Abstract
Broad-scale geographical variation in species richness is strongly correlated with climate, yet the mechanisms underlying this correlation are still unclear. We test two broad classes of hypotheses to explain this pattern. Bottom-up hypotheses propose that the environment determines individual species’ ranges. Ranges then sum up to yield species richness patterns. Top-down hypotheses propose that the environment limits the number of species that occur in a region, but not which ones. We test these two classes of hypotheses using a natural experiment: seasonal changes in environmental variables and seasonal range shifts of 625 migratory birds in the Americas. We show that richness seasonally tracks the environment. By contrast, individual species’ geographical distributions do not. Rather, species occupy different sets of environmental conditions in two seasons. Our results are inconsistent with extant bottom-up hypotheses. Instead, a top-down mechanism appears to constrain the number of species that can occur in a given region.
Keywords: bird migration, climatic niche, species richness carrying capacity, richness–climate relationship, temperature, tropical niche conservatism
1. Introduction
Broad-scale geographical variation in species richness is strongly correlated with climate. At regional scales, across continents and globally, species richness of most taxa covaries strongly with climate [1,2]. Richness–climate relationships are largely consistent among continents [3], suggesting a mechanistic link between climate and species richness that applies very broadly. This hypothesized causal effect of climate on species richness is highly debated in the literature because the high correlation between climate and richness could be due to covariance with other factors, particularly historical ones [4,5].
Natural experiments can be used to test for a causal link between environment and species richness. For example, H-Acevedo & Currie [6] found that the relationship between bird species richness and environmental variables is congruent between the winter and summer season in North America (see also [7]). Similarly, the spatial relationship between climate and butterfly species richness across Canada accurately predicts how richness changes through time because of recent climate changes [8]. The relationship between woody plant richness and water-energy variables at a site in Hungary has remained constant over the past 320 000 years while climate oscillated [9]. These results are consistent with a causal link, either direct or indirect, between species richness and contemporaneous environment.
The environment could impose top-down limits on species richness, independently of species identities. For example, species-energy theory proposed that primary productivity imposes a carrying capacity on the number of individuals, and therefore on species richness [10]. Metabolic theory of biodiversity predicts species richness from a mechanism involving temperature-dependent metabolic rate and a cap on the total number of individuals in a region [11]. Classic island biogeography theory proposes that richness depends upon an equilibrium between colonization and extinction rates [12]. Regardless of their specific mechanism, top-down hypotheses of species richness predict that the richness–environment relationship is congruent through time and space because the mechanism operates independently of factors that vary spatially or temporally, other than climate [13]. In other words, these hypotheses require that the environment limits the number of species that can occur in a given region [13,14], or determines stochastic immigration and extinction rates [12,15].
Alternatively, the environment could control species richness bottom-up by constraining individual species’ ranges. Bottom-up hypotheses attribute richness gradients to mechanisms that create individual species’ environmental niches. These hypotheses predict that the richness–environment relationship is congruent through time and space because individual species’ realized environmental niches are fixed, and they track geographical variations in climatic constraints [16,17]. This is a critical assumption of species distribution models (SDMs) [18,19] and of the approach of stacking these individual models to predict richness [8,20]. The environment could control richness bottom-up by, for example, constraining the number of different physiological configurations that are viable at any given location [21,22]. Another prominent example is the tropical niche conservatism hypothesis, which is the maintenance of ancestral tolerances to tropical (warm and productive) environments in daughter species [23,24].
One way to distinguish between top-down versus bottom-up hypotheses is to investigate a group of species where richness is known to track environmental variables as those variables change temporally, and to ask whether individual species’ ranges also shift in response to the same environmental variables. Bottom-up hypotheses predict that richness–environment relationships persist when environment changes because species geographically track their occupied environmental niche (sensu [25]); the main constraint on species ranges is each species’s abiotic niche [26] such that a species’s occupied niche must either be close to or a predictable subset of its fundamental niche. Top-down hypotheses, on the other hand, do not predict that species temporally track environmental variables. Migratory species could instead occupy different parts of larger fundamental niches when environmental variables change, showing marked differences in habitat use between seasons [27,28].
To test those competing hypotheses, we analysed the summer and winter geographical ranges of birds in the Americas. The environment in many regions changes dramatically between seasons, and many birds apparently respond to this change by migrating between summer and winter ranges. As a consequence of the migration of these species, total bird species richness in any given location varies seasonally, but the overall richness–environment relationship remains approximately constant through time [6,7].
Here, we modelled the environments occupied by 625 migratory bird species from December to February and from May to July. We then measured the overlap of the occupied environmental niche between the two seasons. We tested whether, as predicted by bottom-up hypotheses, this overlap is (i) higher than if species had migrated independently of their occupied environmental niche in the previous season, and (ii) higher than if species had not migrated at all. Although top-down and bottom-up hypotheses are not necessarily mutually exclusive, we can test whether bottom-up mechanisms in isolation are sufficient to account for patterns of richness.
We focus on temperature and enhanced vegetation index (EVI) as our measures of environment; EVI is similar to normalized difference vegetation index (NDVI), but is not likely to saturate when leaf area index is high. Of all environmental variables considered, temperature and EVI contributed the most to the seasonal richness–environment relationship (see Material and methods). Temperature tolerance is generally an important constraint on species ranges [29,30]. Similarly, it is commonly believed that birds migrate because of changes in resource or food availability [31], with EVI reflecting plant productivity and insect abundance [32].
