Abstract
Anthropogenic noise is now recognized as a major global pollutant. Rapidly burgeoning research has identified impacts on individual behaviour and physiology through to community disruption. To date, however, there has been an almost exclusive focus on vertebrates. Not only does their central role in food webs and in fulfilling ecosystem services make imperative our understanding of how invertebrates are impacted by all aspects of environmental change, but also many of their inherent characteristics provide opportunities to overcome common issues with the current anthropogenic noise literature. Here, we begin by explaining why invertebrates are likely to be affected by anthropogenic noise, briefly reviewing their capacity for hearing and providing evidence that they are capable of evolutionary adaptation and behavioural plasticity in response to natural noise sources. We then discuss the importance of quantifying accurately and fully both auditory ability and noise content, emphasizing considerations of direct relevance to how invertebrates detect sounds. We showcase how studying invertebrates can help with the behavioural bias in the literature, the difficulties in drawing strong, ecologically valid conclusions and the need for studies on fitness impacts. Finally, we suggest avenues of future research using invertebrates that would advance our understanding of the impact of anthropogenic noise.
Keywords: environmental change, fitness, hearing, insect, noise quantification, pollution
1. Introduction
The ever-expanding urban world has made anthropogenic (man-made) noise almost ubiquitous across the globe. Noise-generating human activities have increased considerably since the Industrial Revolution, leading to substantial changes in the acoustic landscape both on land and underwater. The prevalence of transportation networks, resource extraction and urban development in terrestrial environments is much greater today than in the past [1,2], while shipping, recreational boating, seismic exploration, sonar and pile-driving are widespread and occur with increasing frequency in aquatic environments [3]. Moreover, the sound generated by human activities is often very different from that arising from natural sources, both in terms of its prominent frequencies and in such acoustic characteristics as constancy, rise time, duty cycle and impulsiveness [4]. Anthropogenic noise therefore presents a very real, and often novel, challenge to animals including ourselves.
In humans, anthropogenic noise causes physiological, neurological and endocrinological problems, increased risk of coronary disease, cognitive impairment and sleep disruption [5,6]. These impacts can be severe and legislation is therefore in place to monitor and manage noise exposure in daily life [7]. Over the last decade, there has also been a growing awareness of the potential impact of anthropogenic noise on non-human animals, with studies on a number of different taxonomic groups demonstrating effects ranging from behavioural and physiological adjustments of individuals to changes at the population and community level [1,3,8–10]. Consequently, anthropogenic noise is now recognized as a major component of environmental change in the twenty-first century and a pollutant of international concern, featuring prominently on international directives and agendas (e.g. inclusion in the United States National Environment Policy Act and the European Commission Marine Strategy Framework Directive, and as a permanent item on the agenda of the International Maritime Organisation).
A comprehensive search of the peer-reviewed literature published on terrestrial species by the end of 2012 (see the electronic supplementary material) highlights a number of trends and issues (see also [11]); we focus here on terrestrial species for brevity, although similar conclusions can be made for aquatic organisms. One striking trend is that only two of the 83 papers considered an invertebrate species. Shieh et al. [12] compared the calling behaviour of the cicada Cryptotympana takasagona in noisy and quiet urban parks, finding positive correlations between noise levels and both call frequency and chorusing. Lampe et al. [13] found that male bow-winged grasshoppers (Chorthippus biguttulus) collected from noisy roadsides sang with a greater low-frequency component than males collected from paired quiet areas nearby. As male singing was recorded in the absence of noise stimuli in anechoic chambers, the differences are unlikely to be the consequence of behavioural plasticity, but instead may result from longer term adaptation. In both studies, modification of call frequency is presented as a mechanism for avoiding masking, although further investigation is needed to determine whether that is indeed achieved and whether the vocal adjustments generate associated costs [14].
The paucity of research on invertebrates does not reflect their general importance, the likelihood that anthropogenic noise will affect them or the potential for such investigations to advance our understanding of this issue. Invertebrates are hugely diverse, constituting the vast majority of species on the Earth and with a large proportion yet to be identified [15]. They are crucial components of food webs and fulfil many ecosystems services, such as pollination, decomposition and nutrient release [16]. Removal of invertebrate species can lead to changes in diversity and modification to ecosystem function [17]. Consequently, our understanding of community structure and resilience, as well as the pressing need for food security, makes it imperative that we study how invertebrates are impacted by environmental change [18], especially as it is clear that they are indeed vulnerable. For example, artificial light can alter invertebrate community composition [19], heavy metals can cause decreased immunity [20], slower development and reduced survival and fecundity [21], and climate change can result in shifts in geographical distribution, population size, phenology, behaviour and genetic composition [16]. As many invertebrates have a proven ability to hear, to use sound for a variety of reasons and to communicate acoustically [22], they are also likely to be affected by the noise introduced into the environment by the activities of humans. Moreover, many inherent characteristics of invertebrates (e.g. their relatively small sizes, short life cycles and ease of study in both laboratory and field conditions) provide the potential to overcome a number of the current issues that can hamper research into the impacts of anthropogenic noise (see [11] and below).
Here, we begin by explaining why invertebrates are likely to be affected by anthropogenic noise—we briefly review their capacity for hearing and provide evidence that they are capable of evolutionary adaptation and behavioural plasticity in response to natural noise sources, such as wind and the chorusing of other organisms. We then discuss the importance of quantifying accurately and fully both auditory ability and noise content, and emphasize considerations of direct relevance to how invertebrates detect sounds. We highlight some current issues identified by our review of the anthropogenic noise literature—a behavioural bias, the difficulty in drawing strong, ecologically valid conclusions, and a need for studies on fitness impacts—and consider whether studying invertebrates can help to resolve them. Finally, we suggest major avenues of future research relating to anthropogenic noise and how invertebrates can be used to advance our understanding of this pervasive global pollutant.
2. Why invertebrates are likely to be affected by anthropogenic noise
There is a considerable body of work on the auditory capabilities of invertebrates and their responses to abiotic and biotic environmental noise, which combined suggest that they have the potential to be impacted by noise sources in an urban environment.
