Abstract
Controversy over the adaptive significance of male hunting in subsistence societies hinges on the relative importance of familial provisioning and mate-quality signalling. This paper examines the proximate and ultimate motivations of hunting behaviour from a neuroendocrine perspective, using salivary testosterone and cortisol data collected before, during and after hunting focal follows from 31 Tsimane hunters aged 18–82 years. Despite circadian declines in hormone levels, testosterone and cortisol of Tsimane hunters increased at the time of a kill, and remained high as successful hunters returned home. Previous studies of hormonal changes during competitions find that high-stakes and success in the presence of relevant audiences result in increased neuroendocrine arousal. If men hunt primarily to provision their families, then an additional audience would not be expected to impact testosterone or cortisol, nor would the size of the animal killed. However, if signalling male quality by ‘showing off’ was a larger relative driver of men's hunting behaviour, one would expect greater hormonal response in cases where men returned with large sharable kills, especially in the presence of community members. Consistent with provisioning models of male hunting motivation, neither kill size nor encountering an audience of villagers while returning from hunting was associated with hormonal changes for successful hunters.
Keywords: Tsimane, hunting, testosterone, cortisol, household-provisioning, costly signalling
1. Introduction
Hunting has been an integral part of human provisioning strategies for hundreds of thousands of years [1]. Hunting success is positively associated with reproductive success across subsistence societies [2–6], including the Tsimane of South America [7]. This pattern is repeated across varied environments, applying to both terrestrial and marine fauna [8,9]. The reproductive success-linked benefits of successful hunting can include increased social status, sharing and support networks, and coalitional memberships; all of which can increase flow of resources to the family as well as result in increased mating opportunities [3,7,8].
Despite the general importance of resource productivity on male reproductive success and on female mate choice [8,10,11], much of the human behavioural endocrinology literature has focused on hormone–behaviour interactions related to other aspects of mate competition, such as direct competition and aggression [12]. When examining acute changes in testosterone during competition, studies generally rely on the ‘challenge hypothesis’ as a framework to understand variation in male testosterone in various reproductive contexts, suggesting a trade-off between the reproductive benefits of higher testosterone, and the side effects of elevated testosterone such as energetic costs and compromising immune function [13]. While this framework has been applied to a number of taxa [14], studies of humans have largely focused on acute increases in testosterone during male–male competition [12] and long-term downregulation of testosterone in relation to fatherhood [15].
Acute increases in cortisol and catecholamines during stress responses can be analysed with the same life-history trade-off framework; acute increases in cortisol can have metabolic and immune benefits, but long-term elevations can have damaging consequences [16,17]. Long-term hypothalamic–pituitary–adrenal axis (HPA) activation can downregulate immune function, increase risk of hypertension and cardiovascular disease, and impair hypothalamic–pituitary–gonadal (HPG) function under some conditions (e.g. [17]). While long-term glucocorticoid exposure can result in decreased testicular testosterone production, testosterone and cortisol often increase together during the first hour following an acute stress or challenge [18]. This interplay between the HPA and HPG axes has stimulated several hypotheses regarding the effects of cortisol and other neuroendocrine markers on testosterone [18,19].
Testosterone and cortisol increase acutely during competitive interactions [12], and in response to heavy physical activity, especially strength-based muscular stress [20,21], including among subsistence populations with high parasite and pathogen loads like the Tsimane [22,23]. Acute increases in testosterone benefit muscle performance, immediately increasing sugar uptake [24,25]. Acute increases in cortisol benefit energy mobilization, disperse immune cells to peripheral tissues and modify memory formation [16]. While increases in these hormones occur during most physical competitions, winning a competitive interaction is often associated with an additional spike in testosterone [26,27], though results with respect to changes in cortisol are mixed [27]. The challenge need not be physical in nature to produce an increase in testosterone following a victory; even chess [28] can result in increased testosterone.
The effect of winning on testosterone and cortisol has not been well characterized in non-industrialized human populations, nor has this relationship been measured under conditions directly associated with increased reproductive success. Hunting offers a socially important activity where success is associated with reproductive fitness among the Tsimane [7]. For hunters, there are two potential opportunities to show signs of a winner effect; when the hunter kills an animal as well as when the hunter returns to his home community and their success is visible to a wider audience. Acute increases in testosterone and cortisol at the time of prey encounter would benefit immediate muscle response and hunting ability [24,25], while increases in testosterone following a successful hunt could modify androgen sensitivity [29] and promote androgenic reward reinforcement [30], potentially resulting in increased future hunting effort, as would be the case for other competitive behavioural strategies [27,31]. These biosocial linkages may help explain the underlying biology facilitating greater resource production, leading to the higher status or reproductive success seen among better hunters [8].
Although associations between reproductive benefits and hunting ability are nearly universal, it is still debated whether fitness benefits, and by implication selective advantages of hunting effort, are due more to familial provisioning or to increased mating success. The familial provisioning hypothesis is based on the premise that meat men acquire is preferentially allocated directly to their families, or indirectly when meat shared with others is returned via reciprocity. Successful hunters would then be expected to marry earlier (being more attractive as a husband), reproduce earlier and have higher reproductive success with their wife or wives through increased fertility and/or offspring survival [7,32]. The mating effort hypothesis, in turn, is based on the premise that meat is diverted to women other than spouses and results in extra-pair copulations [33].