2. Material and methods
(a). Occupancy and climate data
We divided the Americas into equal-area quadrats of 10 000 km2 using a Behrmann projection. After removing quadrats with less than 50% land area, 4141 quadrats remained. For each quadrat, we calculated a 50-year averaged mean temperature and total precipitation for the months of May, June and July (season 1), as well as for the months of December, January and February (season 2), from WorldClim [33]. For both seasons, we also calculated the standard deviation of temperature and precipitation [33], a 25-year averaged mean NDVI from the Advanced Very High Resolution Radiometer data series [34,35], a 2-year averaged EVI from Moderate Resolution Imaging Spectroradiometer on Terra [36], as well as the mean and range in elevation of each quadrat [33].
Breeding and non-breeding ranges for birds of the Americas were obtained from NatureServe [37]. A quadrat was considered occupied by a species when its range overlapped any part of the quadrat. The 3902 bird species with extant ranges overlapping at least one quadrat of the Americas were included.
(b). The richness–environment relationship
For season 1 (May to July) as well as for season 2 (December to February), we tallied the total number of species whose ranges overlapped each quadrat, thus obtaining species richness per quadrat. All species whose geographical ranges include a particular quadrat during the season were included. Species that stayed year round in a particular quadrat were therefore counted in both seasons [6]. For migratory species whose breeding ranges only occurred in the Northern Hemisphere (n = 474) or did not occur south of the Tropic of Capricorn (n = 2), we considered season 1 to be the breeding season and season 2 to be the non-breeding season. Inversely, for species whose breeding ranges only occurred in the Southern Hemisphere (n = 121) or did not occur north of the Tropic of Cancer (n = 19), we considered season 1 to be the non-breeding season and season 2 the breeding season.
We then fitted richness in both seasons as a single function of temperature, precipitation, their standard deviation, NDVI, mean and range in elevation, as these were the variables considered by H-Acevedo & Currie [6]. Additionally, we included EVI as a substitute measure of vegetation density and productivity because it remains sensitive to increases in canopy density beyond the density at which NDVI becomes saturated [38]. We also included season as a categorical variable in the model to test whether richness–environment relationships remain constant between seasons. All second-order interaction terms were considered. We also considered second- and third-order polynomial terms for temperature [6]. We then reduced this model to include only variables with substantial biological effects by sequentially eliminating terms for which the partial r2 was less than 0.01. We did not use p- or AIC-values in model selection because the very high statistical power of our dataset (n = 7810) leads to inclusion of many variables that account for miniscule amounts of variance. To fit the models, we log10-transformed richness to improve homoscedasticity and normality of the residuals. Thus, there are 4141 quadrats represented in each of the two seasons minus a total of 472 quadrats with zero richness (mostly Arctic islands) that were excluded for a total sample size of 7810 quadrats in the richness–environment analyses. We excluded quadrats with richness values of zero to eliminate both the statistical and ecological bound in richness (i.e. richness reaches zero in some harsh climates, but it cannot be lower than zero in more extreme climates). Note that none of our qualitative conclusions are affected by excluding zero-richness quadrats.
Because of high multicollinearity among independent variables, many models had nearly equivalent statistical fits as measured by the R2 and AIC. However, temperature and EVI were uniformly present in the high-performing models. As richness apparently most strongly tracks temperature and EVI, we then measured the occupied environmental niche of individual species considering only those variables. It is certainly possible that individual species’ distributions are constrained by environmental variables other than those retained in our richness–environment model. However, if individual species track variables that richness does not apparently track, then bottom-up hypotheses proposing that richness patterns result from individual species’ environmental tolerances are nevertheless refuted. Precipitation and the standard deviation of temperature were also sometimes retained in the richness–environment model. We therefore present, in the electronic supplementary material, results where the occupied environmental niche of species is modelled with these variables.
(c). Environmental niche overlap
For the 625 migratory species that had distinct ranges in seasons 1 and 2, we fitted a model relating occupancy to the seasonal environment independently for seasons 1 and 2. We related the probability of occupancy (presence or absence in a quadrat) in a given season to the seasonal environmental variables using Gaussian models (e.g. in figure 1). We first fitted occupancy as a Gaussian function of each environmental variable independently (here, temperature and EVI) assuming a binomial error distribution. These models contain three terms: the mean, which represents the optimal environment for the species based on occupancy; the standard deviation of the Gaussian curve, which measures occupied environmental breadth; and a scaling constant that adjusts for the height of the curve, which can vary between 0 and 1. Based on Boucher-Lalonde et al. [15], we assumed that this function generally explains the geographical distribution of species well. There was no pattern in the residuals and all models were highly statistically significant (n = 4141 quadrats). Note that, when presences for a species peak near the limits of existing environmental conditions, the parameters of the Gaussian model are impossible to estimate. Therefore, 32% of the univariate Gaussian models failed to converge (see electronic supplementary material for measures of the occupied niche that do not rely on a fitted model).
Figure 1.