(a). Audition in invertebrates
Although audition is currently documented in detail in relatively few invertebrate species [22,23], the ability to detect sound has evolved multiple times in the insects alone, resulting in a diversity of auditory structures that can be found on nearly any segment of the body and with sensitivities anywhere between 10s of Hz to over 100 kHz [24,25]. Moreover, invertebrate species are known to produce sounds for a variety of reasons, in the same contexts as vertebrates: for example, aggression (e.g. Drosophila, Orthoptera, Coleoptera, Trichoptera; [22]), mate location, attraction and courtship (e.g. Drosophila, mosquitoes, Orthoptera, Hemiptera, Coleoptera; [22]), predator avoidance (e.g. Lepidoptera; [26]) and detection of parasite host species (e.g. tachinid flies; [27]). As many invertebrates rely on communication at frequencies below 10 kHz [24] and are capable of hearing within the main frequency spectrum of much anthropogenic noise (figure 1), their vulnerability to this pollutant is clear.
Figure 1.
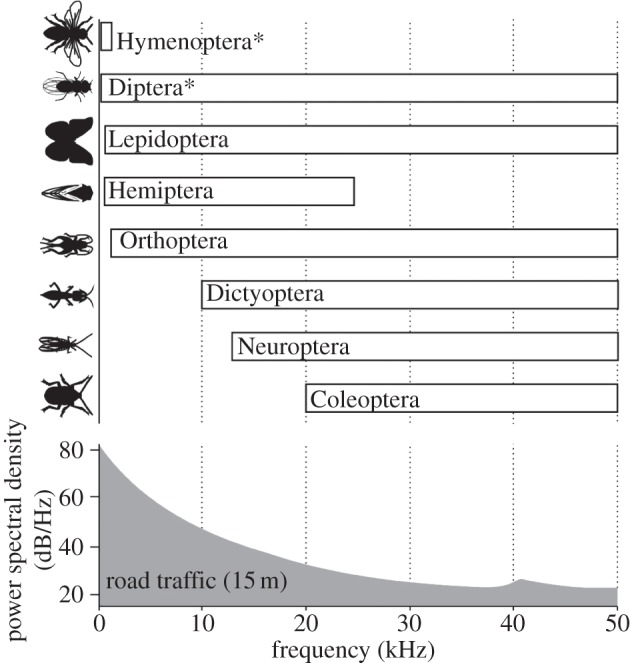
Approximate hearing ranges of insect orders and noise spectrum of road traffic recorded at 15 m. Noise spectra taken from Schaub et al. [28]. Asterisk indicates that species sensitive to particle velocity are also included.
The ability to hear typically refers to the detection of pressure waves; that is, oscillating compressions and rarefactions of the medium (usually air or water). Pressure waves are detected and produced by animals with tympanal ears: thin membranes coupled to mechanosensory cells that transduce the membrane vibration into electrical impulses. Humans, along with other vertebrates and many invertebrates, including the most conspicuously acoustic species, Orthoptera (crickets, katydids, grasshoppers) and cicadas, use tympanal ears [24]; recent work has demonstrated a remarkable example of convergent evolution between the ears of some insects and mammals [29]. As pressure waves dominate the sound field far from the source (greater than 1 wavelength (λ)), animals detecting sound pressure can communicate over considerable distances, but this also makes them vulnerable to noise originating further away. It is this component of sound that has been measured in all anthropogenic noise studies considering terrestrial animals to date.
There is a second distinct component to a sound wave, particle velocity, which comprises the oscillatory motion of particles back and forth within a propagating wave. As particle velocity is not detected by humans, it can be easy to overlook. However, many invertebrates detect this sound element using flagellar mechanosensory structures, such as hairs or antennae, that project into the oscillatory flow [25]. Particle velocity receivers sensitive to air-borne sound have been best characterized in two-winged flies (Diptera), where hair-like flagellar ears are sensitive to low frequencies (less than 1 kHz) [25,30,31]. The particle velocity component of sound attenuates rapidly and dominates only the sound field close to the source (less than 1 λ; for 10 Hz, λ = 34 m; for 1 kHz, λ = 0.34 m) [32]. Animals detecting just particle velocity may therefore be more robust than sound-pressure detectors to the impacts of anthropogenic noise. It must be noted, though, that the mechanosensory cells of both mosquitoes (Toxorhynchites brevipalpis [30]) and fruit flies (Drosophila melanogaster [31]), known to be sensitive to particle velocity, actively amplify quiet stimuli. This may effectively increase their sensitivity to distant sounds and, at the same time, their vulnerability to the effects of noise when compared with those species using a passive receiver system.
Vibrational communication through substrates, such as plants, spider webs and the ground, is also widespread in invertebrates [23]. While the sensory receivers for detecting substrate-borne vibrations are usually distinct from those of audition [22], acoustic stimuli can transmit into and be propagated in substrates, and hence acoustic noise also has the potential to impair vibratory communication. Recent work indicates that vibratory communication in the spider Schizocosa ocreata, for instance, is impacted by air-borne noise [33]. Vibratory communication is used in courtship in this species and when air-borne white noise (0–4 kHz) was played back, signal transmission and mating success in S. ocreata were decreased. The impact of anthropogenic noise on vibratory signals has received little direct attention (see [34] with an exception in Stephen's kangaroo rat (Dipodomys stephensi)) but as this modality is used by many different species both within and beyond the invertebrates, consideration of detrimental effects is important.
(b). Evidence for changes in response to noise
Many abiotic and biotic sound sources, such as wind, rain, running water and the choruses of other animals, can result in naturally noisy environments. To survive and reproduce in these conditions, invertebrates have evolved different mechanisms to cope with noise, incorporating adaptation over evolutionary time-scales and short-term behavioural plasticity.
