Abstract
Many organisms have geographical distributions extending from the tropics to near polar regions or can experience up to 30°C temperature variation within the lifespan of an individual. Two forms of evolutionary adaptation to such wide ranges in ambient temperatures are frequently discussed: local adaptation and phenotypic plasticity. The freshwater planktonic crustacean Daphnia magna, whose range extends from South Africa to near arctic sites, shows strong phenotypic and genotypic variation in response to temperature. In this study, we use D. magna clones from 22 populations (one clone per population) ranging from latitude 0° (Kenya) to 66° North (White Sea) to explore the contributions of phenotypic plasticity and local adaptation to high temperature tolerance. Temperature tolerance was studied as knockout time (time until immobilization, Timm) at 37°C in clones acclimatized to either 20°C or 28°C. Acclimatization to 28°C strongly increased Timm, testifying to adaptive phenotypic plasticity. At the same time, Timm significantly correlated with average high temperature at the clones’ sites of origin, suggesting local adaptation. As earlier studies have found that haemoglobin expression contributes to temperature tolerance, we also quantified haemoglobin concentration in experimental animals and found that both acclimatization temperature (AccT) and temperature at the site of origin are positively correlated with haemoglobin concentration. Furthermore, Daphnia from warmer climates upregulate haemoglobin much more strongly in response to AccT, suggesting local adaptation for plasticity in haemoglobin expression. Our results show that both local adaptation and phenotypic plasticity contribute to temperature tolerance, and elucidate a possible role of haemoglobin in mediating these effects that differs along a cold–warm gradient.
Keywords: Daphnia, temperature, heat tolerance, phenotypic plasticity, local adaptation, haemoglobin
1. Introduction
Among abiotic factors that affect living organisms, temperature plays a unique role because of its profound effect on the organisms’ biochemistry and physiology through fundamental physical and chemical constraints [1,2]. Most species experience daily or seasonal temperature changes and are generally assumed to have temperature ranges in which they perform best, whereas exposure to temperatures outside this range has costs [1,3,4]. Many poikilotherms experience enormous daily and seasonal variation in temperature, which will select for minimizing the fitness costs associated with the exposure to suboptimal temperatures. Selection for tolerance to suboptimal temperatures may take two forms of evolutionary responses: phenotypic plasticity or local adaptation. Plasticity is the ability of a genotype to adjust its metabolism and physiology in response to current environmental conditions [5,6]. In local adaptation, on the other hand, populations show genetic differences for temperature tolerance corresponding to location-specific temperature variations, e.g. local mean or maximum temperature [7–11]. Local adaptation and phenotypic plasticity may evolve together [12–16] and it is often difficult to disentangle their relative contributions [13,17,18]. Furthermore, phenotypic plasticity may also adapt locally. It has been suggested that the interplay between plastic response and local adaptation with regard to temperature will be key in determining the fate of species and populations exposed to global temperature increase [9]. To understand this interplay requires common garden experiments with sufficient acclimatization time and experimental control over genetic variation.
Recent predictions about the likely effects of climate change on plankton communities [19,20] have revitalized intensive research on the response of these organisms to temperature shifts [11,15,21]. Among them, studies of geographical variation in Daphnia have provided evidence for both phenotypic plasticity and genetic population differentiation [22–25]. However, despite ample genetic variation among genotypes and populations, no evidence for local adaptation was found in this system. This is surprising, given that these freshwater planktonic crustaceans are found in standing waters around the world [26]. For example, Daphnia magna, the focus of this study, has a continuous geographical range from South Africa to near-arctic sites with average local temperatures differing by more than 20°C. How a species copes with such strong environmental variation is a key question. If it copes only by phenotypic plasticity, then global temperature changes within the plastic tolerance range will not threaten the survival of local populations. However, if populations show a signature of adaptation to local temperatures, then global warming will put local populations under stress. It is therefore necessary to conduct studies that allow us to disentangle local adaptation and phenotypic plastic response to temperature.
Studies on local adaptation to temperature often use the population's latitude of origin as a proxy for climatic conditions [27–29]. For freshwater organisms, latitude is, however, not a very good estimate of the temperature experienced at local sites, as it does not consider the local climate or the altitude in which populations are found. Furthermore, the growth season may differ geographically. Species that are active during summer in cold climates may be active during winter in warmer climate. All these considerations apply to Daphnia [30–33]. To get a more accurate sense of temperature at local sites, in this study, we use long-term temperature records from local weather stations near our sampled populations. A second problem often encountered in temperature local adaptation studies is the nature of the biological traits assessed. Life-history fitness components such as growth, maturation and fecundity are known to adapt to local demographic conditions such as food availability or predator pressure [34–37] and thus may disguise patterns of local adaptation to temperature. We therefore use short-term ability to tolerate extreme temperatures, a concept used successfully to test for local adaptation in other aquatic systems [10,38,39], where the key variable is the ability to maintain elementary body functions under such extremes. Here, we test the tolerance of D. magna to lethally high temperatures (37°C, which is deadly after several hours), assessing the time span individuals are able to keep swimming when exposed to this temperature.
In this study, we use the time to immobility to test whether D. magna populations are adapted to the local temperature conditions (i.e. local adaptation) and whether they are able to physiologically acclimatize to higher temperature (i.e. adaptive phenotypic plasticity). We found strong evidence for both types of adaptation. Upregulation of haemoglobin expression is a well-known plastic response to elevated temperatures in invertebrates, including Daphnia, serving as a mechanism both to increase oxygen supply and, possibly, to protect tissues from oxidation damage [25,40–45]. Therefore, we also tested the degree to which our results are linked to the animal's ability to upregulate haemoglobin levels in response to higher temperature.
2. Material and methods
Daphnia magna reproduces by cyclic parthenogenesis, which makes it particularly suitable for the studies of both local adaptation and phenotypic plasticity [46–48]. Twenty-two parthenogenetic clones from 22 geographically distinct populations of D. magna were used in this study (table 1), representing a broad range of geographical origins, from a tropical lake in Kenya, to a pond in the extreme continental climate of Yakutia, East Siberia, Russia. Most clones, however, originated from sites in Europe and the Middle East. A single clone from each population was randomly chosen among clones maintained in the Daphnia clone collection at the University of Basel. This may represent a bias in clonal selection, as clones unable to cope with laboratory conditions might have gone extinct. Using public weather archives, we obtained multi-year average monthly high and low air temperatures from the weather station nearest to the sampling sites. All such stations are located within 50 km from the sampling site (see the electronic supplementary material, table S1 for sources and other details of the clones’ origins) and at approximately the same elevation.
Table 1.