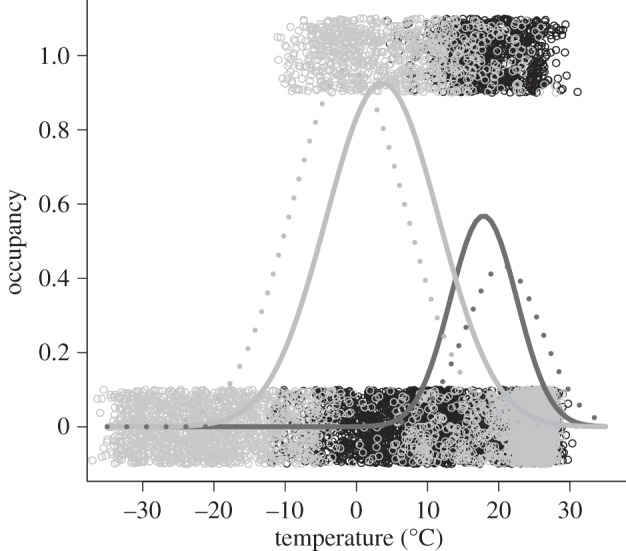
Occupancy of 10 000 km2 quadrats (n = 4141) in the Americas by Accipiter cooperii (Cooper's hawk) in May to July (black dots) and in December to February (grey dots). Presences (occupancy = 1) and absences (occupancy = 0) have here been jittered by 0.1. Seasonal occupancy was related to seasonal temperature by a Gaussian model. The fitted relationship is presented for May to July (dark curve) and December to February (pale curve), and explains, respectively, 21% and 54% of the deviance in occupancy. We also present the fitted relationships assuming no migration—that is, if the species had stayed in its May to July (dark dashed curve) or in its December to February range (pale dashed curve) instead of migrating.
We then measured the overlap in environmental space between the Gaussian models fitted for seasons 1 and 2 for each environmental variable. We measured the overlap in occupied environments by what we hereafter refer to as (i) integral overlap, which is the proportion of the integral of the fitted Gaussian curves for the two seasons that is shared between seasons, and (ii) integral nestedness, which is the maximal proportion of the integral of the fitted relationship for one season that is nested within the integral of the other season. Values of 0 represent no environmental overlap between seasons, whereas values of 1 represent full overlap between seasons. We also used other measures of overlap, which are presented in the electronic supplementary material.
(d). Null models of overlap
Bottom-up hypotheses for the richness–environment relationship predict that species-occupied environmental niches will be stable (i.e. fully overlap) between seasons 1 and 2. Top-down hypotheses predict that the species that occur in a particular climate are independent of the species that occupied that climate in the opposite season. The challenge here is that environmental niches in the two seasons may be independent and nevertheless overlap substantially. Conversely, environmental niches may be highly stable between seasons, but the coarse climate and occupancy data used here as well as the spatial distribution of particular environmental conditions may introduce error in the degree of overlap of environmental niches. Therefore, to test statistically whether occupied environments are seasonally stable, as predicted by bottom-up hypotheses, or are independent, as predicted by top-down hypotheses, we developed null expectations for the measures of overlap.
The null models must retain (i) the extant range of environmental variables, (ii) the cohesive spatial structure of geographical ranges and (iii) the richness–environment relationship which has to be maintained between seasons. We therefore treated the set of observed ranges of all species as the set of possible ranges for any given species. For each species, we tested whether environmental overlap between its ranges in seasons 1 and 2 was greater than the expected environmental overlap with the ranges of other migratory species in the opposite season. When a species’s occupied environmental niche in one season overlapped its own niche in the opposite seasons more than it overlapped with the niche of other species, we considered that the species is tracking its individual environmental niche and that the observed overlap is not merely due to richness tracking climate. Another key prediction of bottom-up hypotheses is that migratory species must conserve a higher niche overlap by migrating than if they had not migrated. Top-down hypotheses do not make this prediction. We therefore used a t-test to test whether migration generally increases niche overlap, with the prediction that the difference between overlap with versus without migration is positive.
To demonstrate that our results and conclusions are robust to the specific type of model used and are unaffected by the description of occupied environments in univariate space, we also fitted a MaxEnt model [39] with four environmental variables for all species: temperature, EVI, precipitation and the standard deviation of temperature. MaxEnt is the most widely used SDM and has been shown to perform among the best when compared with other SDMs [40]. Although MaxEnt was built to model true presence data [39], there is a wide precedent of use with range maps (e.g. [41]). Using the Bray–Curtis distances between MaxEnt suitability scores as our measure of overlap, we confirmed our findings that niche overlap between seasons is typically low, no better than expected under our null model and generally not higher than if species had not migrated (see the electronic supplementary material).
All statistical analyses were performed in R v. 2.14 [42]; MaxEnt models were fitted with the ‘dismo’ package [43] and the area under the receiver operating curve (AUC) with the ‘verification’ package [44].
3. Results
The relationship between bird species richness and environment in the Americas is congruent between season 1 (May to July) and season 2 (December to February) (figure 2). Temperature and EVI together explain 90% of the variance in richness in both seasons, throughout the Americas. Season, and its interaction with temperature and EVI, are statistically significant, but explain less than 1% of additional variance in richness. Similarly, we found that there is no residual effect of continent (North versus South America) in the model, despite the residuals being spatially autocorrelated (Moran's I = 0.57). Moreover, the seasonal change in temperature and EVI explains very well the change in seasonal species richness, and spatial autocorrelation can be eliminated by fitting a simultaneous autoregressive error model (see the electronic supplementary material). Because the seasonal congruence of the richness–environment relationship holds true for the entire Western Hemisphere (figure 2), as expected [6,7], we can proceed with the question: is this because of bottom-up or top-down limits on species richness?
Figure 2.