Changes in auditory tuning mediated by both long-term physiological alterations and short-term behavioural modification are known in crickets and katydids. In noisy rainforests, where acoustic competition levels are high, the cricket Paroecanthus podagrosus has an auditory sensitivity that is relatively sharply tuned to conspecific song [35]. This contrasts with the broader auditory tuning of two species of European cricket, Gryllus bimaculatus and Gryllus campestris, which share their best frequency (the frequency of highest auditory sensitivity) with P. podagrosus, but live in quieter environments. The sharper tuning of P. podagrosus filters out background noise more effectively than in the broadly tuned species, but this may limit the detection of other environmental sounds that fall outside this narrow frequency range, for example those generated by approaching predators. Modifications in auditory tuning are also seen in the Australian bushcricket (Sciarasaga quadrata [36]). This species is able to close down the tracheal system, a system of air-filled tubes linking bilateral ears, to filter out much of the background noise generated by heterospecifics and tune the ear to the lower frequencies used by singing conspecific males. By maintaining a broad auditory sensitivity, these katydids may have a better ability to detect predators, while their flexible auditory response allows tuning into species-specific calls, and thus escape from acoustic competition.
There are also examples where species have evolved robust ways of communicating information even under noisy conditions. In bow-winged grasshoppers, calls include characteristics that allow attractiveness to be assessed even when subjected to high levels of white noise; noise does not appear to impair female choice in this species [37]. In other species, behavioural responses to noise are apparent, both in terms of sound production and recipient response. Römer et al. [38] found modifications to the temporal calling patterns in two sympatric katydid species, Hemisaga dendiculata and Mygalopsis marki, that almost completely overlap in call frequency, with H. dendiculata song suppressed in the presence of calling M. marki. In another species, Mecopoda elongata, which sings in choruses, levels of synchrony were reduced with increasing nocturnal rainforest noise [39]. Background noise can also induce changes in phonotaxis (the ability to move in an orientation with respect to a sound source). The playback of heterospecific calls or random noise interferes with female short-winged meadow katydid (Conocephalus brevipennis) movement towards conspecific male calls [40], while male grey bushcrickets (Platycleis albopunctata) move away from calling M. marki individuals, resulting in a separation of two sympatric species competing for acoustic space [41].
3. Receiver and noise source characterizations
To maximize the usefulness of research into the impact of anthropogenic noise, studies must suitably characterize the particular auditory receiver and noise source under consideration; it is common in the current literature to find that either or both are not done sufficiently to justify the conclusions drawn [11]. In this section, we highlight important general considerations in this regard (see also [10]), with particular reference to aspects of invertebrate sound detection that differ from most vertebrate hearing (see above).
(a). Auditory sensitivities
Determination of whether a given noise stimulus falls within the auditory capabilities of an organism is vital to assess correctly any apparent lack of effect. Characterization of invertebrate hearing should include appropriate consideration of pressure or particle velocity components of sound, as well as potential nonlinear auditory responses (where the sensory system does not respond linearly with input amplitude). Auditory nonlinearities have been demonstrated in mosquitoes [30], fruit flies [42] and the tree cricket Oecanthus henryi [43]; the latter represents the first evidence of nonlinear audition from a tympanal hearing insect. In these systems, the total sound level across frequencies can impact the sensitivity and tuning of the ear, indicating that even noise which does not overlap with the best frequency of the auditory system (frequency of highest sensitivity) may still generate signal masking and impede signal differentiation from the background.
Characterization of the mechanical properties of the ear and of auditory responses and physiological measurement of auditory thresholds are relatively simple to obtain in invertebrates owing to the peripheral location of many auditory structures and ease of access to auditory neurons [22]. This is true for invertebrates sensitive to pressure and particle velocity; for each of these types of receiver, there are good examples of auditory characterization at the mechanical and physiological level (see [29,31,42–44]). Moreover, neurophysiological methods have been developed to measure auditory thresholds both in the laboratory and the field in Orthoptera [45]. Natural habitats have sound fields that are far more complex than laboratory conditions, generating differences in the thresholds of what is perceived by the animal, which makes it important to put laboratory work into an ecologically relevant context.
(b). Noise quantification
To avoid erroneous conclusions, it is critical to quantify the noise source using tools that best reflect the auditory capabilities of the study animal. However, most readily available, and commonly used, audio equipment is designed for human aural sensitivities, and thus studies have often restricted recording and playback to frequencies audible to us (20 Hz–20 kHz) and employed recording filters that emulate human hearing (e.g. A-weighting filter (dBA)). While this approach has been deemed acceptable for birds, which hear in a similar frequency range to us and on which the majority of terrestrial work has so far been conducted, noise quantification ideally needs to cover broad bandwidths extending beyond audible frequencies using unweighted, flat-response recording equipment. A study by Schaub et al. [28] on bat foraging sets a robust standard for quantification of anthropogenic noise in a way relevant to the study species: they measured road traffic noise between 0 and 50 kHz with a flat-response microphone, showing the majority of energy concentrated below 5 kHz. Moreover, Schaub et al. quantified the number of vehicles, vehicle type and distance from the noise source; as the same type of noise source can produce highly variable sounds and the frequency content and amplitude are dependent on the distance from the source, including these factors adds valuable information. In general, studies should ideally report a range of relevant acoustic metrics (e.g. dB, weighting function, maximum power, integration time and order statistics); making high-quality audio recordings of the noise source being studied available for alternative spectral filtering and acoustic analysis would potentially represent the best practice and allow the greatest opportunity for comparative work and generalization (for further details see [10,46]).
For the study of some invertebrates, recording particle velocity or substrate vibration generated by anthropogenic noise, and mimicking these components in playbacks, should be a crucial element of the work. To date, there has been little attempt to quantify these components of terrestrial anthropogenic noise or their impact on animals sensitive to such stimuli (but see [34]), not least because the majority of studies have been conducted on organisms (i.e. vertebrates) for which these considerations are not important. The pressure component of a sound wave, the quantification of which is discussed above, can differ considerably from particle velocity [32] and measuring particle velocity or substrate vibration presents technological challenges. The majority of available microphones are pressure sensitive, but some do detect the pressure gradient, which combined with the use of integrating amplifiers output the particle velocity of a signal. These tools have been used successfully to record particle velocity in studies examining audition, communication and mate location in insects [42]. Likewise, the measurement of substrate vibration is frequently carried out in other contexts by employing accelerometers or non-contact laser Doppler vibrometry [33]. Thus, there is the capacity to measure these aspects of a noise source that are relevant to some invertebrate hearing.