Tsimane men hunt on average twice per week with approximately 61% of these hunts resulting in a kill [34]; hunted game makes up approximately 17% of the Tsimane diet [35]. There is an inherent level of risk in hunting, in terms of the relatively high failure rate, variance in hunting returns and physical danger (e.g. [36]). The animals hunted are often too large to consume in one sitting and are frequently shared with unrelated community members. Given the riskiness of hunting failure and the high demand for meat and its potential exchange value for obtaining other goods and services, questions have been raised as to the motivations behind male hunting. While the goal of hunting is to return with meat, the types of prey items and the skills required to capture prey vary widely. A typical Tsimane hunt involves following a hunting trail into the deep forest, encountering animal signs (e.g. tracks, scat), following those signs until an animal is encountered (seen or heard) and then stalking and killing the animal. If the animal is not killed immediately, then an injured animal may need to be tracked. Some smaller animals, like tortoise, can be gathered when they cross the hunter's path, and do not require any hunting technology or skill. From a behavioural and physiological standpoint, picking up a tortoise is quite different from stalking and killing a peccary, in terms of skill, physical activity and excitement. Because hunters return to their village carrying any game they acquired, community members have the opportunity to appraise the skill of a hunter every time they return.
Several hypotheses have been proposed to explain the benefits of hunting to hunters. The ‘show-off’ hypothesis [33] suggests that hunters gain positive social attention from returning with meat, which is then a widely shared public good. Costly signalling hypotheses argue that successful hunting signals male quality, and receivers of this signal gain honest information about the hunter beyond just the shares of meat [5,37,38]. Men vary in condition, which can include health status, skill and less tangible phenotypic qualities; hunters in better condition pay reduced marginal costs in terms of time and energy needed to hunt the same animals. The visibility (broadcast efficiency) of returning with a kill is high, therefore, hunting can serve as an honest signal of underlying qualities to competitors, allies and potential mates [5,6,39]. Finally, the family provisioning hypothesis posits that food shared with other families results in reciprocal return of meat, ultimately benefiting men's wives and children. [11,36,39–42].
Although time-allocation, hunting returns, interviews, focal follows and various survey methodologies have been implemented in studies of the adaptive significance of hunting [8,36], no studies have measured changes in cortisol, and only one study has examined changes in testosterone during hunting [43], although a small sample size (n = 6), and potential confounding from energetic availability and physical activity preclude strong inferences. Hormone–behaviour interactions offer a different vantage point to examine the role of signalling and provisioning in motivating hunting behaviour. Acute neuroendocrine changes have been used to examine male behaviour in many vertebrate species, including primates and humans [12,14]. Increases in testosterone and cortisol levels in individuals during competitive tasks offer an objective measure of the underlying levels of physiological and often psychological arousal; studies find that individuals with a greater vested interest in a competitive activity tend to express larger increases in testosterone during the course of a competitive encounter [44], especially during high-stakes competition [12,45]. If male hunting is primarily motivated by the potential to use hunting prowess to signal underlying quality to competitors, allies and mates, then one would expect that situations where broadcast efficiency was higher (e.g. returning to a larger audience), or where a large kill could be shared with other community members, would result in larger hormonal increases.
One set of predictions of the above hypotheses are related to physical effort and the winner effect. The physical demands of hunting are expected to increase testosterone and cortisol levels. We thus predict that testosterone and cortisol would be higher after 3 h of hunting than at baseline prior to the hunt (though circadian declines and sustained aerobic exercise can result in reduced testosterone and cortisol [46]; table 1, P1). To determine whether there is an additive winner effect, the following two predictions were tested: testosterone and cortisol will increase more when men kill prey than when they do not (P2); and both hormones will remain elevated as men return home with the meat they killed (P3).
Table 1.
Changes in testosterone (T) and cortisol (C) levels predicted by the provisioning and mate value signalling hypotheses.
| hypotheses | physical activity (P1) | at the time of the kill (P2) | returning home with self-killed meat (P3) | returning home with meat that was gathered, or killed by other hunter (P4) | audience at return (P5) | larger kill (‘show-off’) (P6) |
|---|---|---|---|---|---|---|
| provisioning | increased T and C | increased T and C | increased T and C | increased T and C | no change in T or C | no change in T or C |
| signalling mate value | no change in T or C | increased T and C | increased T and C |
A secondary set of predictions distinguishes the provisioning and mate value signalling models (table 1). When men return with gathered meat (e.g. turtle), or meat killed by another hunter, signalling value should be low, though hunters can still provision their family. Provisioning models would predict that men who return with any meat, even if killed by another hunter, should exhibit increases in cortisol and testosterone, whereas signalling models would only predict increases in testosterone and cortisol when hunters kill an animal themselves (P4). If the underpinnings of hunting motivation stem from signalling male quality, then hunters with higher broadcast efficiency, as measured by directly encountering larger audiences on their return to the village, will experience larger increases in physiological arousal, as measured by testosterone and cortisol (P5). A provisioning model would not predict audience-mediated hormonal differences; hunters returning with any meat are expected to present increases in cortisol and testosterone, regardless of the audience present. The ‘show-off’ hypothesis also predicts that men who return with large animals that could be shared with community members beyond the nuclear family will have increased testosterone or cortisol (P6). The provisioning hypothesis predicts a weaker effect of kill size since both small and large kills contribute meat to the family, though increased kill size could result in greater reciprocal return of meat in the future.