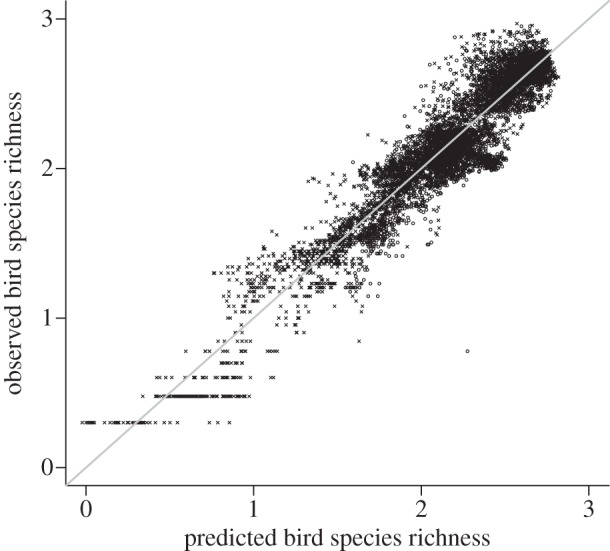
Observed log10-transformed bird species richness versus predicted richness from temperature and EVI. Richness for each 10 000 km2 quadrat is represented for the seasons of May to July (circles) and of December to February (cross symbols), and is calculated from range maps of 3902 birds in the Americas. The 1 : 1 line is shown in grey.
Temperature and EVI describe individual species’ niches reasonably well. We used univariate Gaussian models of temperature and EVI to model the niche of each species in each season (e.g. figure 1), and we found no apparent lack of fit. The AUC was generally high and comparable with that of other studies that have modelled species distribution–environment relationships. For temperature, AUC was higher than 0.5 for all species and higher than 0.8 for 70% of species in season 1 and 97% of species in season 2. For EVI, AUC was higher than 0.8 for only 34% and 13% of species in seasons 1 and 2, respectively. The average explained deviance of the models was 0.30 (0.006 s.e.) for temperature and 0.18 (0.005 s.e.) for EVI. Note that, if species distributions consistently relate poorly to temperature and EVI, which predict species richness very well, that is in itself inconsistent with the hypothesis that individual species’ tolerances drive the richness–environment relationship.
For the 625 migratory bird species, we found that environmental niche overlap between seasons was generally low (figure 3). Niche overlap was not higher than expected under the null hypothesis that individual species do not track their occupied environmental niches when richness tracks the environment (figure 4). Additionally, niche overlap was not typically higher than if species had not migrated (figure 5). Other measures of niche and niche overlap are presented in the electronic supplementary material, and lead to the same conclusions.
Figure 3.
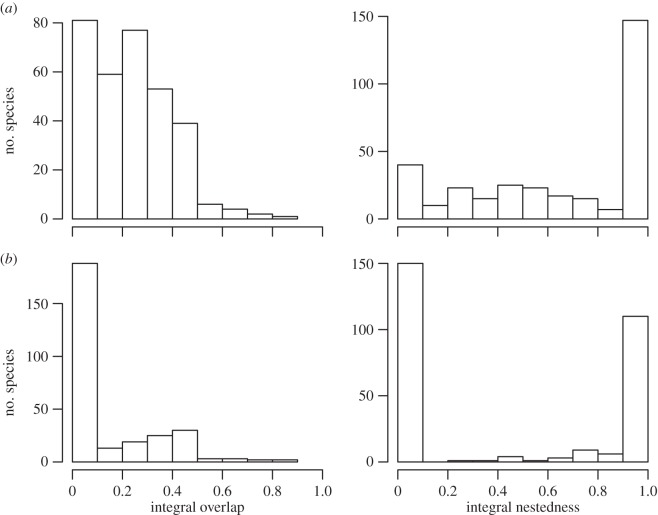
Distribution of measures of environmental niche overlap between May to July and December to February for (a) temperature and (b) EVI. The niche of a species in a given season is modelled by a univariate Gaussian function of the environmental variable (e.g. figure 1). We used two different measures of overlap described in the main text: integral overlap (left column) and integral nestedness (right column). Values of 0 indicate no niche overlap while values of 1 represent perfect niche overlap between seasons. By definition, integral nestedness will always be equal to or higher than integral overlap.
Figure 4.
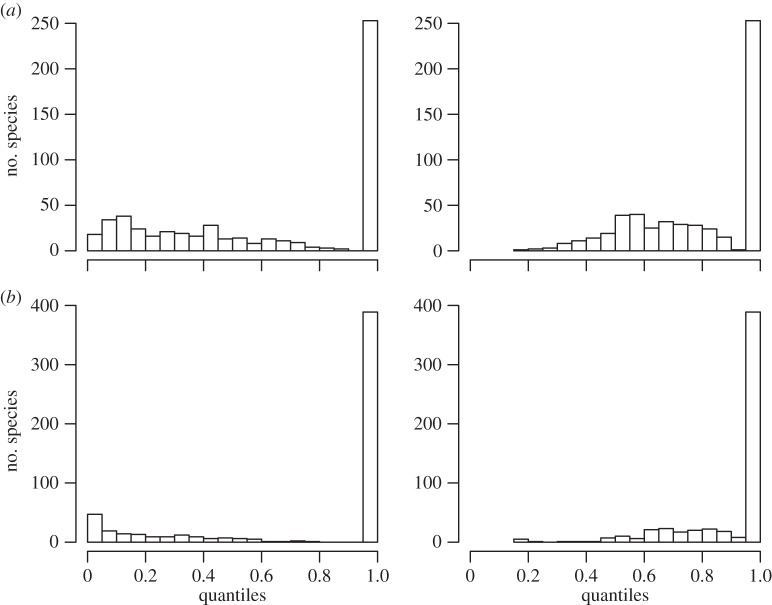
Assume that, when a species migrates between seasons, it could potentially move to the post-migration range of any species. Here, we test whether the environmental conditions in a species’s pre-migration geographical range are more similar to the conditions in its own post-migration range than to the conditions in the ranges occupied by other species post-migration for (a) temperature and (b) EVI. For a given species, the quantile is calculated as the proportion of species for which the occupied niche overlap is equal to or greater than the observed seasonal niche overlap for the species. The quantiles are calculated for two different measures of niche overlap: integral overlap (left column) and integral nestedness (right column).
