar]
- 44.Butts CT, Hunter D, Handcock MS.2012. network: classes for relational data (1.7–1 edn). See http://cran.r-project.org/web/packages/network/
- 45.Albert R, Barabasi AL. 2002. Statistical mechanics of complex networks. Rev. Mod. Phys. 74, 47–97 (doi:10.1103/RevModPhys.74.47) [Google Scholar]
- 46.Halvorsen K.2012. ElemStatLearn: data sets, functions and examples from the book: ‘The elements of statistical learning, data mining, inference, and prediction’ (2012.04–0 edn). See http://cran.r-project.org/web/packages/ElemStatLearn/
- 47.Opsahl T, Panzarasa P. 2009. Clustering in weighted networks. Soc. Netw. 31, 155–163 (doi:10.1016/j.socnet.2009.02.002) [Google Scholar]
- 48.Croft DP, Madden JR, Franks DW, James R. 2011. Hypothesis testing in animal social networks. Trends Ecol. Evol. 26, 502–507 (doi:10.1016/j.tree.2011.05.012) [DOI] [PubMed] [Google Scholar]
- 49.McCullagh PN, Nelder JA. 1983. Generalized linear models p. 532, 2nd edn London, UK: Chapman & Hall [Google Scholar]
- 50.Freeman LC. 1979. Centrality in social networks conceptual clarification. Soc. Netw. 1, 215–239 (doi:10.1016/0378-8733(78)90021-7) [Google Scholar]
- 51.Bates D, Maechler M, Bolker B.2012. lme4: Linear mixed-effects models using S4 classes. (0.999999–0 edn). See http://cran.r-project.org/web/packages/lme4/index.html .
- 52.Skaug HFD, Nielsen A, Magnusson A, Bolker B.2013. GlmmADMB package. (0.6.7.1 edn). See http://glmmadmb.r-forge.r-project.org/
- 53.Wickham H, Chang W.2012. ggplot2: an implementation of the grammar of graphics. (0.9.3 edn). See http://ggplot2.org/
- 54.Vazquez DP, Melian CJ, Williams NM, Bluthgen N, Krasnov BR, Poulin R. 2007. Species abundance and asymmetric interaction strength in ecological networks. Oikos 116, 1120–1127 (doi:10.1111/j.2007.0030-1299.15825.x) [Google Scholar]
- 55.de Visser SN, Freymann BP, Olff H. 2011. The Serengeti food web: empirical quantification and analysis of topological changes under increasing human impact. J. Anim. Ecol. 80, 484–494 (doi:10.1111/j.1365-2656.2010.01787.x) [DOI] [PubMed] [Google Scholar]
- 56.O'Gorman EJ, Fitch JE, Crowe TP. 2012. Multiple anthropogenic stressors and the structural properties of food webs. Ecology 93, 441–448 (doi:10.1890/11-0982.1) [DOI] [PubMed] [Google Scholar]
- 57.Beyer K, Gozlan RE, Copp GH. 2010. Social network properties within a fish assemblage invaded by non-native sunbleak Leucaspius delineatus. Ecol. Model. 221, 2118–2122 (doi:10.1016/j.ecolmodel.2010.06.002) [Google Scholar]
- 58.Laliberte E, Tylianakis JM. 2010. Deforestation homogenizes tropical parasitoid-host networks. Ecology 91, 1740–1747 (doi:10.1890/09-1328.1) [DOI] [PubMed] [Google Scholar]
- 59.Albrecht M, Duelli P, Schmid B, Muller CB. 2007. Interaction diversity within quantified insect food webs in restored and adjacent intensively managed meadows. J. Anim. Ecol. 76, 1015–1025 (doi:10.1111/j.1365-2656.2007.01264x) [DOI] [PubMed] [Google Scholar]
- 60.Thiollay JM. 1999. Frequency of mixed species flocking in tropical forest birds and correlates of predation risk: an intertropical comparison. J. Avian Biol. 30, 282–294 (doi:10.2307/3677354) [Google Scholar]
- 61.Knowlton JL, Graham CH. 2011. Species interactions are disrupted by habitat degradation in the highly threatened Tumbesian region of Ecuador. Ecol. Appl. 21, 2974–2986 (doi:10.1890/10-1886.1) [Google Scholar]