Geographical origins of Daphnia magna clones used in temperature tolerance experiments.
| clone | location | latitude | longitude | meters above-sea level | average highest temperature (°C) |
|---|---|---|---|---|---|
| KE-1-1 | Kenya | 0° 26′ 25″ | 35° 18′ 16″ | 2172 | 25 |
| IL-M1-8 | Jerusalem, Israel | 31° 42’ 52" | 35° 3’ 03" | 603 | 30 |
| IR-GG1-7 | Lake Guru-göl, Iran | 37° 54’ 55" | 46° 41’ 58" | 1905 | 31 |
| FR-C1-1 | Camargue, France | 43° 35’ 30" | 4° 35’ 19" | 5 | 27 |
| IT-ISR1-8 | San Rossore, Pisa, Italy | 43° 41’ 31" | 10° 17’ 20" | 2 | 30 |
| CH-H-149 | Frauenfeld, Switzerland | 47° 33’ 29" | 8° 51’ 43" | 439 | 25 |
| DE-Iinb-1a | Ismaning, carp pond, Germany | 48° 12’ 22" | 11° 42’ 03" | 497 | 24 |
| DE-K35-Mu11 | Ismaning, pond K-3-35, Germany | 48° 12’ 24" | 11° 42’ 35" | 497 | 24 |
| BE-OM-2 | Heverlee, Belgium | 50° 51’ 48’’ | 4° 43’ 17’’ | 26 | 23 |
| GB-EL75-69 | London, UK | 51° 30’ 26’’ | −0° 7’ 39’’ | 17 | 20 |
| BY-G1-9 | Gomel, Belarus | 52° 25’ 05’’ | 31° 00’ 26’’ | 140 | 25 |
| RU-RM1-009 | Moscow, Russia | 55° 45’ 49" | 37° 34’ 54" | 146 | 23 |
| FI-N-47-20 | Tvärminne, Finland | 59° 49’ 30’’ | 23° 14’ 00’’ | 4 | 20 |
| FI-Xinb-3a,b | Tvärminne, Finland | 59° 49’ 30’’ | 23° 14’ 00’’ | 5 | 20 |
| FI-FAT-1-6 | Aland Island, Trebaren, Finland | 60° 02’ 00’’ | 19° 54’ 00’’ | 4 | 21 |
| FI-FSP1-16-2 | Suur-Pellinki, Finland | 60° 10’ 04’’ | 25° 47’ 41’’ | 4 | 23 |
| FI-FHS2-11-8 | Haapasaari, Finland | 60° 16’ 26’’ | 27° 13’ 08’’ | 3 | 21 |
| FI-FUT1-2-1 | Ulko-Tammio, Finland | 60° 20’ 50’’ | 27° 28’ 44’’ | 19 | 21 |
| SE-G1-9b | east coast island, Sweden | 60° 25’ 18’’ | 18° 30’ 37’’ | 10 | 21 |
| RU-YAK1-1 | Yakutia, Russia | 61° 57’ 43" | 129° 39’ 51" | 101 | 25 |
| RU-BOL1-1 | Bolshoj Asafiy Island, White Sea, Russia | 66° 25’ 17" | 33° 50’ 55" | 3 | 17 |
| RU-KOR1-1 | Korablik Island, White Sea, Russia | 66° 25’ 52" | 33° 47’ 00" | 3 | 17 |
aClones used in short-term nutritional status assay.
bClones used in long-term nutritional status assay.
(a). General experimental procedure
Neonates from mass cultures of each of the 22 Daphnia clones were placed into 400 ml jars with ADAM water medium [49]. For each clone-by-acclimatization temperature treatment combination, 12 400 ml replicate jars were set up. Jars were stored in constant temperature incubators at two acclimatization temperatures (hereafter AccT), 20°C and 28°C. The 28°C treatment was chosen as the upper limit at which all clones continued to reproduce and complete a life cycle for at least two generations based on a pilot experiment. Water was replaced in the jars twice weekly, and 5 × 107 cells of chemostat grown green algae Scenedesmus sp. were supplied daily. Neonates produced in these acclimatization jars were collected within a 24 h window and transferred to fresh jars with 20 individuals per 400 ml jar. These individuals were used in the experiments as adults (females carrying their second–fourth clutch). Thus, the acclimatization to temperature period lasted for one whole generation. For each of the experimental blocks, we used only one animal per jar per experiment to avoid pseudo-replication.
The knockout time or time until immobilization (hereafter Timm) was measured as in [25]. Briefly, up to 3 h before Timm was assessed, individual adult females were each transferred into a 50 ml cell culture flask, containing medium at the AccT. To estimate Timm, all flasks were placed simultaneously into a water bath adjusted to 37°C with spatial randomization over clones and treatments. The water bath allowed us to monitor up to 46 animals in a single block. For experiments with more than 46 replicates, we performed a block design, with all treatment and clone combination being present in every block. The Daphnia's swimming activity was monitored every 1–2 min, starting at 20 min after the flasks were immersed into the water bath (i.e. approx. 10 min after the water inside the flasks reached 37°C). Timm was recorded as the time from first placing the flasks into the 37°C water bath until the time when the Daphnia lay with no movement visible to the naked eye on the bottom of the flask.
(b). Main experiment on local adaptation and adaptive plasticity
Our main experiment consisted of a series of 11 blocks to test for the effect of Daphnia clone (n = 22) and AccTs, resulting in a total of 484 measurements. We performed up to four blocks a day, with all blocks of an experiment run within a 4 day interval.
(c). Testing for the effect of potentially confounding factors
Keeping animals at two different temperatures is not possible without introducing confounding factors that covary with temperature. For example, at higher temperatures, animals have higher food demands, differ in body size at a given age, and vary in age and size at maturity. Therefore, we performed experiments to test for the influence of age and nutritional condition on Timm. We used only subsets of the 22 clones to adjust for availability of material and logistical limitations.
Feeding rate and caloric requirements of Daphnia are also strongly dependent on water temperature [50]. Furthermore, we may also have caused short-term food stress when transferring Daphnia from their acclimatization jars to the bottles in which we assessed Timm, as the animals received no food in the test bottles. Therefore, we conducted two experiments in which we manipulated food levels for a subset of three clones in each AccT treatment. Short-term nutritional status was manipulated by transferring acclimatized adult females to fresh water in 400 ml medium 8 h before their Timm assessment. They were then fed either 50 × 106 green algae cells or kept unfed. For long-term manipulation of the nutritional status, neonates from acclimatized cultures were collected, placed into fresh water and supplied with either 1 × 108 cells per day (high nutrition treatment) or 2.50 × 106 cells per day (low nutrition treatment) of the green algae Scenedesmus sp. Neonates produced in these different feeding and temperature acclimatization conditions were collected within a 24 h window, transferred to jars with fresh water and kept under the same food regimes. These individuals were then used in the experiment as adults.