2. Material and methods
From August to October 2011, 31 Tsimane men provided saliva specimens before, during and immediately following single-day hunts in lowland Bolivia. All participants provided initial specimens early in the morning shortly after waking and before leaving their house, a second specimen after 3 h of hunting for use as a physically active baseline and a final specimen 10 min after returning home. Specimens were also collected in the event that the hunter used a firearm, 10–15 min after firing a shot (whether resulting in a kill or a miss). Previous studies report diminished hormonal response to continued stimuli [47], thus specimens were only collected in the first instance of a miss and first instance of a kill in cases where the hunter made multiple kills. ActiTrainer tri-axial accelerometer and heart rate monitors (Pensacola, FL, USA) were attached to participants before they began their hunts, and men were asked what animal they would prefer to encounter that day. Hunters were followed by the lead author and Tsimane field assistant until their return home. Prior to re-entering the community following the hunt, all hunters were asked whether they were pleased with the results of their hunt and whether they would have been happier to encounter the animal they noted as being preferential prior to the hunt. All family, community members or other individuals who witnessed the hunter returning were noted.
Complete heart rate measurements were available in 21 cases. In 10 cases, the heart rate monitor shifted because of the physical activity of hunting, resulting in incomplete heart rate data. During each hunt, identical lunches were provided (canned fish and crackers) to control energetic intake.
Saliva was frozen in liquid nitrogen immediately upon returning to camp. At the end of the study, specimens were shipped on dry ice to the University of Washington and assayed for salivary testosterone and then shipped on dry ice to the University of California, Santa Barbara, where cortisol was run [48,49]. Specimens were thawed and centrifuged (1500×g) for 20 min, and the aqueous layer aliquoted for assay. All specimens had gone through two freeze–thaw cycles when initially assayed for testosterone. Specimens were run in duplicate, with each participant's samples run on the same plate to reduce bias due to inter-plate variation. Individuals were randomized between plates to ensure that successful and unsuccessful hunters were evenly mixed across plates. The within and between assay coefficients of variation (CVs) for testosterone (n = 4 plates) were 5.2 and 7.2% for the low (292.5 pg ml−1) and 6.7 and 9.3% for the high (696.8 pg ml−1) controls, respectively. For cortisol (n = 4 plates), the CV were 11.9 and 2.3% for the low (96.2 pg ml−1) and 7.6 and 3.2% for the high (920.1 pg ml−1), respectively. All participants provided informed consent and all procedures were approved by the University of Washington Internal Review Board.
(a). Statistical methods
Salivary hormone values were log transformed for normality before statistical modelling. Linear mixed effects models were used to examine absolute changes in log testosterone and cortisol at up to five different time points: (i) before leaving on a single-day hunt (baseline), (ii) after 3 h of hunting, (iii) if a shot missed, (iv) if a kill was made and (v) upon returning home. Individuals were modelled as random effects to control for non-independence of multiple specimens collected from the same participant. Linear regression models examined per cent change in cortisol and testosterone. Previous studies report an inverted U-shaped association between age and hunting success [34]; logistic regressions examining the probability of success varied parabolically by age in this sample (p = 0.003), and thus age2 was included as a covariate. Testosterone and cortisol follow diurnal rhythms with peak concentrations at waking and decreased levels over the course of the day; thus hours since the beginning of the hunt were used as a proxy to control for diurnal variation in hormones as all hunters left early in the morning. Log-likelihood ratio tests and Akaike's information criteria were used to determine the inclusion of covariates; all models included body mass index (BMI), age2 and time hunting as covariates.
3. Results
All participants left shortly after waking between 5.28 and 9.11, and returned between 9.03 and 19.40, for a mean ± s.d. hunting time of 8.4 ± 2.8 h (range 2.8–13.1 h). The mean distance travelled was 17.9 ± 5.7 km (range 6.0–27.5 km). The mean age of the hunters was 37.8 ± 15.1 years (range 18–82). These men reported hunting an average of 1.3 ± 0.5 times per week, although the median time since last hunt was 14 days (range 1–140 days). All of the hunters were married and two men were polygynous. All men but one had dependent offspring. On six of the 31 hunts, there was a second hunter present, either the man's son (n = 5) or cousin (n = 1). At least one animal was killed on 61% (19/31) of the hunts. There were 22 cases where a hunter returned with meat: 18 subjects killed an animal, in three cases the second hunter present killed an animal, and in three cases hunters captured a tortoise without using a firearm. There were 10 cases where men killed an animal large enough to be shared with others (peccary, gray-brocket deer), though post-hunt food sharing data were not collected. Three men did not provide a 3-h control specimen because they made a kill before the 3 h mark. Fifteen men hunted with shotguns, and 16 men hunted with 0.22 caliber rifles; four men hunted with dogs in addition to their firearm, and eight men also gathered resources along the way, including fish (n = 3), tortoise (n = 3) or honey (n = 2).
(a). Changes in testosterone
Despite potential circadian declines in testosterone, testosterone was on average higher than baseline after 3 h of hunting, suggesting an exercise effect (figure 1a). Linear mixed effects models revealed evidence of increased salivary testosterone at the time of the kill, relative to other time points (β = 0.13, p = 0.04; figure 1a), when controlling for age2, BMI and time hunting. Regression models comparing unsuccessful hunters with those returning with any meat (killed by the hunter, collected without a firearm or killed by another hunter) revealed that those returning with meat exhibited a larger per cent change in testosterone over the course of the day (β = 36.1, p = 0.03); those who made a kill themselves also showed larger per cent increases in testosterone than those who did not make a kill (β = 24.87, p = 0.09), controlling for age2, BMI and time hunting. Absolute log testosterone at the time of a pursuit-kill was significantly higher than the 3 h testosterone measure, suggesting that physiological arousal owing to a successful kill modifies testosterone above and beyond the physical act of hunting (β = 0.17, p = 0.025). Of the six men who missed a shot, there was no evidence of testosterone change following a missed shot (p = 0.99), though the sample size was small (figure 1a).