4. Can invertebrates provide model systems to investigate the impact of anthropogenic noise?
Our review of the current anthropogenic noise literature has identified three key issues that we believe need resolving (see [11]): a behavioural bias, the difficulty in drawing strong, ecologically valid conclusions and a need to determine the effects on individual fitness. In this section, we outline these issues and then consider whether invertebrates can help with their resolution.
(a). Behavioural bias
The majority of studies (60 out of 83) investigating the impact of anthropogenic noise on terrestrial species have considered behavioural responses (see the electronic supplementary material). The most commonly researched behaviour is acoustic communication and particularly ways in which animals might minimize the risk that their auditory signals are masked; masking occurs when there is an increase in the threshold for detection or discrimination of one sound in the presence of another. Loss of clear and efficient transmission of acoustic information can create potential fitness costs, including those related to mate attraction and territory defence if song is masked, increased predation risk if detection of alarm calls is impaired and reduced reproductive success if parent–offspring or parent–parent communication is disrupted (see [14]). Consequently, anthropogenic noise has resulted in alterations to the vocal parameters (frequency, amplitude, rate and duration) or the timing of signalling in many birds and anurans, either through behavioural plasticity or evolutionary adaptation [14,47,48]. Some studies have also considered the impact of masking on adventitious signals [28,49]. For instance, greater mouse-eared bats (Myotis myotis), which listen for prey-generated sounds to locate food, avoid foraging when exposed to playback of road traffic noise and exhibit reduced foraging efficiency when noise is unavoidable. There is also some evidence that noise can mimic communicatory signals [34] and that vigilance behaviour is modified [50].
In contrast to behavioural adaptations, relatively little research has considered how anthropogenic noise impacts physiology ([8]; but see [51,52]), and there have been virtually no investigations with respect to development, neurobiology or genetics. Assessing how noise affects processes in addition to behaviour is vital for a full understanding of both proximate and ultimate impacts on fitness [8]. There is a long history of studying such fundamental processes in invertebrates in other contexts [53,54]. For example, by using genetic techniques and physiological and mechanical measurement, the molecular genetic and neural components required for an ear to receive and actively amplify sounds are being pieced together in Drosophila (see [53]). Moreover, there are good examples where invertebrate physiology, development and genetics have been studied with respect to global changes other than anthropogenic noise. For instance, considerable research has focused on the potential impacts of climate change on development in insects [55,56], as well as genetic effects in mosquitoes and fruit flies (for an overview see [57]). Physiological responses to climate change have also been measured in many invertebrates (for discussion see [58]). Such approaches should be equally applicable to studies examining the impact of anthropogenic noise.
(b). Difficulties in drawing strong, ecologically valid conclusions
Strong conclusions about the impact of anthropogenic noise are often not possible because suitable controls are lacking [11]. For example, roads are noisy, but they also have high levels of disturbance, chemical pollution and light, and provide an edge habitat. Studies comparing the responses of animals near a noisy road with those in a control area, either a quieter road or a site at a greater distance from the road, do not allow any differences to be conclusively attributed to noise. An experimental approach where noise is the only factor that differs is ideally required to tease out the direct effect of noise from potentially confounding factors.
Studies by Francis et al. [59] and Bayne et al. [60], for example, have highlighted that it is possible to provide strong evidence for the impact of noise using natural experiments: they have taken advantage of areas containing gas wells that either have or do not have noisy compressors to show that anthropogenic noise affects birds at both the species and community level. As the wells are comparable in both structure and surrounding habitat, and thus differ only in noise production, this system provides an excellent test of the impact of anthropogenic noise under field conditions. Such natural experimental situations may be rare, however, and manipulations are usually required. Careful controls are often the easiest in laboratory experiments, where more detailed data collection than in the wild is also potentially feasible [28,49,61], but care must be taken when extrapolating results to meaningful implications for free-ranging animals in natural conditions; the ecological validity of laboratory-based work can be questioned. Field experiments are becoming more common (e.g. [62,63]), but can be logistically more difficult, with the same level of control and detailed data collection harder to achieve than that in the laboratory, and characterization of some responses (e.g. neurological) particularly challenging. Studies that pair different types of work in different settings [48,64] offer the best solution, allowing the benefits of each approach to be used.
Invertebrates are amenable to a combined laboratory and field approach; they are small enough to be kept in large numbers in captivity and they can be manipulated in the wild. Römer et al. [38] provide an excellent example of this in their work with katydids, examining the influence of the acoustic environment on signal transmission. Investigating responses to masking by heterospecific noise, this study pairs both behavioural and neurophysiological measurements of auditory neurons in the field and laboratory settings, providing ecological validation for the laboratory work and technical controls for any confounding variables in the fieldwork. Further examples of experiments conducted in both the field and laboratory can be found in other orthopteran species. Schmidt & Römer [45] investigated neurophysiological detection thresholds for conspecific song in tropical crickets under noisy conditions, while studies of directional sensitivity in grasshopper audition [65] and katydid discrimination between background noise and calls of approaching predators [66] also used this paired laboratory and field approach.
(c). Need to evaluate effects of noise on individual fitness
Ultimately what is needed for successful policy-making and mitigation is consideration of how anthropogenic noise impacts individual survival and reproductive success, and consequently population and community structure. However, the vast majority of experimental studies to date have considered relatively short-term effects (see the electronic supplementary material), which do not necessarily have clear implications for fitness; at best, most of the current literature reports fitness proxies (see [11]). Some short-term effects (e.g. increased predation risk) can be translated relatively easily into ultimate consequences. However, others (e.g. foraging behaviour, signalling characteristics, movement patterns) need more careful consideration because animals may be able to compensate in quieter periods, the implications of the behavioural change are unclear or there may be costs associated with the noise-induced adjustment [14], and thus there may be no direct link between short-term effects and long-term consequences (see [67]). That is not to say changes in fitness do not result, but rather that the experiments required to determine them have rarely been carried out (but see [59,64,68,69]). A multi-year study by Francis et al. [59] demonstrated that some species might actually gain from additional noise if, for instance, potential predators avoid the area, and thus implications for individual fitness and community structure are not necessarily easy to predict.