(d). Haemoglobin concentration
Haemoglobin concentration is known to play a role in high temperature tolerance in Daphnia [42,43,51]. To quantify the contribution of haemoglobin expression on both acclimatization and geographical differences in Timm, we measured haemoglobin concentration in whole bodies of Daphnia from the same 22 clones acclimatized to the experimental temperatures. Single Daphnia (four replicates per clone per AccT) were homogenized on ice in 1.5 microcentrifuge vials containing 50 µl of Tris–HCl buffer, pH 7.2. After centrifuging the vials at 14 000 rpm for 6 min, we transferred 50 µl of supernatant from each vial into a 384-well plate and measured the absorbance spectrum on a Tecan Infinite M200 spectrophotometer from 230 to 660 nm at 2 nm using a 3 × 3 grid of beams, five flashes in each within 20 min after centrifugation. Tris–HCl buffer was used as the blank, and commercial haemoglobin samples as standards. Total protein concentration was measured with samples from the same supernatant using the Bradford method (Sigma-Aldrich).
From the recorded spectra, we calculated total haem-containing protein (globins, P450 cytochromes, peroxidases) concentration from absorbance at 414 nm and relative height of haemoglobin-specific peak at 576 nm as H576 = A576 – (A560 + A600)/2.
Peak height was then normalized by total protein concentration. We report here only the results obtained using relative peak height at 576 nm (H576), as results based on relative peak heights at 414 nm and 540 nm were, similar to those obtained with the 576 nm absorbance.
(e). Statistical analysis
Using temperature records from weather stations nearest to each Daphnia clones’ sites of origin, we calculated three proxies of climatic conditions likely to represent exposure to high temperatures in Daphnia clones’ recent evolutionary history: (i) average high temperature of the warmest month (AHT_warmest); (ii) average high temperature of the warmest month when Daphnia populations are planktonic; and (iii) the midpoint between average high and low temperatures of the months when Daphnia are planktonic. The choice of the proxy serving as the best predictor of time until immobilization was accomplished by the stepwise multiple regression platform in the JMP statistical package [52] with AccT as a covariable, using the ‘forward’ model building option and minimized corrected Akaike information criterion as the stopping rule. This analysis resulted in the inclusion of AHT_warmest effect into the model (p < 0.002) and exclusion of the two other proxies (p > 0.5 for each). In this and further analyses, AccT was treated as the fixed effect, AHT_warmest as a continuous covariable and Timm values were log-transformed to reach a normal distribution. Analysis of local adaptation assumes that populations are the units of replication and using multiple observations per clone would generate pseudo-replication. Therefore, we used the means of Timm per clone and treatment group for the analysis. Because AHT_warmest was chosen as the strongest effect of three correlated climatic proxies, a Bonferroni correction was applied to the p-value reported for this factor by multiplying the raw p-value by the factor of 3.
Feeding condition experiments conducted on subsets of clones were analysed in a similar manner, except that we used clones and their interactions as random effects in the initial analysis. These random effects were found to be non-significant (the lowest observed p > 0.15) and therefore were removed from the model, thus pooling the random variance associated with clones with the residual variance. Response variable Timm was log-transformed in all cases.
3. Results
(a). Main experiment on local adaptation and adaptive plasticity
Time until immobilization at 37°C (Timm) increased strongly with AccT (20°C versus 28°C; figure 1 and table 2). It also increased with AHT_warmest at the sites of origin of our D. magna clones (figure 1 and table 2). The linear model predicting Timm by AccT and AHT_warmest at the geographical origin of clones explain 81% of the total variance (table 2). Because the difference between the two AccTs (20°C versus 28°C) represent the range between approximately optimal and stressful temperatures for most clones studied, we believe that the magnitude of the AccT effect would not increase if additional, more extreme temperature treatments were added. Some of our clones do not reproduce at temperatures above 29°C.
Figure 1.
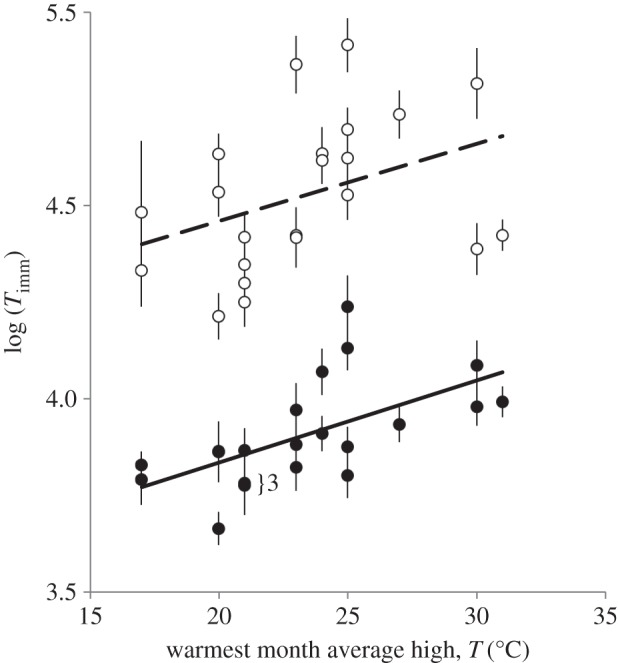
Time until immobilization (±1 s.e.; Timm, min., natural logarithm-transformed) as a function of average high temperature of the warmest month at the clone's site of origin and acclimatization temperature (open symbols, 28°C; filled symbols, 20°C).
Table 2.
General linear model of the effects of acclimatization temperature (AccT) on time until immobilization, Timm, using average high temperature of the warmest month of each clone's site of geographical origin (AHT_warmest) as a covariable. 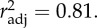
| source | d.f. | MS | F ratio | p |
|---|---|---|---|---|
| AccT | 1 | 4.2348 | 179.02 | 2.4 × 10−16 |
| AHT_warmest | 1 | 0.2620 | 11.08 | 0.0057a |
| AccT × AHT_warmest | 1 | 0.0002 | 0.0102 | 0.92 |
| error | 40 | 0.0237 |
aBonferroni factor of 3 applied to account for the choice of climatic proxy out of three correlated aggregates.
Among the three climate proxies tested, only the AHT_warmest was a significant predictor of temperature tolerance. Although correlated with AHT_warmest, the climate aggregates limited to the months when Daphnia are present in the plankton are not good predictors of temperature tolerance. Likewise, latitude of the site of Daphnia clone origin is not a strong predictor of temperature tolerance. If latitude were to be used instead of AHT_warmest in the analysis presented in table 2, then it would only approach significance.