Figure 1.
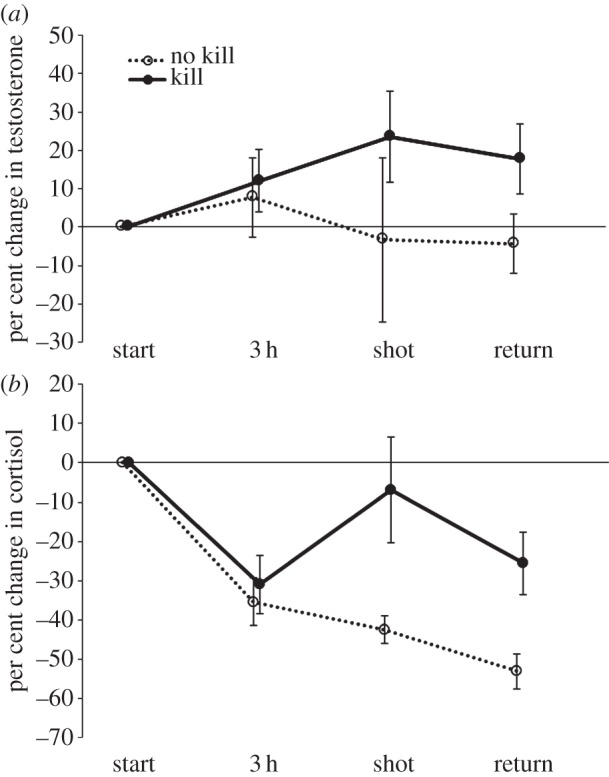
Mean per cent change (±s.e.) in (a) testosterone and (b) cortisol from baseline at each time point over the course of the study for Tsimane hunters who made a kill (solid line with filled circles, n = 18) and those who did not make a kill (dashed line with open circles, n = 13). At the time point labelled ‘shot’, 18 hunters made a kill, six missed a shot and seven hunters did not take a shot (and thus did not provide saliva at this time point).
(b). Changes in cortisol
Cortisol followed similar patters to testosterone (figure 1b), although circadian-related decreases in cortisol across the day were not offset by sustained physical activity to the same degree as testosterone [46], and thus cortisol at the time of the kill was lower than cortisol at the beginning of the hunt. Compared with unsuccessful hunters, men who returned with meat showed attenuated reductions in cortisol (β = 36.15, p = 0.007) as did those who made a kill (β = 30.07, p = 0.012), both models controlling for age2, BMI and time hunting. Like testosterone, absolute cortisol at the time of the kill was significantly higher than 3 h cortisol levels (β = 0.237, p = 0.014).
(c). Interactions between testosterone and cortisol
Baseline cortisol and testosterone were trended towards a positive correlation (r = 0.34, p = 0.056). At the time a kill was made, the per cent change in cortisol was positively associated with the per cent change in testosterone (r = 0.88, p < 0.001; electronic supplementary material, figure S1). Upon returning home at the end of the hunt, relative changes in testosterone and cortisol also trended towards positive association (r = 0.35, p = 0.067; electronic supplementary material, figure S1).
(d). Heart rate and accelerometery
Heart rate and accelerometery data (see electronic supplementary material, figure S2) were used to test whether increases in testosterone and cortisol during successful pursuits were due to changes in physical activity during the hunt rather than hunting success. A one-tailed paired t-test showed that the mean heart beats per minute (BPM) during a pursuit that ended in a kill (104.3 BPM) was significantly higher than the mean heart rate in the 5 min prior to the pursuit (91.7 BPM) (p = 0.014). This increase in heart rate occurred despite a significant decrease in physical activity as measured by accelerometery during the same time period as men slowed down to stalk their prey, with hunters averaging 383.6 accelerometer vector magnitude units (VMU) 5 min prior to the pursuit versus 295.5 VMU during the pursuits that ended in a kill (p = 0.004), suggesting that the change in heart rate was not because of physical activity. Among men who made a kill, those returning with larger animals had higher heart rates, (β = 11.25, p < 0.001), but this may have been because of the heavier weight of the animals.