Figure 5.
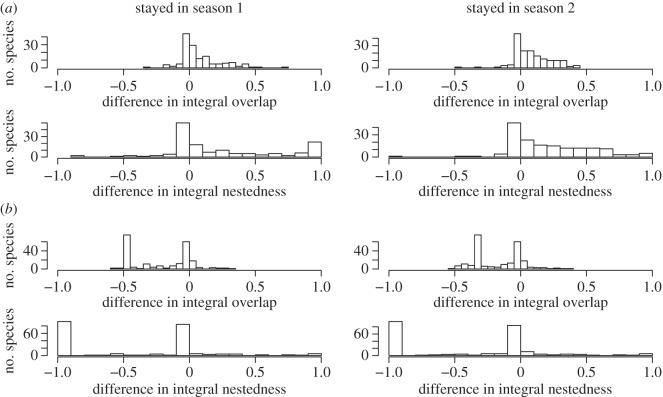
Distribution of the differences in niche overlap if migratory species had stayed year round in either the range they occupy from May to July (left column) or from December to February (right column) instead of migrating. Specifically, we measured overlap given migration minus overlap assuming no migration. If species migrate to track their environmental niche, positive values are predicted. Results are presented for (a) temperature and (b) EVI, and two measures of overlap: integral overlap and integral nestedness.
Specifically, the occupied temperature niche of most species only partially overlaps in the two seasons, although one is nearly always nested within the other (figure 3a). For a given species, the overlap of its observed temperature niches between the two seasons was generally not higher than the overlap with the occupied temperature niches of other species (figure 4a). We tested whether this is an artefact caused by many species having similar climatic niches. If this were the case, then niche overlap for a pair of species between seasons should be strongly related to their overlap within a given season, with a slope of 1 and an intercept of 0. However, we find that the overlap of species pairs within a season poorly predicts their overlap between seasons (R2 = 0.13), with a slope of only 0.37 and an intercept of 0.44. Thus, species that do not overlap at all within a given season (i.e. share none of their climate niche as here defined) still have, on average, 44% overlap between seasons 1 and 2. The predictive power of nestedness as a measure of overlap is only slightly higher (table 1). We also found that most species would conserve a similar niche overlap if they did not migrate (figure 5a). Migration increases the average integral overlap and nestedness for temperature by only 8% and 23%, respectively.
Table 1.
Linear regression models of the overlap of species pairs between seasons as a function of their overlap within a given season. For all pairs of species, the overlap between seasons 1 and 2 is therefore predicted by their overlap within season 1 and within season 2 (i.e. two x-values for each y-value). If all species fully conserved their niches between seasons, the regression would have a slope of 1, an intercept of 0 and an R2 of 1. Here, we present the observed coefficients of the fitted relationships for the environmental variables temperature and EVI, and for two different measures of niche overlap: integral overlap and integral nestedness. The standard errors are in parentheses.
| temperature |
EVI |
|||
|---|---|---|---|---|
| integral overlap | integral nestedness | integral overlap | integral nestedness | |
| slope | 0.37 (0.0018) | 0.43 (0.00037) | 0.49 (0.0019) | 0.34 (0.0014) |
| intercept | 0.44 (0.0014) | 0.09 (0.0016) | 0.25 (0.0017) | 0.03 (0.00026) |
| R2 | 0.13 | 0.22 | 0.22 | 0.17 |
EVI niche overlap was also typically very low (figure 3b) and was not higher than expected under our null model (figure 4b). Additionally, for all pairs of species, within-season overlap was a very poor predictor of between-season overlap (table 1). Finally, species would on average conserve higher niche overlap for EVI if they stayed year round in either their May to July or December to February range instead of migrating (figure 5b). Migration decreases average integral niche overlap and nestedness for EVI by 19% and 40%, respectively. Therefore, we reject the hypothesis that species generally track the environmental variables that richness is tracking. Instead, species migrate largely independently of their occupied niches in the previous season.
4. Discussion
We have here found that, while richness strongly tracks seasonal changes in temperature and productivity measured by EVI, the vast majority of species do not track these variables. Temperature and EVI apparently impose top-down limits on species richness independently of either factor's effect on the location of individual species’ range boundaries. Bottom-up hypotheses proposing that richness tracks the environment through the sum of the effects on individual species’ environmental tolerances (e.g. tropical niche conservatism) may explain, for example, why entire biogeographic provinces have differing numbers of species, and contribute to understanding the origins of species pools, but we found no evidence that they account for regional variations in richness such as those commonly represented in global maps of species richness.