(b). Testing for the effect of potentially confounding factors
Because temperature affects certain Daphnia life-history and physiological traits that may influence the experimental result for phenotypic plasticity, we tested for the effect of Daphnia nutritional condition at the time of the experiment. Short-term food availability (females fed versus unfed within 8 h of the experiment) had no effect on Timm (figure 2a and table 3). On the other hand, long-term nutritional manipulation had a significant effect on Timm: Daphnia reared on limited food had about 25–30% higher Timm than those reared on excess food (figure 2b and table 3). AccT was highly significant in both experiments, but in neither comparison was the feeding condition by AccT interaction significant. Given that the higher AccT caused some nutritional stress and that both factors (nutritional stress and high temperature) cause an increase in Timm, our estimate of the plastic response to higher AccT might be overestimated. However, given that the effect of long-term food stress was smaller than the overall effect observed for AccT (27.5% increase from low-to-high food versus 49.6% increase from low-to-high AccT in the same clones), we conclude that the AccT effect observed in our main experiments is only slightly overestimated by the interaction with long-term feeding conditions.
Figure 2.
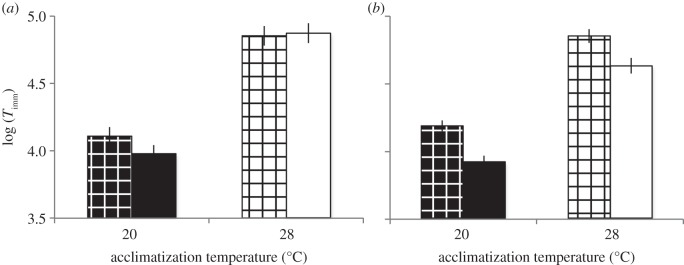
Time until immobilization (Timm) tested under short-term (8 h, (a)) and long-term (one generation, (b)) nutritional manipulation. Black/white symbols as in figure 1. Solid black or white, Daphnia fed at high food level; cross-hatched pattern, Daphnia fed limited food.
Table 3.
General linear mixed model for the effects of acclimatization temperature (AccT) and (a) short-term and (b) long-term nutritional manipulation. In all tests, the clone effect was not significant and was pooled with the residual variance.
| source | d.f. | MS | F | p |
|---|---|---|---|---|
(a) short-term nutritional (food) manipulation (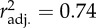 ) ) | ||||
| AccT | 1 | 8.033 | 138.9 | 3.3 × 10−15 |
| food | 1 | 0.036 | 0.62 | 0.43 |
| AccT × food | 1 | 0.067 | 1.15 | 0.29 |
| error | 44 | 0.058 | ||
(b) long-term nutritional (food) manipulation (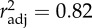 ) ) | ||||
| AccT | 1 | 5.648 | 195.4 | 8.6 × 10−18 |
| food | 1 | 0.708 | 24.5 | 1.1 × 10−5 |
| AccT × food | 1 | 0.006 | 0.201 | 0.65 |
| error | 44 | 0.29 | ||
(c). Haemoglobin concentration
Timm increased with haemoglobin absorption peak height across the two AccTs (figure 3a). This effect differed locally, as haemoglobin peak height correlated positively with AHT_warmest (figure 3b and table 4). Interestingly, the haemoglobin-specific absorption peak height showed a significant AHT_warmest by AccT interaction (figure 3b,c). In 28°C, clones from warmer climates showed a significantly higher degree of haemoglobin upregulation than clones from colder climates, whereas at 20°C, this effect was not observed (figure 3b).
Figure 3.
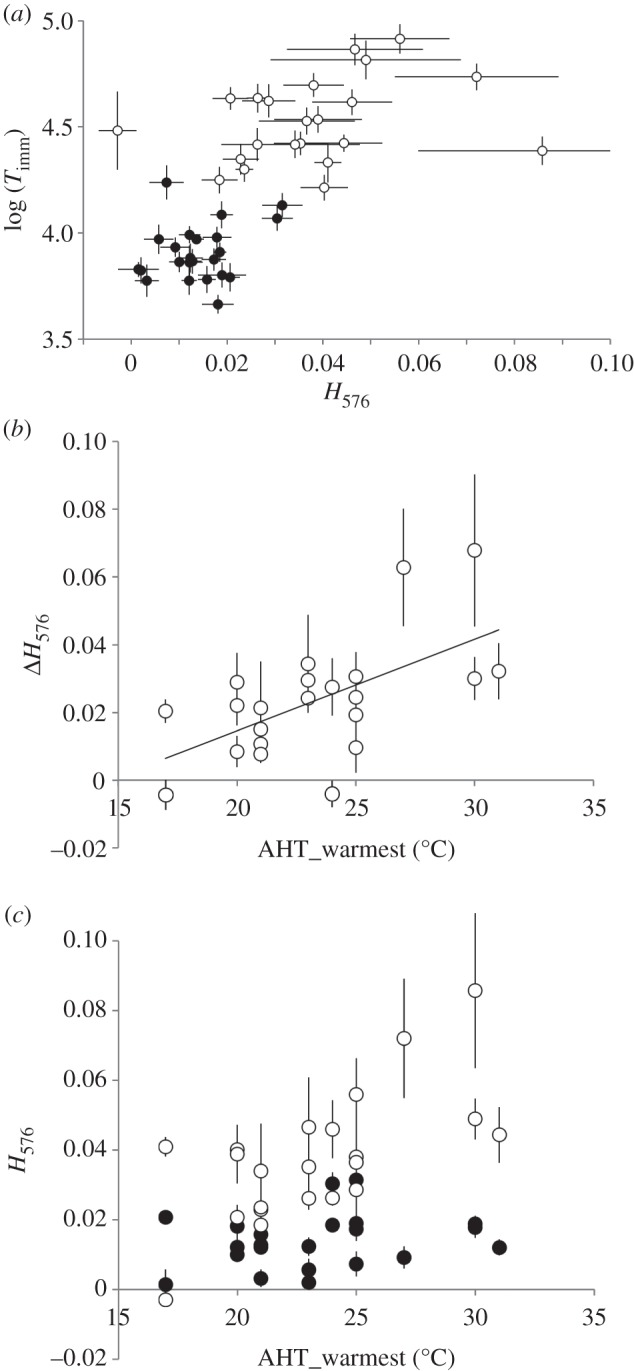
(a) Time until immobilization (Timm; ±1 s.e.) in Daphnia acclimatized to either 20°C (filled circles) or 28°C (open circles) plotted against the relative haemoglobin absorbance peak height at 576 nm (H576; ±1 s.e.). (b) The difference between the relative haemoglobin absorbance peak height at 576 nm in 28°C and in 20°C (ΔH576 = H57628–H57620) as a function of average high temperature of the warmest month at the clones’ site of origin. Bars are standard errors. Coefficient of correlation = 0.60, p < 0.0011. (Bonferroni factor of 3 applied to account for the choice of climatic proxy out of three correlated aggregates.) (c) Same data as on (b) plotted for each acclimatization temperature separately. Linear regression coefficients for 20°C and 28°C are 0.00049 (s.e. = 0.00045, p > 0.1) and 0.00319 (s.e. = 0.00081, p < 0008); heterogeneity of slopes p < 0.006.
Table 4.