(e). Provisioning and signalling models
There was no difference in per cent changes in testosterone (t = −0.163, p = 0.87) or cortisol (t = −0.956, p = 0.35) when comparing men who made a kill with those who gathered meat or returned with meat killed by another hunter. Regression models controlling for age2, BMI and time hunting showed that hunters returning with large kills (n = 10) did not differ from men who killed smaller game in absolute (p = 0.41) or per cent change in testosterone (p = 0.13) at the time of the kill, nor in absolute or per cent change in testosterone upon returning home (p = 0.93, p = 0.99, respectively). Absolute log cortisol (β = 0.36, p = 0.01) and per cent change in cortisol (β = 54.22, p = 0.002) were higher for men killing a larger animal at the time of the kill, but not upon returning home (p = 0.41, p = 0.17, respectively) controlling for age2, BMI and time hunting. Regression models found no evidence of differences in absolute (p = 0.86) or change in testosterone (p = 0.78) for successful hunters who encountered individuals other than their nuclear family on the way home, or at their house (p = 0.94, p = 0.93), controlling for age2, BMI and time hunting. Identical models examining audience effect on absolute cortisol and cortisol change find no differences during the return trip (p = 0.35, p = 0.95), or later at home (p = 0.74, p = 0.47). Interactions between returning with meat and audience, or animal size and audience did not affect absolute levels of testosterone or cortisol, or changes in testosterone or cortisol. Regression models showed no association between reports of being content with their hunt and per cent change in testosterone (p = 0.17), or cortisol (p = 0.20), controlling for age2, BMI and time hunting. Men who reported that they would have been happier if they killed the animal they mentioned at the beginning of the hunt did not show any differences in testosterone per cent change (p = 0.82) from baseline although these men trended towards decreased cortisol (p = 0.08). Neither time since last hunt nor individual frequency of hunting was associated with change in testosterone or cortisol upon returning home (all p > 0.19).
4. Discussion
Consistent with the hypothesis that successful hunting would elicit a winner effect response, hunters’ salivary testosterone and cortisol increased at the time of the kill and remained high upon returning home. Hunters who missed a shot had no increase in testosterone or cortisol at the time they took their shot. Unsuccessful hunters tended towards a decrease in testosterone and cortisol over the course of the day, while those returning with meat exhibited relatively increased testosterone and cortisol. These results are consistent with previous studies examining economic production [23,50] and hunting behaviour [43], and with the Steroid-Peptide Theory of Social Bonds, an extension of the challenge hypothesis framework suggesting that increases in testosterone can be beneficial while parenting, a period generally associated with lower testosterone [51]. Parenting is made up of a diverse set of behaviours and activities, some of which, including resource gathering and offspring protection, can benefit from acute increases in testosterone [51].
(a). Winner effect
The increases in cortisol and testosterone during a successful hunt that were observed in this study are similar to those seen following successful competition, similar to winner effects in other contexts, including sports [12], competitive games [28], occupational gains [50] and even low-investment psychological tasks [26]. Research using animal models finds evidence for androgen based reward reinforcement, thus repeated increases in testosterone following successful hunts could potentially reward or reinforce hunting behaviour [30]. Indeed, in several populations, it has been reported that better hunters spend more time hunting than poor hunters [52,53].
Unsuccessful hunters experienced decreases in cortisol and testosterone over the course of the day. This probably reflects the diurnal patterns of these hormones and is also consistent with studies of multi-hour endurance exercise, which tend to report diminished testosterone and cortisol during sustained or repeated aerobic activity [21,54]. Similar decreases in testosterone were reported on unsuccessful hunting days in a previous study of hunting among the !Kung San [43].
(b). Provisioning and signalling models
Although men returning home with meat had significantly higher levels of testosterone and cortisol than those returning without meat, the number and size of kills were not associated with hormonal levels upon return, nor did the presence of an audience beyond immediate family have an effect on cortisol or testosterone levels. In this sample, being the hunter to actually make the kill was not necessary to stimulate testosterone; men who gathered meat, or were with another hunter who killed animals, showed increases in testosterone and cortisol similar to those who were personally successful, regardless of the audience. These results are consistent with the provisioning model of male hunting motivation; if men hunt primarily to provision their families, then the audience would not be expected to impact testosterone or cortisol, nor would they have needed to kill the animal themselves. However, if signalling male quality was a larger relative driver of men's hunting behaviour, one would expect higher levels of cortisol and testosterone in cases where men returned with large shareable kills that they themselves had killed, especially in the presence of community members.
(c). Interactions between physical activity, testosterone and cortisol
Although heart rates rose while men stalked prey, physical activity (as measured by accelerometer) decreased; thus the acute increases in testosterone and cortisol at the time of the kill appear to reflect physiological arousal above and beyond physical activity. The arousal and excitement of the encounter could trigger a stress response, which can swiftly increase cortisol [26]. Although cortisol decreases over the course of the day, aerobic activity can elicit acute increases in cortisol throughout the day [21]. There is not yet consensus of the mechanism by which testosterone increases acutely following competitive activity in humans [45]. The rapid nature of these increases at the time of a kill probably rules out the HPG axis, as luteinizing hormone stimulation does not increase testosterone for 45–75 min [55]. Increases in testosterone reported here occurred within minutes of a pursuit, and thus are unlikely owing to decreased liver clearance [56]. While heart rates were elevated during animal pursuits, physical activity decreased as men stalked their prey, and testosterone was negatively correlated with heart rate during a pursuit-kill. In sum, the evidence presented here best supports the causal influence of psychological arousal. The evidence does not support the alternative argument that increased aerobic activity alone is responsible for acute elevations in testosterone and cortisol following a successful kill.
Though high cortisol levels can diminish testosterone response to a challenge in some [19], though not all, cases [18], here we found that baseline testosterone and cortisol were positively associated, as were the per cent changes in testosterone and cortisol following a kill (see the electronic supplementary material for additional analyses). Experimental studies in animal models report similar increases in testosterone and cortisol during acute stress, implicating catecholamines as one potential moderator of these changes [18,57]; human studies also find associations between acute changes in catecholamines and testosterone during sports competition [58]. Thus, while long-term exposure to glucocorticoids can downregulate testicular testosterone production, the role of HPA axis in mediating acute changes in testosterone needs further study.