- 62.Dolby AS, Grubb TC. 1999. Functional roles in mixed-species foraging flocks: a field manipulation. Auk 116, 557–559 (doi:10.2307/4089392) [Google Scholar]
- 63.Stouffer PC, Bierregaard RO, Strong C, Lovejoy TE. 2006. Long-term landscape change and bird abundance in Amazonian rainforest fragments. Conserv. Biol. 20, 1212–1223 (doi:10.1111/j.1523-1739.2006.00427.x) [DOI] [PubMed] [Google Scholar]
- 64.Şekercioğlu CH, Ehrlich PR, Daily GC, Aygen D, Goehring D, Sandi RF. 2002. Disappearance of insectivorous birds from tropical forest fragments. Proc. Natl Acad. Sci. USA 99, 263–267 (doi:10.1073/pnas.012616199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stouffer PC. 2007. Density, territory size, and long-term spatial dynamics of a guild of terrestrial insectivorous birds near Manaus, Brazil. Auk 124, 291–306 (doi:10.1642/0004-8038(2007)124[291:DTSALS]2.0.CO;2) [Google Scholar]
- 66.Maldonado-Coelho M, Marini MA. 2004. Mixed-species bird flocks from Brazilian Atlantic forest: the effects of forest fragmentation and seasonality on their size, richness and stability. Biol. Conserv. 116, 19–26 (doi:10.1016/s0006-3207(03)00169-1) [Google Scholar]
- 67.Goetz SJ, Steinberg D, Betts MG, Holmes RT, Doran PJ, Dubayah R, Hofton M. 2010. Lidar remote sensing variables predict breeding habitat of a Neotropical migrant bird. Ecology 91, 1569–1576 (doi:10.1890/09-1670.1) [DOI] [PubMed] [Google Scholar]
- 68.Whitfeld TJS, Kress WJ, Erickson DL, Weiblen GD. 2012. Change in community phylogenetic structure during tropical forest succession: evidence from New Guinea. Ecography 35, 821–830 (doi:10.1111/j.1600-0587.2011.07181.x) [Google Scholar]
- 69.Walther BA. 2002. Grounded ground birds and surfing canopy birds: variation of foraging stratum breadth observed in neotropical forest birds and tested with simulation models using boundary constraints. Auk 119, 658–675 [Google Scholar]
- 70.Comín FA. 2010. Ecological restoration: a global challenge, p. 381 New York, NY: Cambridge University Press [Google Scholar]
- 71.Chazdon RL, Peres CA, Dent D, Sheil D, Lugo AE, Lamb D, Stork NE, Miller SE. 2009. The potential for species conservation in tropical secondary forests. Conserv. Biol. 23, 1406–1417 (doi:10.1111/j.1523-1739.2009.01338.x) [DOI] [PubMed] [Google Scholar]
- 72.Thiollay JM. 1985. Composition of Falconiforms commmunities along successional gradients from primary rain forest to secondary habitats. In Conservation studies of raptors (ed. Chancellor INR.), p. 181 Cambridge, UK: International Council for Bird Preservation [Google Scholar]
- 73.Couzin ID, Krause J, James R, Ruxton GD, Franks NR. 2002. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11 (doi:10.1006/yjtbi.3065) [DOI] [PubMed] [Google Scholar]
- 74.Jullien M, Clobert J. 2000. The survival value of flocking in neotropical birds: reality or fiction? Ecology 81, 3416–3430 (doi:10.1890/0012-9658(2000)081[3416:TSVOFI]2.0.CO;2) [Google Scholar]
- 75.Pimm SL, Raven P. 2000. Biodiversity: extinction by numbers. Nature 403, 843–845 (doi:10.1038/35002708) [DOI] [PubMed] [Google Scholar]
- 76.Stouffer PC, Strong C, Naka LN. 2009. Twenty years of understorey bird extinctions from Amazonian rain forest fragments: consistent trends and landscape-mediated dynamics. Divers. Distrib. 15, 88–97 (doi:10.1111/j.1472-4642.2008.00497.x) [Google Scholar]