The effect of acclimatization temperature (AccT) and average high monthly temperature of the warmest month at the clone's site of origin (AHT_warmest) on relative height of haemoglobin absorption peak at 576 nm (log-transformed). 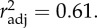
| Source | d.f. | MS | F | p |
|---|---|---|---|---|
| AccT | 1 | 0.0061 | 46.02 | 3.8 × 10−8 |
| AHT_warmest | 1 | 0.0021 | 15.70 | 0.0003 |
| AccT × AHT_warmest | 1 | 0.0011 | 8.46 | 0.018a |
| error | 40 | 0.00013 |
aThe p-value for AHT_warmest was Bonferroni corrected to account for the choice of climatic proxy out of three correlated aggregates.
4. Discussion
Our study found that the strongest factor influencing high temperature tolerance of Daphnia magna was AccT, which corroborates with previous results both for Daphnia [51,53,54] and other aquatic poikilotherms (for review, see [9,55]). We also found a strong signature of local adaptation, with clones from warmer climatic backgrounds having higher tolerance. There was no significant interaction between the effects of our climate proxy and of the AccT, indicating that the acclimatization effect did not differ systematically with the local temperature estimate (figure 1).
(a). Temperature tolerance shows local adaptation
In selection experiments, different temperature regimes can quickly shape genotype composition, life-history traits and temperature tolerance in Daphnia [56–58]. Because the geographical range of D. magna covers a broad span of climate conditions, we expected to find evidence for local adaptation to temperatures. Indeed, we observed that a high tolerance to temperature correlates with the average high air temperature of the warmest month at the site of clone origin, suggesting that D. magna from warmer climates are able to tolerate a lethal temperature longer than those from colder climates, even though all animals were pre-acclimatized in the laboratory to the same temperatures. Given the wide geographical range (in terms of latitude, longitude and elevation) from which our 22 genotypes were collected, it is unlikely that this pattern is caused by chance events (genetic drift, bottlenecks). Furthermore, D. magna is known to exhibit extensive gene flow and very little geographical structure across Europe [59], making it unlikely that temperature tolerance reflects common ancestry. Thus, we strongly believe that the described pattern of high temperature tolerance is the result of local natural selection.
It should be noted that we do not know which phenotype—heat tolerance or heat sensitivity—is ancestral in D. magna. In a geographically more restricted study [25] with North American Daphnia pulex, it was possible to use glacial history to conclude that cold-water-adapted, heat-sensitive genotypes represent the ancestral condition, but this approach is not possible for the current set of populations. It is therefore impossible to exclude a scenario implying the loss of tolerance in populations from the coldest habitats rather than gain of tolerance in those from the warmest habitats.
Our finding is consistent with the north–south difference in high temperature tolerance observed in North American D. pulex [25], although it is not fully consistent with other traits, such as growth and metabolic rates previously observed in clones of different latitudinal origins [24,25]. The likelihood of observing a latitudinal correlation of temperature tolerance probably depends strongly on the choice of the tolerance indicator and climatic proxy used. For example, Mitchell & Lampert [25] observed that southern clones have a higher growth rate at 20–23°C, but do not differ from the northern clones in the shape of growth rate reaction norm, which led them to conclude that among-population differences are not a manifestation of local adaptation. Chopelet et al. [24] found evidence of higher oxygen consumption by southern clones at higher temperatures and vice versa, but this correlation was evident for only one of the two southern locations they sampled. Thus, local thermal adaptation and its possible mechanisms have been elusive in Daphnia studies. Our study improved on previous attempts in two ways: we used locally recorded long-term averaged temperature data, which reflect local conditions better than latitude as a proxy for temperature. Second, we used a different variable to estimate high temperature tolerance: time to immobility at a temperature deadly in the longer run (37°C). Both these improvements are easy to implement and may be used in further studies.
Local adaptation to high temperature tolerance has been observed in a large number of studies, in particular in Drosophila, in which heat tolerance within a species has been repeatedly observed to negatively correlate with latitude and altitude [60–62] (see Schoville et al. [39] for a review). A similar pattern has been observed in a variety of other terrestrial and aquatic ectotherms, including crustaceans [39,63,64]. One pattern emerging from these studies is that short-term exposure measurements (lethal temperatures, knockout times) typically correlate with climate of populations’ origin better than long-term measurements such as thermal optima [65]. Our finding of a correlation between heat tolerance in an acute experiment and climate at the geographical origin of our clones is in good concordance with these previous studies.
Much attention has been given to the question of whether local adaptation to heat tolerance implies trade-offs causing poorer performance at lower temperatures. While such trade-offs have been observed in selection experiments [66,67], they seem to be too weak to shift the temperature optimum and to affect life-history traits at non-extreme temperatures more than tolerance to lethal ones [68]. It is difficult to test this trade-off hypothesis using nature-derived genotypes with completely different genetic backgrounds. However, the observed local adaptation in Daphnia opens the possibility for the study of trade-offs across extreme high and low temperatures, taking advantage of Daphnia's clonal mode of reproduction.
(b). Haemoglobin expression
Haemoglobin has been shown to play an important role in temperature acclimatization in Daphnia [42,43,51] and other aquatic organisms [69,70]. Our haemoglobin data are consistent with these studies. However, we uncovered a further effect: the upregulation of haemoglobins during acclimatization to higher temperature revealed significant genotypic variation (table 4 and figure 3) in which some clones acclimatized to 28°C did not respond by increasing haemoglobin, whereas others increased it three- to fourfold. The strength of this upregulation correlates positively with temperatures at the sites of origin (figure 3), suggesting that plasticity in upregulation of haemoglobin might be locally adapted. While our results observed in Daphnia clones from warmer climates corroborate previous findings on the role of haemoglobin upregulation in coping with high temperature for Daphnia [42,43,51,71], they raise a question about the nature of heat acclimatization in genotypes from cold environments, which to the best of our knowledge had previously not been tested. Daphnia magna from cold climates are able to acclimatize to high temperatures equally well as D. magna from warm climates, but without upregulation of haemoglobin. Thus, haemoglobin upregulation is not the only, or perhaps even not the most important, mechanism of heat acclimatization in this system, and there are apparently different mechanisms that Daphnia from different climatic regions use to differing degrees.
Why is high haemoglobin expression not constitutive in Daphnia? An obvious speculation is that haemoglobin synthesis is costly. Selection, therefore, should favour plasticity in haemoglobin expression in habitats with variable temperatures (i.e. in seasonal environments), and constitutive expression in more constant habitats (less seasonal environments). This is opposite of what we found. Northern populations undergo extreme seasonal fluctuations but show the least plasticity in haemoglobin expression in our study. It has also been suggested that the higher visibility of haemoglobin-rich Daphnia is costly in the presence of visual fish predators (cf. [72]), so that haemoglobin production should be avoided as much as possible in habitats with such predators. Again, this is not in line with our findings. Our northern Daphnia populations all come from fishless ponds, whereas the more southern populations differed widely in this respect. The costs of over-expressing haemoglobins at low temperatures may also include other factors than the direct metabolic costs of haemoglobin synthesis. For example, overexpression of haemoglobin may cause an oversupply of oxygen to tissues in normoxic conditions. Indeed, heat-acclimatized Daphnia were found to perform worse than cold-acclimatized ones at lower temperatures [51], but it is not clear if the higher haemoglobin level in the heat-acclimatized animals caused this result.