As the life cycle of invertebrates is relatively short, it enables individual fitness and population viability to be assessed directly in a way that is logistically difficult in many vertebrates. Research into climate change provides good examples of how potential impacts of environmental modification on insects can be developed [70]. For example, an intergenerational study on the pitcher-plant mosquito (Wyeomyia smithii) has revealed large decreases in fitness in response to changes in photoperiod and climate over evolutionary time-scales [71]. In a tropical butterfly (Bicyclus anynana), resource availability and temperature were found to modify fitness-related traits, with implications for the impacts of climate change on this species [72].
It is also possible to use data on individual fitness consequences to parametrize theoretical models making predictions about outcomes at a population level. Such agent-based modelling has previously been applied to environmental resource management, and to ecological and conservation issues [73]. If modelling such as this can be introduced to anthropogenic noise research, individual-based fitness studies would be able to indicate conservation priorities without the immediate requirement for long-term data that are not likely to become available in the near future. However, validation of such models is a crucial element of the process, and this step is also feasible with short-lived invertebrate species: successive generations, with appropriate controls, could be bred under different noise conditions.
5. The future
In addition to the suggestions inherent in the previous sections, there are three main areas that we consider are in need of particular attention if research into anthropogenic noise is to move forward substantially. First, experimental studies to date have concentrated efforts on the impact of a single, acute noise exposure in isolation (e.g. [63,74]; but see [52,59,60,62]). While this is understandable from a logistical perspective, organisms in most natural situations are likely to experience either chronic or repeated exposure to noise, which might lead to changes in response through such processes as sensitization, habituation or tolerance [75]. Moreover, it is currently unclear precisely how the impacts of anthropogenic noise are affected by simultaneous exposure to such situations as high disturbance or light and chemical pollution; potential synergistic effects arising from the combination of noise with other stressors require investigation.
Second, the majority of (experimental) studies to date have tackled the simple, but important question: is there an immediate impact of noise? It is clear from the rapidly expanding literature that this is indeed the case across a range of taxa (see the electronic supplementary material). What is required now is consideration of additional issues that build on this knowledge. For example, what is the spatial scale of impact and the dose-dependent relationship between noise and responses? What characteristics of anthropogenic noises are most problematic; it is unlikely that it is simply the amplitude that matters, but do such aspects as predictability, rise time, and frequency range and modulation also play a key role? How quickly do animals recover to pre-exposure levels and do they show compensation for any noise-induced responses? How are different members of the same species affected by the same noise; are there, for example, age-, sex-, size- and condition-dependent responses?
Third, it is clear that the same noise may not affect different species in the same way. Such variation in impact could have consequences at the dyadic level (i.e. when two species interact). For example, if a predator is affected in a more detrimental manner than its prey [49], the reproductive success of the latter may be enhanced in noisy environments. There could also be consequences in terms of community structure. Francis et al. [59] have found, for instance, that the nest success of certain bird species increased at noisy treatment sites compared with a quiet control, owing to a decrease in the abundance of predators. To date, there have been relatively few attempts to consider how anthropogenic noise affects biodiversity per se (but see [59,60,76]) and findings are mixed and potentially taxon specific. For instance, Herrera-Montez & Aide [76] found that although avian biodiversity declined in noisy areas, anuran biodiversity was not significantly affected. Finally, recent work has provided, to our knowledge, the first evidence that anthropogenic noise could affect ecosystem services: Francis et al. [77] showed that noise could influence pollination and seed dispersal. Interactions at the community and ecosystem level are clearly more complex than when considering single species, but are crucial for a full understanding of the potential impact of anthropogenic noise.
Although the issues outlined above can potentially be addressed using vertebrates, intergenerational studies considering the impacts of chronic or repeated exposure, as well as the possibilities for recovery and compensation, are achievable within relatively short time-frames using invertebrates. Likewise, their small size and the relative ease of maintaining populations in the laboratory make it possible to examine the impacts of complex interactions with other stressors, dose- and condition-dependence and intrapopulation differences in response. Moreover, as invertebrates can be good bioindicators of impacts of environmental change [78], they offer an ideal opportunity to track the impact of anthropogenic noise on wildlife in natural habitats. Not only are invertebrates useful as models and indicators, but their ubiquity in ecosystems throughout the world makes it important to assess how noise is affecting them per se together with their interactions with other species within the ecosystem.
6. Conclusion
Anthropogenic noise is an issue of international concern and studies of its potential impacts are important and becoming more prevalent. For brevity, this review has focused on terrestrial species, but there is also increasing awareness of the effects of such noise in aquatic environments [3,9]. Little direct work has so far investigated how invertebrates, despite their probable vulnerability, are impacted (but see [12,13,79,80]). One potential reason for this is that regulators and policymakers are intrinsically more interested in how noise affects charismatic vertebrates. However, research on invertebrates is not only important (invertebrates are critical elements of all ecosystems, not least in providing the food for most vertebrates), but also has the potential both to assist with some of the current issues apparent in the literature and to drive the field forward, thus establishing the full impact of this global pollutant. Unlike, for example, climate change and ocean acidification, where studies are considering future predicted changes, anthropogenic noise is an issue in the present day. Advancing our knowledge of its impacts and developing mitigation measures is therefore of pressing importance, and we argue that the study of invertebrates, perhaps within the valuable framework recently outlined by Francis & Barber [10], can play a crucial, yet currently underused role.
Acknowledgements
We are grateful to Martin McVay, Hilary Notley and the Bristol Bioacoustics and Behavioural Ecology research group for valuable discussions, and to Jesse Barber and two anonymous referees for helpful comments on the manuscript.