Although the environmental niches of most species at least partly overlapped between seasons, this overlap was no greater than expected under the null hypothesis that species migrate independently of their previously occupied environmental niche. Like other studies [28,45,46], we find that, for the majority of species, the overlap between seasons is higher than if presences were randomly located within the study region (see the electronic supplementary material). However, we extend these findings and show that species seasonal niches can partly overlap simply because this is what is expected when species migrate within a bounded environmental space with a species richness gradient that is controlled top-down by the environment. Moreover, contrary to predictions from bottom-up hypotheses, the seasonal overlap between species' environmental niches was typically not higher than if the species had stayed year round in either its breeding or non-breeding range.
Species’ ranges may nevertheless be constrained by tolerances to environmental variables. Perhaps species require different environmental conditions at different stages of their life history, such as between spring breeding and overwintering periods [27]; or perhaps individual species’ ranges are constrained by different environmental variables than those we considered here. For example, temperature extremes, rather than mean temperatures, could constrain species richness and/or species ranges [47]. Here, it would be difficult to distinguish their partial effects because the spatial variation of seasonal mean, minimum or maximum temperatures across the Americas is very highly correlated (r = 0.99). We acknowledge that we have not modelled all dimensions of a species’s environmental niche (nor did we intend to).
That said, recall that bottom-up hypotheses propose that spatial variation in species richness is very strongly correlated with temperature and EVI because individual species’ ranges are strongly constrained by their tolerances for those variables. If, instead, individual species track different sets of conditions during the breeding and non-breeding seasons, and if richness variation reflects such tolerances, then richness must correlate with different sets of variables in the two seasons. We observed the opposite: species’ ranges do not seasonally track temperature and EVI, but richness does. For illustrative purposes, imagine that species were strongly constrained by soil type. This cannot possibly explain why richness is strongly related to temperature. Similarly, if species ranges are constrained by temperature during the breeding season but by soil type during the non-breeding season, these limits could not explain why richness seasonally tracks temperature. Finally, one could argue that bottom-up hypotheses could explain richness–environment relationships at other temporal or spatial scales. However, the most parsimonious explanation for richness patterns that prove consistent across temporal [6,7,48] and spatial scales [2,49], and across continents [50,51] and taxa [49], is that they share a common explanation.
There is a great deal of evidence consistent with the most general prediction of top-down hypotheses: that the richness–environment relationship should be consistent across space and time [6,7,48–51]. However, specific top-down mechanisms have been less successful. Consider, for example, the ‘species-energy hypothesis’: that energy determines the number of individuals that can occur in a region, and thereby the number of species [10]. It has been shown that richness is more highly correlated with climate than with abundance, including for American birds [52,53]. Thus, the specific mechanism is insufficient [47,52,53]. Similarly, other top-down hypotheses [13,14] implicitly raise the question: why more species and not more individuals?
One example of a broadly successful top-down hypothesis is the equilibrium theory of island biogeography [12]. According to this theory, species richness on islands represents the equilibrium between immigration and local extinction rates. The species involved are not individually distinguished. It is possible that a similar mechanism operates on continents. Evolution may have provided pools of species within biogeographic provinces that are much larger than regional species assemblages. If rates of immigration to, or local extinction within, regions are climate-dependent, then correlations between richness and climate would result.
Here, we have rejected both predictions derived from the hypothesis that bottom-up mechanisms explain contemporary richness–environment relationships among birds in the Americas. This cannot be because our test was too conservative because our conclusions do not depend on the threshold used (i.e. the vast majority of species are not tracking their own occupied niche better than the niche of other species; figure 4). But perhaps the environmental niches of migratory birds of the Americas are simply all too similar for our test to be valid. However, if this were the case, then species’ environmental niches could not account for seasonal variations in species richness.
Niche conservatism may largely determine the identities of the species occurring in a given region without strongly affecting species richness. For instance, Algar et al. [54] showed that hylid frog phylogenetic structure is independent of the environmental factors that best explain richness. Similarly, Hawkins et al. [55] have shown that the relationship between richness and contemporary climate is highly congruent for birds and mammals, but that it cannot be explained by similar evolutionary trajectories. Here, we do not argue that evolutionary or historical [56] processes are never important, but rather that the numbers of species in regional species assemblages are apparently primarily (but not necessarily exclusively) controlled by contemporary top-down effects. We conclude that environmental change leads to a reorganization of species where the warmest and most productive environment can sustain more species, regardless of their identities.
Acknowledgements
We thank David Storch, Allen Hurlbert, Kamran Safi and an anonymous reviewer for providing useful comments on previous versions of this manuscript.