5. Conclusion
Thermal acclimatization and local adaptation are believed to determine the fate of species facing climatic change [9,73]. We demonstrated that high temperature tolerance in geographically diverse genotypes of D. magna shows evidence for local, climatically determined adaptation. Animals from warmer climates show tolerance, but also higher plasticity for haemoglobin upregulation. This raises questions about the mechanisms and costs of such adaptation and about the species’ ability to evolve to new conditions, which will be important for predicting the evolutionary response of plankton to rising temperatures.
Daphnia, an ecologically important component of freshwater plankton, seems to possess a sufficient safety zone for future adaptation to changing temperatures, as local selection appears to shape its temperature response rather well, operating both on plastic responses and on the mean ability to cope with temperature. Along with the well-documented upregulation of haemoglobin expression, Daphnia also seems to use other responses. These may include metabolic compensation [74,75] or membrane restructuring [76], which have been documented in other organisms, but are not yet well characterized in Daphnia. The further step in the studies of Daphnia thermal acclimatization may be addressing the question of whether the observed acclimatization changes incur a cost in terms of adjusting physiology and biochemistry or a cost of miss-acclimatization in the case of rapidly changing environment.
Acknowledgements
We thank Jürgen Hottinger for assistance in the laboratory.
Data accessibility
All data necessary to recreate the analysis reported here are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.sjzhv.
Funding statement
D.E. is supported by the Swiss National Science Foundation; D.E. and T.S. are supported by the ERC, and L.Y. is supported by NSF grant no. 1136706 and NSF/EDEN grant no. 0955517.
References
- 1.Sibly RM, Calow P. 1986. Physiological ecology of animals: an evolutionary approach, p. 179 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 2.Schmidt-Nielsen K. 1997. Animal physiology: adaptation and environment, p. 612 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692 (doi:10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw WE, Holzapfel CM. 2010. Light, time, and the physiology of biotic response to rapid climate change in animals. Annu. Rev. Physiol. 72, 147–166 (doi:10.1146/annurev-physiol-021909-135837) [DOI] [PubMed] [Google Scholar]
- 5.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 6.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture, p. 306 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 7.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241 (doi:10.1111/j.1461-0248.2004.00684.x) [Google Scholar]
- 8.Hoffmann A, Willi Y. 2008. Detecting genetic responses to environmental change. Nat. Rev. Genet. 9, 421–432 (doi:10.1038/nrg2339) [DOI] [PubMed] [Google Scholar]
- 9.Somero GN. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine 'winners’ and 'losers’. J. Exp. Biol. 213, 912–920 (doi:10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 10.Barrett RD, Paccard A, Healy TM, Bergek S, Schulte PM, Schluter D, Rogers SM. 2011. Rapid evolution of cold tolerance in stickleback. Proc. R. Soc. B 278, 233–238 (doi:10.1098/rspb.2010.0923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas MK, Kremer CT, Klausmeier CA, Litchman E. 2012. A global pattern of thermal adaptation in marine phytoplankton. Science 338, 1085–1088 (doi:10.1126/science.1224836) [DOI] [PubMed] [Google Scholar]
- 12.Calosi P, Bilton DT, Spicer JI. 2008. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 4, 99–102 (doi:10.1098/rsbl.2007.0408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen LF, Hansen MM, Pertoldi C, Holdensgaard G, Mensberg KL, Loeschcke V. 2008. Local adaptation in brown trout early life-history traits: implications for climate change adaptability. Proc. R. Soc. B 275, 2859–2868 (doi:10.1098/rspb.2008.0870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottin D, Roussel D, Foucreau N, Hervant F, Piscart C. 2012. Disentangling the effects of local and regional factors on the thermal tolerance of freshwater crustaceans. Naturwissenschaften 99, 259–264 (doi:10.1007/s00114-012-0894-4) [DOI] [PubMed] [Google Scholar]
- 15.Dam HG. 2012. Evolutionary adaptation of marine zooplankton to global change. Annu. Rev. Mar. Sci. 5, 349–370 (doi:10.1146/annurev-marine-121211-172229) [DOI] [PubMed] [Google Scholar]
- 16.Kelly MW, Sanford E, Grosberg RK. 2012. Limited potential for adaptation to climate change in a broadly distributed marine crustacean. Proc. R. Soc. B 279, 349–356 (doi:10.1098/rspb.2011.0542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulgar JM, Bozinovic F, Ojeda FP. 2005. Local distribution and thermal ecology of two intertidal fishes. Oecologia 142, 511–320 (doi:10.1007/s00442-004-1755-4) [DOI] [PubMed] [Google Scholar]
- 18.Bedulina DS, Zimmer M, Timofeyev MA. 2010. Sub-littoral and supra-littoral amphipods respond differently to acute thermal stress. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 155, 413–418 (doi:10.1016/j.cbpb.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 19.Richardson A. 2008. In hot water: zooplankton and climate change. ICES J. Mar. Sci. Technol. 65, 279–295 (doi:10.1093/icesjms/fsn028) [Google Scholar]
- 20.Daufresne M, Lengfellner K, Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12 788–12 793 (doi:10.1073/pnas.0902080106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forster J, Hirst AG, Woodward G. 2011. Growth and development rates have different thermal responses. Am. Nat. 178, 668–678 (doi:10.1086/662174) [DOI] [PubMed] [Google Scholar]
- 22.Mitchell SE, Lampert W. 2000. Temperature adaptation in a geographically widespread zooplankter, Daphnia magna. J. Evol. Biol. 13, 371–382 (doi:10.1046/j.1420-9101.2000.00193.x) [Google Scholar]