Funding statement
We are grateful to Defra, who funded the initial literature search and assessment.
References
- 1.Barber JR, Crooks KR, Fristrup KM. 2010. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189 (doi:10.1016/j.tree.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 2.Watts RD, Compton RW, McCammon JH, Rich CL, Wright SM, Owens T, Ouren DS. 2007. Roadless space of the conterminous United States. Science 316, 736–738 (doi:10.1126/science.1138141) [DOI] [PubMed] [Google Scholar]
- 3.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427 (doi:10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 4.Hildebrand J. 2009. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Progr. Ser. 395, 5–20 (doi:10.3354/meps08353) [Google Scholar]
- 5.Le Prell CG, Henderson D, Fay RR, Popper AN. (eds) 2012. Noise-induced hearing loss: scientific advances. New York, NY: Springer [Google Scholar]
- 6.World Health Organisation 2011. Burden of disease from environmental noise: quantification of healthy life years lost in Europe. Geneva, Switzerland: World Health Organisation Regional Office for Europe [Google Scholar]
- 7.European Parliament 2002. Directive 2002/49/EC of the European Parliament and of the Council. Official J. Eur. Communities L 189, 12–26 [Google Scholar]
- 8.Kight CR, Swaddle JP. 2011. How and why environmental noise impacts animals: an integrative, mechanistic review. Ecol. Lett. 14, 1052–1061 (doi:10.1111/j.1461-0248.2011.01664.x) [DOI] [PubMed] [Google Scholar]
- 9.Tyack PL. 2008. Implications for marine mammals of large-scale changes in the marine acoustic environment. J. Mammol. 89, 549–558 (doi:10.1644/07-MAMM-S-307R.1) [Google Scholar]
- 10.Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305–313 (doi:10.1890/120183) [Google Scholar]
- 11.Radford AN, Jones G, Morley EL.2012. The effects of noise on biodiversity. Defra Report NO0235.
- 12.Shieh B-S, Liang S-H, Chen C-C, Loa H-H, Liao C-Y. 2012. Acoustic adaptations to anthropogenic noise in the cicada Cryptotympana takasagona Kato (Hemiptera: Cicadidae). Acta Ethol. 15, 33–38 (doi:10.1007/s10211-011-0105-x) [Google Scholar]
- 13.Lampe U, Schmoll T, Franzke A, Reinhold K. 2012. Staying tuned: grasshoppers from noisy roadside habitats produce courtship signals with elevated frequency components. Funct. Ecol. 26, 1348–1354 (doi:10.1111/1365-2435.12000) [Google Scholar]
- 14.Read J, Jones G, Radford AN. In press Fitness costs as well as benefits are important when considering responses to anthropogenic noise. Behav. Ecol. (doi:10.1093/beheco/art102) [Google Scholar]
- 15.Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. 2011. How many species are there on Earth and in the ocean? PLoS Biol. 9, e1001127 (doi:10.1371/journal.pbio.1001127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prather CM, et al. 2012. Invertebrates, ecosystem services and climate change. Biol. Rev. 88, 328–348 (doi:10.1111/brv.12002) [DOI] [PubMed] [Google Scholar]
- 17.Mulder CPH, Koricheva J, Huss-Danell K, Högberg P, Joshi J. 1999. Insects affect relationships between plant species richness and ecosystem processes. Ecol. Lett. 2, 237–246 (doi:10.1046/j.1461-0248.1999.00070.x) [Google Scholar]
- 18.Losey JE, Vaughan M. 2006. The economic value of ecological services provided by insects. BioScience 56, 311–323 (doi:10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2) [Google Scholar]
- 19.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764–767 (doi:10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorvari J, Rantala LM, Rantala MJ, Hakkarainen H, Eeva T. 2007. Heavy metal pollution disturbs immune response in wild ant populations. Environ. Pollut. 145, 324–328 (doi:10.1016/j.envpol.2006.03.004) [DOI] [PubMed] [Google Scholar]
- 21.Moe SJ, Stenseth NC, Smith RH. 2001. Effects of a toxicant on population growth rates: sublethal and delayed responses in blowfly populations. Funct. Ecol. 15, 712–721 (doi:10.1046/j.0269-8463.2001.00575.x) [Google Scholar]
- 22.Ewing AW. 1989. Arthropod bioacoustics: neurobiology and behaviour. Ithaca, NY: Cornell University Press [Google Scholar]
- 23.Hill PSM. 2009. How do animals use substrate-borne vibrations as an information source? Naturwissenschaften 96, 1355–1371 (doi:10.1007/s00114-009-0588-8) [DOI] [PubMed] [Google Scholar]
- 24.Hoy RR, Robert D. 1996. Tympanal hearing in insects. Annu. Rev. Entomol. 41, 433–450 (doi:10.1146/annurev.en.41.010196.002245) [DOI] [PubMed] [Google Scholar]
- 25.Tautz J. 1979. Reception of particle oscillation in a medium: an unorthodox sensory capacity. Naturwissenschaften 66, 452–461 (doi:10.1007/BF00399002) [Google Scholar]
- 26.Spangler HG. 1988. Moth hearing, defense and communication. Annu. Rev. Entomol. 33, 59–81 (doi:10.1146/annurev.en.33.010188.000423) [Google Scholar]
- 27.Hoy RR, Popper AN, Fay RR. 1998. Comparative hearing: insects. New York, NY: Springer [Google Scholar]
- 28.Schaub A, Ostwald J, Siemers BM. 2009. Foraging bats avoid noise. J. Exp. Biol. 211, 3174–3180 (doi:10.1242/jeb.022863) [DOI] [PubMed] [Google Scholar]
- 29.Montealegre-Z F, Jonsson T, Robson-Brown KA, Postles M, Robert D. 2012. Convergent evolution between insect and mammalian audition. Science 338, 968–971 (doi:10.1126/science.1225271) [DOI] [PubMed] [Google Scholar]