Funding statement
V.B.-L. was supported by a postgraduate scholarship, while J.T.K. and D.J.C. were supported by Discovery Grants from the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Currie DJ. 1991. Energy and large-scale patterns of animal-species and plant-species richness. Am. Nat. 137, 27–49 (doi:10.1086/285144) [Google Scholar]
- 2.Field R, et al. 2009. Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147 (doi:10.1111/j.1365-2699.2008.01963.x) [Google Scholar]
- 3.Currie DJ, Francis AP. 2004. Taxon richness and climate in angiosperms: is there a globally consistent relationship that precludes region effects? Reply. Am. Nat. 163, 780–785 (doi:10.1086/383596) [DOI] [PubMed] [Google Scholar]
- 4.Latham RE, Ricklefs RE. 1993. Global patterns of tree species richness in moist forests—energy-diversity theory does not account for variation in species richness. Oikos 67, 325–333 (doi:10.2307/3545479) [Google Scholar]
- 5.Fine PVA, Ree RH. 2006. Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am. Nat. 168, 796–804 (doi:10.1086/508635) [DOI] [PubMed] [Google Scholar]
- 6.H-Acevedo D, Currie D. 2003. Does climate determine broad-scale patterns of species richness? A test of the causal link by natural experiment. Glob. Ecol. Biogeogr. 12, 461–473 (doi:10.1046/j.1466-822X.2003.00058.x) [Google Scholar]
- 7.Hurlbert AH, Haskell JP. 2003. The effect of energy and seasonality on avian species richness and community composition. Am. Nat. 161, 83–97 (doi:10.1086/345459) [DOI] [PubMed] [Google Scholar]
- 8.Algar AC, Kharouba HM, Young ER, Kerr JT. 2009. Predicting the future of species diversity: macroecological theory, climate change, and direct tests of alternative forecasting methods. Ecography 32, 22–33 (doi:10.1111/j.1600-0587.2009.05832.x) [Google Scholar]
- 9.Willis KJ, Kleczkowski A, New M, Whittaker RJ. 2007. Testing the impact of climate variability on European plant diversity: 320 000 years of water-energy dynamics and its long-term influence on plant taxonomic richness. Ecol. Lett. 10, 673–679 (doi:10.1111/j.1461-0248.2007.01056.x) [DOI] [PubMed] [Google Scholar]
- 10.Wright DH. 1983. Species-energy theory—an extension of species-area theory. Oikos 41, 496–506 (doi:10.2307/3544109) [Google Scholar]
- 11.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (doi:10.1890/03-9000) [Google Scholar]
- 12.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Brown JH, Ernest SKM, Parody JM, Haskell JP. 2001. Regulation of diversity: maintenance of species richness in changing environments. Oecologia 126, 321–332 (doi:10.1007/s004420000536) [DOI] [PubMed] [Google Scholar]
- 14.O'Brien EM. 1998. Water-energy dynamics, climate, and predicting of woody plant species richness: an interim general model. J. Biogeogr. 25, 379–398 (doi:10.1046/j.1365-2699.1998.252166.x) [Google Scholar]
- 15.Boucher-Lalonde V, Morin A, Currie D. 2012. How are tree species distributed in climatic space? A simple and general pattern. Glob. Ecol. Biogeogr. 21, 1157–1166 (doi:10.1111/j.1466-8238.2012.00764.x) [Google Scholar]
- 16.La Sorte FA, Jetz W. 2012. Tracking of climatic niche boundaries under recent climate change. J. Anim. Ecol. 81, 914–925 (doi:10.1111/j.1365-2656.2012.01958.x) [DOI] [PubMed] [Google Scholar]
- 17.Tingley MW, Monahan WB, Beissinger SR, Moritz C. 2009. Birds track their Grinnellian niche through a century of climate change. Proc. Natl Acad. Sci. USA 106, 19 637–19 643 (doi:10.1073/pnas.0901562106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharouba HM, Algar AC, Kerr JT. 2009. Historically calibrated predictions of butterfly species’ range shift using global change as a pseudo-experiment. Ecology 90, 2213–2222 (doi:10.1890/08-1304.1) [DOI] [PubMed] [Google Scholar]
- 19.Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 20.Guisan A, Rahbek C. 2011. SESAM: a new framework integrating macroecological and species distribution models for predicting spatio-temporal patterns of species assemblages. J. Biogeogr. 38, 1433–1444 (doi:10.1111/j.1365-2699.2011.02550.x) [Google Scholar]
- 21.Kleidon A, Adams J, Pavlick R, Reu B. 2009. Simulated geographic variations of plant species richness, evenness and abundance using climatic constraints on plant functional diversity. Environ. Res. Lett. 4, 1–5 (doi:10.1088/1748-9326/4/1/014007) [Google Scholar]
- 22.Kleidon A, Mooney HA. 2000. A global distribution of biodiversity inferred from climatic constraints: results from a process-based modelling study. Glob. Change Biol. 6, 507–523 (doi:10.1046/j.1365-2486.2000.00332.x) [Google Scholar]
- 23.Wiens JJ, Donoghue MJ. 2004. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644 (doi:10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 24.Wiens JJ, et al. 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 (doi:10.1111/j.1461-0248.2010.01515.x) [DOI] [PubMed] [Google Scholar]
- 25.Pearson R. 2007. Species’ distribution modeling for conservation educators and practitioners. New York, NY: Network of Conservation Educators and Practitioners. [Google Scholar]
- 26.Soberon J, Nakamura M. 2009. Niches and distributional areas: concepts, methods, and assumptions. Proc. Natl Acad. Sci. USA 106, 19 644–19 650 (doi:10.1073/pnas.0901637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagan JM, Johnston DW. 1992. Ecology and conservation of neotropical migrant landbirds. Washington, DC: Smithsonian Institution Press [Google Scholar]