- 23.Wojewodzic MW, Kyle M, Elser JJ, Hessen DO, Andersen T. 2011. Joint effect of phosphorus limitation and temperature on alkaline phosphatase activity and somatic growth in Daphnia magna. Oecologia 165, 837–846 (doi:10.1007/s00442-010-1863-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopelet J, Blier PU, Dufresne F. 2008. Plasticity of growth rate and metabolism in Daphnia magna populations from different thermal habitats. J. Exp. Zool. A, Ecol. Genet. Physiol. 309, 553–562 (doi:10.1002/jez.488) [DOI] [PubMed] [Google Scholar]
- 25.Williams P, Dick KB, Yampolsky LY. 2012. Heat tolerance, temperature acclimation and canalization of haemoglobin expression in Daphnia. Evol. Ecol. 26, 591–609 (doi:10.1007/s10682-011-9506-6) [Google Scholar]
- 26.Benzie JAH. 2005. Cladocera, the genus Daphnia (including Daphniopsis). Leiden, The Netherlands: Backhuys Publishers [Google Scholar]
- 27.Schmidt PS, et al. 2008. Ecological genetics in the North Atlantic: environmental gradients and adaptation at specific loci. Ecology 89, S91–S107 (doi:10.1890/07-1162.1) [DOI] [PubMed] [Google Scholar]
- 28.Nilsson-Ortman V, Stoks R, De Block M, Johansson F. 2012. Generalists and specialists along a latitudinal transect: patterns of thermal adaptation in six species of damselflies. Ecology 93, 1340–1352 (doi:10.1890/11-1910.1) [DOI] [PubMed] [Google Scholar]
- 29.Pitchers W, Pool JE, Dworkin I. 2012. Altitudinal clinal variation in wing size and shape in African Drosophila melanogaster: one cline or many? Evolution 67, 438–452 (doi:10.1111/j.1558-5646.2012.01774.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajunen VI, Pajunen I. 2003. Long-term dynamics in rock pool Daphnia metapopulations. Ecography 26, 731–738 (doi:10.1111/j.0906-7590.2003.03542.x) [Google Scholar]
- 31.Dufresne F, Marková S, Vergilino R, Ventura M, Kotlík P. 2011. Diversity in the reproductive modes of European Daphnia pulicaria deviates from the geographical parthenogenesis. PLoS ONE 6, e20049 (doi:10.1371/journal.pone.0020049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goren L, Ben-Ami F. 2012. Ecological correlates between cladocerans and their endoparasites from permanent and rain pools: patterns in community composition and diversity. Hydrobiologia 701, 13–23 (doi:10.1007/s10750-012-1243-5) [Google Scholar]
- 33.Roulin AC, Routtu J, Hall MD, Janicke T, Colson I, Haag C, Ebert D. 2013. Local adaptation of sex-induction in a facultative sexual crustacean: insights from QTL mapping and natural population of Daphnia magna. Mol. Ecol. 22, 3567–3579 (doi:10.1111/mec.12308) [DOI] [PubMed] [Google Scholar]
- 34.Boersma M, De Meester L, Spaak P. 1999. Environmental stress and local adaptation in Daphnia magna. Limnol. Oceanogr. 44, 393–402 (doi:10.4319/lo.1999.44.2.0393) [Google Scholar]
- 35.Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F. 2001. Rapid, local adaptation of zooplankton behaviour to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA 98, 6256–6260 (doi:10.1073/pnas.111606798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeClerck S, Cousyn C, De Meester L. 2001. Evidence for local adaptation in neighbouring Daphnia populations: a laboratory transplant experiment. Freshw. Biol. 46, 187–198 (doi:10.1046/j.1365-2427.2001.00632.x) [Google Scholar]
- 37.Jansen M, De Meester L, Cielen A, Buser CC, Stoks R. 2011. The interplay of past and current stress exposure on the water flea Daphnia. Funct. Ecol. 25, 974–982 (doi:10.1111/j.1365-2435.2011.01869.x) [Google Scholar]
- 38.Lockwood BL, Somero GN. 2011. Invasive and native blue mussels (genus Mytilus) on the California coast: the role of physiology in a biological invasion. J. Exp. Mar. Biol. Ecol. 400, 167–174 (doi:10.1016/j.jembe.2011.02.022) [Google Scholar]
- 39.Schoville SD, Barreto FS, Moy GW, Wolff A, Burton RS. 2012. Investigating the molecular basis of local adaptation to thermal stress: population differences in gene expression across the transcriptome of the copepod Tigriopus californicus . BMC Evol. Biol. 12, 170 (doi:10.1186/1471-2148-12-170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi M, Hoshi T. 1984. Analysis of respiratory role of haemoglobin in Daphnia magna. Zool. Sci. 1, 523–532 [Google Scholar]
- 41.Kimura S, Tokishita SI, Ohta T, Kobayashi M, Kobayashi M, Yamagata H. 1999. Heterogeneity and differential expression under hypoxia of two-domain hemoglobin chains in the water flea, Daphnia magna. J. Biol. Chem. 274, 10 649–10 653 (doi:10.1074/jbc.274.15.10649) [DOI] [PubMed] [Google Scholar]
- 42.Pirow R, Bäumer C, Paul RJ. 2001. Benefits of haemoglobin in the cladoceran crustacean Daphnia magna. J. Exp. Biol. 204, 3425–3441 [DOI] [PubMed] [Google Scholar]
- 43.Seidl MD, Pirow R, Paul RJ. 2005. Acclimation of the micro-crustacean Daphnia magna to warm temperatures is dependent on haemoglobin expression. J. Therm. Biol. 30, 532–544 (doi:10.1016/j.jtherbio.2005.06.004) [Google Scholar]
- 44.Zeis B, et al. 2009. Acclimatory responses of the Daphnia pulex proteome to environmental changes. I. Chronic exposure to hypoxia affects the oxygen transport system and carbohydrate metabolism. BMC Physiol. 9, 7 (doi:10.1186/1472-6793-9-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Park Y, Choi K. 2009. Phototoxicity and oxidative stress responses in Daphnia magna under exposure to sulfathiazole and environmental level ultraviolet B irradiation. Aquat. Toxicol. 91, 87–94 (doi:10.1016/j.aquatox.2008.10.006) [DOI] [PubMed] [Google Scholar]
- 46.Woltereck R. 1909. Weitere experimentelle Untersuchungen über Artverinderung, speziell über das Wesen quantitativer Artunterschiede bei Daphniden. Verhand Deut Zool Ges 1909, 110–172 [Google Scholar]
- 47.Ebert D, Yampolsky LY, van Noordwijk AJ. 1993. Genetics of life-history traits in Daphnia magna. 2. Phenotypic plasticity. Heredity 70, 344–352 (doi:10.1038/hdy.1993.49) [Google Scholar]
- 48.De Meester L. 1993. Genotype, fish-mediated chemicals, and phototactic behavior in Daphnia magna. Ecology 74, 1467–1474 (doi:10.2307/1940075) [Google Scholar]
- 49.Kluettgen B, Duelmer U, Engels M, Ratte HT. 1994. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 28, 743–746 (doi:10.1016/0043-1354(94)90157-0) [Google Scholar]