- 30.Göpfert MC, Robert D. 2001. Active auditory mechanics in mosquitoes. Proc. R. Soc. Lond. B 268, 333–339 (doi:10.1098/rspb.2000.1376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Göpfert MC, Humphris ADL, Albert JT, Robert D, Hendrich O. 2005. Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc. Natl Acad. Sci. USA 102, 325–330 (doi:10.1073/pnas.0405741102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossing TD. (eds) 2007. Springer handbook of acoustics. New York, NY: Springer [Google Scholar]
- 33.Gordon SD, Uetz GW. 2012. Environmental interference: impact of acoustic noise on seismic communication and mating success. Behav. Ecol. 23, 707–714 (doi:10.1093/beheco/ars016) [Google Scholar]
- 34.Shier DM, Lea AJ, Owen MA. 2012. Beyond masking: endangered Stephen's kangaroo rats respond to traffic noise with footdrumming. Biol. Conserv. 150, 53–58 (doi:10.1016/j.biocon.2012.03.007) [Google Scholar]
- 35.Schmidt AKD, Riede K, Römer H. 2011. High background noise shapes selective auditory filters in a tropical cricket. J. Exp. Biol. 214, 1754–1762 (doi:10.1242/jeb.053819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Römer H, Bailey W. 1998. Strategies for hearing in noise: peripheral control over auditory sensitivity in the bushcricket Sciarasaga quadrata (Austrosaginae: Tettigoniidae). J. Exp. Biol. 201, 1023–1033 [DOI] [PubMed] [Google Scholar]
- 37.Einhaupl A, Stange N, Hennig RM, Ronacher B. 2011. Attractiveness of grasshopper songs correlates with their robustness against noise. Behav. Ecol. 22, 791–799 (doi:10.1093/beheco/arr064) [Google Scholar]
- 38.Römer H, Bailey W, Dadour I. 1989. Insect hearing in the field. III. Masking by noise. J. Comp. Physiol. A 164, 609–620 (doi:10.1007/BF00614503) [Google Scholar]
- 39.Hartbauer M, Siegert ME, Fertschai I, Römer H. 2012. Acoustic signal perception in a noisy habitat: lessons from synchronising insects. J. Comp. Physiol. A 198, 397–409 (doi:10.1007/s00359-012-0718-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey WJ, Morris GK. 2010. Confusion of phonotaxis by masking sounds in the bushcricket Conocephalus brevipennis (Tettigoniidae: Conocephalinae). Ethology 73, 19–28 (doi:10.1111/j.1439-0310.1986.tb00996.x) [Google Scholar]
- 41.Latimer W. 1981. Acoustic competition in bush crickets. Ecol. Entomol. 6, 35–45 (doi:10.1111/j.1365-2311.1981.tb00970.x) [Google Scholar]
- 42.Göpfert MC, Robert D. 2002. The mechanical basis of Drosophila audition. J. Exp. Biol. 205, 1199–208 [DOI] [PubMed] [Google Scholar]
- 43.Mhatre N, Robert D. 2013. A tympanal insect ear exploits a critical oscillator for active amplification and tuning. Curr. Biol. 23, 1952–1957 (doi:10.1016/j.cub.2013.08.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kostarakos K, Römer H. 2010. Sound transmission and directional hearing in field crickets: neurophysiological studies outdoors. J. Comp. Physiol. A 196, 669–681 (doi:10.1007/s00359-010-0557-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt AKD, Römer H. 2011. Solutions to the cocktail party problem in insects: selective filters, spatial release from masking and gain control in tropical crickets. PLoS ONE 6, e28593 (doi:10.1371/journal.pone.0028593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pater LL, Grubb TG, Delaney DK. 2009. Recommendations for improved assessment of noise impacts on wildlife. J. Wildl. Manage. 73, 788–795 (doi:10.2193/2006-235) [Google Scholar]
- 47.Brumm H, Slabbekoorn H. 2005. Acoustic communication in noise. Adv. Stud. Behav. 35, 151–209 [Google Scholar]
- 48.Cunnington GM, Fahrig L. 2010. Plasticity in the vocalizations of anurans in response to traffic noise. Acta Oecol. 36, 463–470 (doi:10.1016/j.actao.2010.06.002) [Google Scholar]
- 49.Siemers BM, Schaub A. 2011. Hunting at the highway: traffic noise reduces foraging efficiency in acoustic predators. Proc. R. Soc. B 278, 1646–1652 (doi:10.1098/rspb.2010.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabin L, Coss R, Owings D. 2006. The effects of wind turbines on antipredator behavior in California ground squirrels (Spermophilus beecheyi). Biol. Conserv. 131, 410–420 (doi:10.1016/j.biocon.2006.02.016) [Google Scholar]
- 51.Payne CJ, Jessop TS, Guay P-J, Johnstone M, Feore M, Mulder RA. 2012. Population, behavioural and physiological responses of an urban population of black swans to an intense annual noise event. PLoS ONE 7, e45014 (doi:10.1371/journal.pone.0045014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blickley JL, Word KR, Krakauer AH, Phillips JL, Sells SN, Taff CC, Wingfield JC, Patricelli GL. 2012. Experimental chronic noise is related to elevated fecal corticosteroid metabolites in lekking male greater sage-grouse (Centrocercus urophasianus). PLoS ONE 7, e50462 (doi:10.1371/journal.pone.0050462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu Q, Senthilan PR, Effertz T, Nadrowski B, Göpfert MC. 2009. Using Drosophila for studying fundamental processes in hearing. Integr. Comp. Biol. 49, 674–680 (doi:10.1093/icb/icp072) [DOI] [PubMed] [Google Scholar]
- 54.Scharrer B. 1987. Insects as models in neuroendocrine research. Annu. Rev. Entomol. 32, 1–16 (doi:10.1146/annurev.en.32.010187.000245) [DOI] [PubMed] [Google Scholar]