5. Limitations
The study design required saliva specimens be collected at exact times under naturalistic circumstances, and thus suffers from a relatively small sample size (n = 31). That said, this study is larger than other studies examining economic productivity and hormone–behaviour interactions (n = 17) [50], and five times larger than a previous study of hormonal change during hunting (n = 6) [43]. Given the paucity of detailed hunting data, and with a shrinking number of populations engaging in subsistence hunting, opportunities to examine physiological changes during hunting under naturalistic conditions are increasingly rare. While previous studies found no researcher effect on Tsimane testosterone change while resting [23], it is possible that the presence of the researcher and a field assistant modified hunter behaviour or hormone–behaviour relationships. However, the researcher and field assistant were kept constant to minimize potential effects. It is also possible that the broadcast efficiency of hunting relies not on people seeing the hunter returning with meat, but on word-of-mouth following the hunter's return. Future work will examine whether the presence of an audience seeing a returning hunter is important, or whether word spreads regardless of who views the hunter carrying meat. Additionally, all but one of these hunters had dependent offspring; previous work with Ache forager-horticulturalists suggests that men without dependent offspring were more likely to engage in signalling behaviours [39], thus these results may not be generalizable to younger unmarried men.
6. Conclusion
Using neuroendocrine data offers an approach to study motivations underlying complex evolved behaviours. Our study suggests that success in non-directly competitive male resource production can result in ‘winner effect’ increases in hormone levels similar to those seen during more direct male–male competition. The ubiquity of hunting behaviour across subsistence societies suggests that throughout most of human evolution, men probably experienced a series of acute increases in testosterone and cortisol as a part of everyday life. How repeated exposure to acute changes in steroid hormones may impact immune function, body composition and behaviour needs to be assessed.
Overall, our results are consistent with models suggesting that familial provisioning is a key contributor of male hunting (and status-seeking) behaviour. In concert with previous studies [23] and theoretical models suggesting increased testosterone during familial provisioning [51], our findings suggest further expanding the current mating effort and competition-centric focus of hormone–behaviour interactions to include family provisioning activities that are also important aspects of female mate choice.
Acknowledgements
We thank the Tsimane participants, S. Lero Vie, A. Cari Lero, R. Roldan Acosta, C. von Rueden, J. Stieglitz, D. Cummings, A. Guyton, M. Costa, A. Jaeggi, E. Miner, two anonymous reviewers and R. Knapp.
Funding statement
Support for this research came from NICHD R24HD042828 and 5T32HD007543 to the Center for Studies in Demography and Ecology, and NIA R01AG024119-01, R56AG024119-06, R01AG024119-07.
References
- 1.Stiner MC. 2002. Carnivory, coevolution, and the geographic spread of the genus Homo. J. Archaeol. Res. 10, 1–63 (doi:10.1023/a:1014588307174) [Google Scholar]
- 2.Kaplan H, Hill K. 1985. Hunting ability and reproductive success among male Ache foragers: preliminary results. Curr. Anthropol. 26, 131–133 (doi:10.1086/203235) [Google Scholar]
- 3.Wiessner PW, Schiefenhövel W. 1996. Food and the status quest: an interdisciplinary perspective, vol. viii, p. 294 Providence, RI: Berghahn Books [Google Scholar]
- 4.Marlowe F. 2004. Mate preferences among Hadza hunter–gatherers. Hum. Nat. 15, 365–376 (doi:10.1007/s12110-004-1014-8) [DOI] [PubMed] [Google Scholar]
- 5.Bliege BR, Smith E, Bird D. 2001. The hunting handicap: costly signaling in human foraging strategies. Behav. Ecol. Sociobiol. 50, 9–19 (doi:10.1007/s002650100338) [Google Scholar]
- 6.Smith EA, Bird RB, Bird DW. 2003. The benefits of costly signaling: Meriam turtle hunters. Behav. Ecol. 14, 116–126 (doi:10.1093/beheco/14.1.116) [Google Scholar]
- 7.Gurven M, von Rueden C. 2006. Hunting, social status and biological fitness. Soc. Biol. 53, 81–99 (doi:10.1080/19485565.2006.9989118) [DOI] [PubMed] [Google Scholar]
- 8.Smith EA. 2004. Why do good hunters have higher reproductive success? Hum. Nat. 15, 343–364 (doi:10.1007/s12110-004-1013-9) [DOI] [PubMed] [Google Scholar]
- 9.Alvard MS, Gillespie A. 2004. Good Lamalara whale hunters accrue reproductive benefits. Res. Econ. Anthropol. 23, 223–245 (doi:10.1016/S0190-1281(04)23009-8) [Google Scholar]
- 10.Pillsworth EG. 2008. Mate preferences among the Shuar of Ecuador: trait rankings and peer evaluations. Evol. Hum. Behav. 29, 256–267 (doi:10.1016/j.evolhumbehav.2008.01.005) [Google Scholar]