- 28.Nakazawa Y, Peterson AT, Martinez-Meyer E, Navarro-Siguenza AG. 2004. Seasonal niches of Nearctic–Neotropical migratory birds: implications for the evolution of migration. Auk 121, 610–618 (doi:10.1642/0004-8038(2004)121[0610:SNONMB]2.0.CO;2) [Google Scholar]
- 29.Root T. 1988. Energy constraints on avian distributions and abundances. Ecology 69, 330–339 (doi:10.2307/1940431) [Google Scholar]
- 30.Gaston KJ. 2003. The structure and dynamics of geographic ranges. New York, NY: Oxford University Press [Google Scholar]
- 31.Berthold P. 1995. Control of bird migration. London, UK: Chapman & Hall [Google Scholar]
- 32.Szep T, Moller AP, Piper S, Nuttall R, Szabo ZD, Pap PL. 2006. Searching for potential wintering and migration areas of a Danish Barn Swallow population in South Africa by correlating NDVI with survival estimates. J. Ornithol. 147, 245–253 (doi:10.1007/s10336-006-0060-x) [Google Scholar]
- 33.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (doi:10.1002/joc.1276) [Google Scholar]
- 34.Pinzon J, Brown ME, Tucker CJ. 2005. Satellite time series correction of orbital drift artifacts using empirical mode decomposition. In Hilbert–Huang transform: introduction and applications (ed. Huang N.), pp. 167–186 College Park, MD: Global Land Cover Facility, University of Maryland [Google Scholar]
- 35.Tucker CJ, Pinzon JE, Brown ME, Slayback DA, Pak EW, Mahoney R, Vermote EF, El Saleous N. 2005. An extended AVHRR 8-km NDVI dataset compatible with MODIS and SPOT vegetation NDVI data. Int. J. Remote Sens. 26, 4485–4498 (doi:10.1080/01431160500168686) [Google Scholar]
- 36.Justice CO, et al. 1998. The Moderate Resolution Imaging Spectroradiometer (MODIS): land remote sensing for global change research. Ieee T. Geosci. Remote Sens. 36, 1228–1249 (doi:10.1109/36.701075) [Google Scholar]
- 37.Ridgely RS, Allnutt TF, Brooks T, McNicol DK, Mehlman DW, Young BE, Zook JR. 2007 Digital distribution maps of the birds of the Western Hemisphere, version 3.0. Arlington, VA: Nature Serve. [Google Scholar]
- 38.Huete A, Didan K, Miura T, Rodriguez EP, Gao X, Ferreira LG. 2002. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 83, 195–213 (doi:10.1016/s0034-4257(02)00096-2) [Google Scholar]
- 39.Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259 (doi:10.1016/j.ecolmodel.2005.03.026) [Google Scholar]
- 40.Elith J, et al. 2006. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29, 129–151 (doi:10.1111/j.2006.0906-7590.04596.x) [Google Scholar]
- 41.Hof C, Araujo MB, Jetz W, Rahbek C. 2011. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480, U516–U519 (doi:10.1038/nature10650) [DOI] [PubMed] [Google Scholar]
- 42.R Development Core Team 2012. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical computing; See http://www.R-project.org/ [Google Scholar]
- 43.Hijmans R, Phillips S, Leathwick J, Elith J. 2012. dismo: Species distribution modeling R package version 0.7–17 See http://CRANR-project.org/package=dismo
- 44.NCAR - Research Application Program 2012. verification: Forecast verification utilities R package version 1.32 See http://CRANR-project.org/package=verification
- 45.Martinez-Meyer E, Peterson AT, Navarro-Siguenza AG. 2004. Evolution of seasonal ecological niches in the Passerina buntings (Aves: Cardinalidae). Proc. R. Soc. Lond. B 271, 1151–1157 (doi:10.1098/rspb.2003.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papes M, Peterson AT, Powell GVN. 2012. Vegetation dynamics and avian seasonal migration: clues from remotely sensed vegetation indices and ecological niche modelling. J. Biogeogr. 39, 652–664 (doi:10.1111/j.1365-2699.2011.02632.x) [Google Scholar]
- 47.Simova I, Storch D, Keil P, Boyle B, Phillips OL, Enquist BJ. 2011. Global species-energy relationship in forest plots: role of abundance, temperature and species climatic tolerances. Glob. Ecol. Biogeogr. 20, 842–856 (doi:10.1111/j.1466-8238.2011.00650.x) [Google Scholar]
- 48.Yasuhara M, Hunt G, Dowsett H, Robinson M, Stoll D. 2012. Latitudinal species diversity gradient of marine zooplankton for the last three million years. Ecol. Lett. 15, 1174–1179 (doi:10.1111/j.1461-0248.2012.01828.x) [DOI] [PubMed] [Google Scholar]
- 49.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (doi:10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 50.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (doi:10.1890/03-8006) [Google Scholar]
- 51.Francis AP, Currie DJ. 2003. A globally consistent richness–climate relationship for angiosperms. Am. Nat. 161, 523–536 (doi:10.1086/368223) [DOI] [PubMed] [Google Scholar]
- 52.Currie DJ, et al. 2004. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 (doi:10.1111/j.1461-0248.2004.00671.x) [Google Scholar]
- 53.Hurlbert AH. 2004. Species-energy relationships and habitat complexity in bird communities. Ecol. Lett. 7, 714–720 (doi:10.1111/j.1461-0248.2004.00630.x) [Google Scholar]
- 54.Algar AC, Kerr JT, Currie DJ. 2009. Evolutionary constraints on regional faunas: whom, but not how many. Ecol. Lett. 12, 57–65 (doi:10.1111/j.1461-0248.2008.01260.x) [DOI] [PubMed] [Google Scholar]
- 55.Hawkins BA, et al. 2012. Different evolutionary histories underlie congruent species richness gradients of birds and mammals. J. Biogeogr. 39, 825–841 (doi:10.1111/j.1365-2699.2011.02655.x) [Google Scholar]
- 56.Ricklefs RE. 2006. Evolutionary diversification and the origin of the diversity–environment relationship. Ecology 87, S3–S13 (doi:10.1890/0012-9658(2006)87[3:EDATOO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]