- 55.Piyaphongkul J, Pritchard J, Bale J. 2012. Heat stress impedes development and lowers fecundity of the brown planthopper Nilaparvata lugens (Stål). PLoS ONE 7, e47413 (doi:10.1371/journal.pone.0047413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Régnière J, St-Amant R, Duval P. 2010. Predicting insect distributions under climate change from physiological responses: spruce budworm as an example. Biol. Invasions 14, 1571–1586 (doi:10.1007/s10530-010-9918-1) [Google Scholar]
- 57.Bradshaw WE, Holzapfel CM. 2008. Genetic response to rapid climate change: it's seasonal timing that matters. Mol. Ecol. 17, 157–166 (doi:10.1111/j.1365-294X.2007.03509.x) [DOI] [PubMed] [Google Scholar]
- 58.Chown SL, Terblanche JS. 2006. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 33, 50–152 (doi:10.1016/S0065-2806(06)33002-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francis CD, Ortega CP, Cruz A. 2009. Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419 (doi:10.1016/j.cub.2009.06.052) [DOI] [PubMed] [Google Scholar]
- 60.Bayne EM, Habib L, Boutin S. 2008. Impacts of chronic anthropogenic noise from energy-sector activity on abundance of songbirds in the boreal forest. Conserv. Biol. 22, 1186–1193 (doi:10.1111/j.1523-1739.2008.00973.x) [DOI] [PubMed] [Google Scholar]
- 61.Bee MA, Swanson EM. 2007. Auditory masking of anuran advertisement calls by road traffic noise. Anim. Behav. 74, 1765–1776 (doi:10.1016/j.anbehav.2007.03.019) [Google Scholar]
- 62.Blickley JL, Blackwood D, Patricelli GL. 2012. Experimental evidence for the effects of chronic anthropogenic noise on abundance of greater sage-grouse at leks. Conserv. Biol. 26, 461–471 (doi:10.1111/j.1523-1739.2012.01840.x) [DOI] [PubMed] [Google Scholar]
- 63.McLaughlin KE, Kunc HP. 2013. Experimentally increased noise levels change spatial and singing behaviour. Biol. Lett. 9, 20120771 (doi:10.1098/rsbl.2012.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halfwerk W, Bot S, Buikx J, van der Velde M, Komdeur J, ten Cate C, Slabbekoorn H. 2011. Low-frequency songs lose their potency in noisy urban conditions. Proc. Natl Acad. Sci. USA 108, 14 549–14 554 (doi:10.1073/pnas.1109091108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbert F, Elsner N. 2000. Directional hearing of a grasshopper in the field. J. Exp. Biol. 203, 983–993 [DOI] [PubMed] [Google Scholar]
- 66.Hartbauer M, Radspieler G, Römer H. 2010. Reliable detection of predator cues in afferent spike trains of a katydid under high background noise levels. J. Exp. Biol. 213, 3036–3046 (doi:10.1242/jeb.042432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bejder L, et al. 2006. Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conserv. Biol. 20, 1791–1798 (doi:10.1111/j.1523-1739.2006.00540.x) [DOI] [PubMed] [Google Scholar]
- 68.Francis CD, Paritsis J, Ortega CP, Cruz A. 2011. Landscape patterns of avian habitat use and nest success are affected by chronic gas well compressor noise. Landscape Ecol. 26, 1269–1280 (doi:10.1007/s10980-011-9609-z) [Google Scholar]
- 69.Kight CR, Saha MS, Swaddle JP. 2012. Anthropogenic noise is associated with reductions in the productivity of breeding eastern bluebirds (Sialia sialis). Ecol. Appl. 22, 1989–1996 (doi:10.1890/12-0133.1) [DOI] [PubMed] [Google Scholar]
- 70.Bradshaw WE, Holzapfel CM. 2006. Evolutionary response to rapid climate change. Science 312, 1477–1478 (doi:10.1126/science.1127000) [DOI] [PubMed] [Google Scholar]
- 71.Bradshaw WE, Zani PA, Holzapfel CM. 2004. Adaptation to temperate climates. Evolution 58, 1748–1762 [DOI] [PubMed] [Google Scholar]
- 72.Karl I, Stoks R, De Block M, Janowitz SA, Fischer K. 2011. Temperature extremes and butterfly fitness: conflicting evidence from life history and immune function. Glob. Change Biol. 17, 676–687 (doi:10.1111/j.1365-2486.2010.02277.x) [Google Scholar]
- 73.McLane AJ, Semeniuk C, McDermid GJ, Marceau DJ. 2011. The role of agent-based models in wildlife ecology and management. Ecol. Model. 222, 1544–1556 (doi:10.1016/j.ecolmodel.2011.01.020) [Google Scholar]
- 74.Halfwerk W, Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307 (doi:10.1016/j.anbehav.2009.09.015) [Google Scholar]
- 75.Bejder L, Samuels A, Whitehead H, Finn H, Allen S. 2009. Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Progr. Ser. 395, 177–185 (doi:10.3354/meps07979) [Google Scholar]
- 76.Herrera-Montes MI, Aide TM. 2011. Impacts of traffic noise on anuran and bird communities. Urban Ecosyst. 14, 415–427 (doi:10.1007/s11252-011-0158-7) [Google Scholar]
- 77.Francis CD, Kleist NJ, Ortega CP, Cruz A. 2012. Noise pollution alters ecological services: enhanced pollination and disrupted seed dispersal. Proc. R. Soc. B 279, 2727–2735 (doi:10.1098/rspb.2012.0230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jennings N, Pocock MJO. 2009. Relationships between sensitivity to agricultural intensification and ecological traits of insectivorous mammals and arthropods. Conserv. Biol. 23, 1195–1203 (doi:10.1111/j.1523-1739.2009.01208.x) [DOI] [PubMed] [Google Scholar]
- 79.Wale MA, Simpson SD, Radford AN. 2013. Size-dependent physiological responses of shore crabs to single and repeated playback of ship noise. Biol. Lett. 9, 20121194 (doi:10.1098/rsbl.2012.1194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wale MA, Simpson SD, Radford AN. 2013. Noise negatively affects foraging and antipredator behaviour in shore crabs. Anim. Behav. 86, 111–118 (doi:10.1016/j.anbehav.2013.05.001) [Google Scholar]