- 11.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. Issues News Rev. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::aid-evan5>3.0.co;2-7) [Google Scholar]
- 12.Archer J. 2006. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 30, 319–345 (doi:10.1016/j.neubiorev.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 13.Wingfield JC, Hegner RE, Dufty J, Alfred M, Ball GF. 1990. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 (doi:10.1086/285134) [Google Scholar]
- 14.Hirschenhauser K, Oliveira RF. 2006. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 71, 265–277 (doi:10.1016/j.anbehav.2005.04.014) [Google Scholar]
- 15.Gettler LT, McDade TW, Feranil AB, Kuzawa CW. 2011. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc. Natl Acad. Sci. USA 108, 16 194–16 199 (doi:10.1073/pnas.1105403108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwen BS. 2001. From molecules to mind. Ann. NY Acad. Sci. 935, 42–49 (doi:10.1111/j.1749-6632.2001.tb03469.x) [PubMed] [Google Scholar]
- 17.Sapolsky RM. 2004. Social status and health in humans and other animals. Annu. Rev. Anthropol. 33, 393–418 (doi:10.2307/25064859) [Google Scholar]
- 18.Wingfield JC, Sapolsky RM. 2003. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 15, 711–724 (doi:10.1046/j.1365-2826.2003.01033.x) [DOI] [PubMed] [Google Scholar]
- 19.Mehta PH, Josephs RA. 2010. Testosterone and cortisol jointly regulate dominance: evidence for a dual-hormone hypothesis. Horm. Behav. 58, 898–906 (doi:10.1016/j.yhbeh.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 20.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. 2010. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. (Auckland, NZ) 40, 1037–1053 (doi:10.2165/11536910-000000000-00000) [DOI] [PubMed] [Google Scholar]
- 21.Hackney A, Viru A. 1999. Twenty-four-hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin. Physiol. 19, 178–182 (doi:10.1046/j.1365-2281.1999.00157.x) [DOI] [PubMed] [Google Scholar]
- 22.Trumble B, Cummings D, Von Rueden C, O'Connor K, Smith E, Gurven M, Kaplan H. 2012. Physical competition increases testosterone among Amazonian forager-horticulturalists: a test of the ‘challenge hypothesis’. Proc. R. Soc. B 279, 2907–2912 (doi:10.1098/rspb.2012.0455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trumble BC, Cummings DK, O'Connor KA, Holman DJ, Smith EA, Kaplan H, Gurven M. 2013. Age-independent increases in male salivary testosterone during physical activity among Tsimane forager horticulturalists. Evol. Hum. Behav. 34, 350–357 (doi:10.1016/j.evolhumbehav.2013.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai LW, Sapolsky RM. 1996. Rapid stimulatory effects of testosterone upon myotubule metabolism and sugar transport, as assessed by silicon microphysiometry. Aggress. Behav. 22, 357–364 (doi:10.1002/(sici)1098-2337) [Google Scholar]
- 25.Crewther BT, Cook C, Cardinale M, Weatherby RP, Lowe T. 2011. Two emerging concepts for elite athletes: the short-term effects of testosterone and cortisol on the neuromuscular system and the dose–response training role of these endogenous hormones. Sports Med. 41, 103–123 (doi:10.2165/11539170) [DOI] [PubMed] [Google Scholar]
- 26.Booth A, Shelley G, Mazur A, Tharp G, Kittok R. 1989. Testosterone, and winning and losing in human competition. Horm. Behav. 23, 556–571 (doi:10.1016/0018-506X(89)90042-1) [DOI] [PubMed] [Google Scholar]
- 27.Oyegbile TO, Marler CA. 2005. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm. Behav. 48, 259–267 (doi:10.1016/j.yhbeh.2005.04.007) [DOI] [PubMed] [Google Scholar]
- 28.Mazur A, Booth A, Dabbs J., Jr 1992. Testosterone and chess competition. Soc. Psychol. Q 55, 70–77 (doi:10.2307/2786687) [Google Scholar]
- 29.Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. 2010. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc. Natl Acad. Sci. USA 107, 12 393–12 398 (doi:10.1073/pnas.1001394107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood RI. 2004. Reinforcing aspects of androgens. Physiol. Behav. 83, 279–289 (doi:10.1016/j.physbeh.2004.08.012) [DOI] [PubMed] [Google Scholar]
- 31.Hirschenhauser K, Gahr M, Goymann W. 2013. Winning and losing in public: audiences direct future success in Japanese quail. Horm. Behav. 63, 625–633 (doi:10.1016/j.yhbeh.2013.02.010) [DOI] [PubMed] [Google Scholar]
- 32.Kaplan HS, Hill K, Hurtado AM, Lancaster JB. 2001. The embodied capital theory of human evolution. In Reproductive ecology and human evolution (ed. Ellison PT.), pp. 293–317 Hawthorne, NY: Aldine de Gruyter [Google Scholar]
- 33.Hawkes K. 1991. Showing off: tests of an hypothesis about men's foraging goals. Ethol. Sociobiol. 12, 29–54 (doi:10.1016/0162-3095(91)90011-e) [Google Scholar]
- 34.Gurven M, Kaplan H, Gutierrez M. 2006. How long does it take to become a proficient hunter? Implications for the evolution of extended development and long life span. J. Hum. Evol. 51, 454–470 (doi:10.1016/j.jhevol.2006.05.003) [DOI] [PubMed] [Google Scholar]