- 50.Mourelatos S, Lacroix G. 1990. In situ filtering rates of Cladocera: effect of body length, temperature, and food concentration. Limnol. Oceanogr. 35, 1101–1111 (doi:10.4319/lo.1990.35.5.1101) [Google Scholar]
- 51.Paul RJ, Lamkemeyer T, Maurer J, Pinkhaus O, Pirow R, Seidl M, Zeis B. 2004. Thermal acclimation in the microcrustacean Daphnia: a survey of behavioural, physiological and biochemical mechanisms. J. Therm. Biol. 29, 655–662 (doi:10.1016/j.jtherbio.2004.08.035) [Google Scholar]
- 52.SAS Institute Inc 2010. JMP 9. Cary, NC: SAS Institute Inc [Google Scholar]
- 53.MacIsaac HJ, Hebert PDN, Schwartz SS. 1985. Inter- and intraspecific variation in acute thermal tolerance of Daphnia. Physiol. Zool. 58, 350–355 [Google Scholar]
- 54.Zeis B, Maurer J, Pinkhaus O, Bongartz E, Paul RJ. 2004. A swimming activity assay shows that the thermal tolerance of Daphnia magna is influenced by temperature acclimation. Can. J. Zool. 82, 1605–1613 (doi:10.1139/z04-141) [Google Scholar]
- 55.Pörtner HO. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A 132, 739–761 (doi:10.1016/S1095-6433(02)00045-4) [DOI] [PubMed] [Google Scholar]
- 56.Scheiner SM, Yampolsky LY. 1998. The evolution of Daphnia pulex in temporally varying environment. Genet. Res. 72, 25–37 (doi:10.1017/S0016672398003322) [Google Scholar]
- 57.Van Doorslaer W, Stoks R, Duvivier C, Bednarska A, De Meester L. 2009. Population dynamics determine genetic adaptation to temperature in Daphnia. Evolution 63, 1867–1878 (doi:10.1111/j.1558-5646.2009.00679.x) [DOI] [PubMed] [Google Scholar]
- 58.Van Doorslaer W, Stoks R, Swillen I, Feuchtmayr H, Atkinson D, Moss B, De Meester . 2010. Experimental thermal microevolution in community-embedded Daphnia populations . Clim. Res. 43, 81–89 (doi:10.3354/cr00894) [Google Scholar]
- 59.De Gelas K, De Meester L. 2005. Phylogeography of Daphnia magna in Europe. Mol. Ecol. 14, 753–764 (doi:10.1111/j.1365-294X.2004.02434.x) [DOI] [PubMed] [Google Scholar]
- 60.Sorensen JG, Norry FM, Scannapieco AC, Loeschcke V. 2005. Altitudinal variation for stress resistance traits and thermal adaptation in adult Drosophila buzzatii from the New World. J. Evol. Biol. 18, 829–837 (doi:10.1111/j.1420-9101.2004.00876.x) [DOI] [PubMed] [Google Scholar]
- 61.Hoffmann AA, Anderson A, Hallas R. 2002. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol. Lett. 5, 614–618 (doi:10.1046/j.1461-0248.2002.00367.x) [Google Scholar]
- 62.Sgro CM, Overgaard J, Kristensen TN, Mitchell KA, Cockerell FE, Hoffmann AA. 2010. A comprehensive assessment of geographic variation in heat tolerance and hardening capacity in populations of Drosophila melanogaster from eastern Australia. J. Evol. Biol. 23, 2484–2493 (doi:10.1111/j.1420-9101.2010.02110.x) [DOI] [PubMed] [Google Scholar]
- 63.Gaston KJ, Spicer JI. 1998. Do upper thermal tolerances differ in geographically separated populations of the beachflea Orchestia gammarellus (Crustacea: Amphipoda)? J. Exp. Mar. Biol. Ecol. 22, 265–276 (doi:10.1016/S0022-0981(98)00057-4) [Google Scholar]
- 64.Stillman JH, Somero GN. 2000. A comparative analysis of the upper thermal tolerance limits of eastern Pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol. Biochem. Zool. 73, 200–208 (doi:10.1086/316738) [DOI] [PubMed] [Google Scholar]
- 65.Angilletta MJ. 2009. Thermal adpatation. A theoretical and empirical synthesis. Oxford, UK: Oxford University Press [Google Scholar]
- 66.Huey RB, Partridge L, Fowler K. 1991. Thermal sensitivity of Drosophila melanogaster responds rapidly to laboratory natural selection. Evolution 45, 751–756 (doi:10.2307/2409925) [DOI] [PubMed] [Google Scholar]
- 67.James AC, Partridge L. 1995. Thermal evolution of rate of larval development in Drosophila melanogaster in laboratory and field populations. J. Evol. Biol. 8, 315–330 (doi:10.1046/j.1420-9101.1995.8030315.x) [Google Scholar]
- 68.Gilchrist GW, Huey RB, Partridge L. 1997. Thermal sensitivity of Drosophila melanogaster: evolutionary responses of adults and eggs to laboratory natural selection at different temperatures. Physiol. Zool. 70, 403–414 (doi:10.1086/515853) [DOI] [PubMed] [Google Scholar]
- 69.Wittmann AC, Schroeer M, Bock C, Steeger HU, Paul RJ, Pörtner HO. 2008. Indicators of oxygen- and capacity-limited thermal tolerance in the lugworm Arenicola marina. Clim. Res. 37, 227–240 (doi:10.3354/cr00763) [Google Scholar]
- 70.Beers JM, Sidell BD. 2011. Thermal tolerance of Antarctic notothenioid fishes correlates with level of circulating haemoglobin. Physiol. Biochem. Zool. 84, 353–362 (doi:10.1086/660191) [DOI] [PubMed] [Google Scholar]
- 71.Zeis B, Becker D, Gerke P, Koch M, Paul RJ. 2013. Hypoxia-inducible haemoglobins of Daphnia pulex and their role in the response to acute and chronic temperature increase. Biochim. Biophys. Acta Proteins Proteomics 1834, 1704–1710 (doi:10.1016/j.bbapap.2013.01.036) [DOI] [PubMed] [Google Scholar]
- 72.Tollrian R, Heibl C. 2004. Phenotypic plasticity in pigmentation in Daphnia induced by UV radiation and fish kairomones. Funct. Ecol. 18, 497–502 (doi:10.1111/j.0269-8463.2004.00870.x) [Google Scholar]
- 73.Stoks R, Geerts AN, De Meester L. In press. Evolutionary and plastic responses of freshwater invertebrates to climate change: realized patterns and future potential. Evol. Appl. (doi:10.1111/eva.12108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bullock TH. 1955. Compensation for temperature in the metabolism and activity of poikilotherms. Biol. Rev. 30, 311–342 (doi:10.1111/j.1469-185X.1955.tb01211.x) [Google Scholar]
- 75.Hochachka PW, Somero GN. 2002. Biochemical adaptation. Oxford, UK: Oxford University Press [Google Scholar]
- 76.Seebacher F, Brand MD, Else P, Guderley H, Hulbert AJ, Moyes CD. 2010. Plasticity of oxidative metabolism in variable climates: molecular mechanisms. Physiol. Biochem. Zool. 83, 721–732 (doi:10.1086/649964) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary to recreate the analysis reported here are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.sjzhv.