- 35.Martin MA, et al. 2012. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Matern. Child Nutr. 8, 404–418 (doi:10.1111/j.1740-8709.2012.00412.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurven M, Hill K. 2009. Why do men hunt? A reevaluation of ‘man the hunter’ and the sexual division of labor. Curr. Anthropol. 50, 51–62; (doi:10.1086/595620) discussion 62–74 [DOI] [PubMed] [Google Scholar]
- 37.Bliege BR, Smith EA. 2005. Signaling theory, strategic interaction, and symbolic capital. Curr. Anthropol. 46, 221–248 (doi:10.1086/427115) [Google Scholar]
- 38.Hawkes K, Bliege BR. 2002. Showing off, handicap signaling, and the evolution of men's work. Evol. Anthropol. Issues News Rev. 11, 58–67 (doi:10.1002/evan.20005) [Google Scholar]
- 39.Wood B, Hill K. 2000. A test of the ‘showing-off’ hypothesis with Ache hunters. Curr. Anthropol. 41, 124–125 (doi:10.1086/300111) [PubMed] [Google Scholar]
- 40.Wood BM, Marlowe FW. 2011. Dynamics of postmarital residence among the Hadza. Hum. Nat. 22, 128–138 (doi:10.1007/s12110-011-9109-5) [DOI] [PubMed] [Google Scholar]
- 41.Lancaster JB. 1978. Carrying and sharing in human evolution. Hum. Nat. 1, 82–89 [Google Scholar]
- 42.Woods BM, Marlowe FW. 2013. Household and kin provisioning by Hadza men. Hum. Nat. 24, 280–317 (doi:10.1007/s12110-013-9173-0) [DOI] [PubMed] [Google Scholar]
- 43.Worthman C, Konner M. 1987. Testosterone levels change with subsistence hunting effort in Kung San men. Psychoneuroendocrinology 12, 449–458 (doi:10.1016/0306-4530(87)90079-5) [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Bono E, Salvador A, Serrano MA, Ricarte J. 1999. Testosterone, cortisol, and mood in a sports team competition. Horm. Behav. 35, 55–62 (doi:10.1006/hbeh.1998.1496) [DOI] [PubMed] [Google Scholar]
- 45.Flinn M, Ponzi D, Muehlenbein M. 2012. Hormonal mechanisms for regulation of aggression in human coalitions. Hum. Nat. 23, 68–88 1–21 (doi:10.1007/s12110-012-9135-y) [DOI] [PubMed] [Google Scholar]
- 46.Tremblay MS, Copeland JL, Van Helder W. 2004. Effect of training status and exercise mode on endogenous steroid hormones in men. J. Appl. Physiol. 96, 531–539 (doi:10.1152/japplphysiol.00656.2003) [DOI] [PubMed] [Google Scholar]
- 47.Levine S. 1978. Cortisol changes following repeated experiences with parachute training. New York, NY: Academic Press [Google Scholar]
- 48.Muir C, Spironello-Vella E, Pisani N, de Catanzaro D. 2001. Enzyme immunoassay of 17 beta-estradiol, estrone conjugates, and testosterone in urinary and fecal samples from male and female mice. Horm. Metab. Res. 33, 653–658 (doi:10.1055/s-2001-18692) [DOI] [PubMed] [Google Scholar]
- 49.Munro C, Stabenfeldt G. 1985. Development of a cortisol enzyme immunoassay in plasma. Clin. Chem. 31, 956 [Google Scholar]
- 50.Coates JM, Herbert J. 2008. Endogenous steroids and financial risk taking on a London trading floor. Proc. Natl Acad. Sci. USA 105, 6167–6172 (doi:10.1073/pnas.0704025105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Anders SM, Goldey KL, Kuo PX. 2011. The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology 36, 1265–1275 (doi:10.1016/j.psyneuen.2011.06.001) [DOI] [PubMed] [Google Scholar]
- 52.Smith EA. 1987. On fitness maximization, limited needs, and hunter–gatherer time allocation. Ethol. Sociobiol. 8, 73–85 (doi:10.1016/0162-3095(87)90059-8) [Google Scholar]
- 53.Hawkes K, O'Connell JF, Hill K, Charnov EL. 1985. How much is enough? Hunters and limited needs. Ethol. Sociobiol. 6, 3–15 (doi:10.1016/0162-3095(85)90037-8) [Google Scholar]
- 54.Keizer H, Janssen GME, Menheere P, Kranenburg G. 1989. Changes in basal plasma testosterone, cortisol, and dehydroepiandrosterone sulfate in previously untrained males and females preparing for a marathon. Int. J. Sports Med. 10, S139–S145 (doi:10.1055/s-2007-1024962) [DOI] [PubMed] [Google Scholar]
- 55.Veldhuis JD, Iranmanesh A. 2004. Pulsatile intravenous infusion of recombinant human luteinizing hormone under acute gonadotropin-releasing hormone receptor blockade reconstitutes testosterone secretion in young men. J. Clin. Endocrinol. Metab. 89, 4474–4479 (doi:10.1210/jc.2004-0203) [DOI] [PubMed] [Google Scholar]
- 56.Cadoux-Hudson T, Few J, Imms F. 1985. The effect of exercise on the production and clearance of testosterone in well trained young men. Eur. J. Appl. Physiol. Occup. Physiol. 54, 321–325 (doi:10.1007/bf00426153) [DOI] [PubMed] [Google Scholar]
- 57.Sapolsky RM. 1986. Stress-induced elevation of testosterone concentration in high ranking baboons: role of catecholamines. Endocrinology 118, 1630–1635 (doi:10.1210/endo-118-4-1630) [DOI] [PubMed] [Google Scholar]
- 58.Fry AC, Schilling BK, Fleck SJ, Kraemer WJ. 2011. `ips between competitive wrestling success and neuroendocrine responses. J. Strength Cond. Res. 25, 40–45 (doi:10.1519/JSC.1510b1013e3181fef1562f) [DOI] [PubMed] [Google Scholar]


