Abstract
Kin selection is a fundamentally important process that affects the evolution of social behaviours. The genomics revolution now provides the opportunity to test kin selection theory using genomic data. In this commentary, we discuss previous studies that explored the link between kin selection and patterns of variation within the genome. We then present a new theory aimed at understanding the evolution of genes involved in the development of social insects. Specifically, we investigate caste-antagonistic pleiotropy, which occurs when the phenotypes of distinct castes are optimized by different genotypes at a single locus. We find that caste-antagonistic pleiotropy leads to narrow regions where polymorphism can be maintained. Furthermore, multiple mating by queens reduces the region in which worker-favoured alleles fix, which suggests that multiple mating impedes worker caste evolution. We conclude by discussing ways to test these and other facets of kin selection using newly emerging genomic data.
Keywords: antagonistic selection, eusocial insect caste, molecular evolution, sexual selection, social conflict
1. William D. Hamilton and kin selection theory
William D. Hamilton revolutionized the study of sociality [1]. Arguably, Hamilton's most important work focused on the process of kin selection. Kin selection occurs when alleles for social behaviours are selected because these behaviours affect the fitness of relatives [2]. Fundamentally, the idea underlying kin selection is that an allele can be transmitted not only through personal reproduction, but also through the reproduction of kin.
Kin selection is responsible for the evolution of many of the remarkable actions displayed by social animals, such as the extreme helping behaviours displayed by social insects (figure 1) [3–4]. Kin selection also underlies the social actions of microbes, including the production of public goods [5]. Remarkably, even plants show evidence of kin-selected ‘behaviours’, such as competition through root growth, which may vary based on kinship [6]. Indeed, the evolution of many of the cooperative actions among entities at all levels of biological organization relied on kin selection-like processes [7]. Thus, kin selection represents a fundamentally important mechanism governing biological group formation.
Figure 1.

The behaviours of social insects, such as (a) the honeybee (Apis mellifera), (b) the fire ant (Solenopsis invicta) and (c) the yellowjacket wasp (Vespula maculifrons) have been shaped extensively by the process of kin selection. Social insects often exhibit phenotypically distinct castes, the evolution of which may be hampered by caste-antagonistic pleiotropy, particularly in species where queens mate with multiple males such as A. mellifera and V. maculifrons (see text for details). (Online version in colour.)
2. Kin selection and molecular evolution
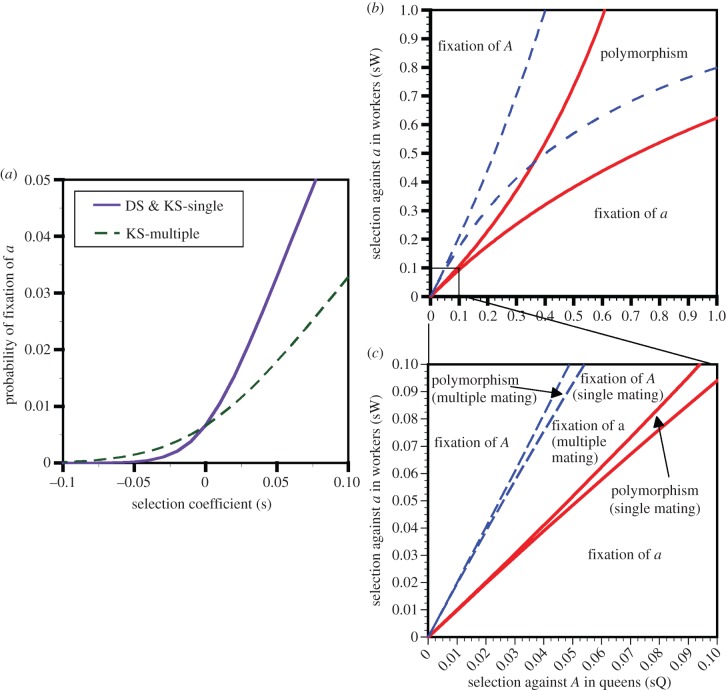
Kin selection theory has been primarily applied to explain the evolution of social behaviours at the phenotypic level. However, the signatures of kin selection should be seen at the genomic level as well. For example, Linksvayer & Wade [8] and Hall & Goodisman [9] determined the effects of kin selection on molecular evolution. They showed that the strength of kin selection, measured as the probability of fixation of a newly arising allele experiencing directional selection, could equal that of direct selection, but only when relatedness was high. As relatedness declined, so too did the strength of kin selection (figure 2a). Consequently, genes experiencing kin selection and direct selection may exhibit different rates of molecular evolution depending on the social system of the species in question.
Figure 2.
The outcome of selection for genes experiencing directional selection or caste-antagonistic selection. (a) The probability of fixation of newly arising additive alleles as a function of the strength of selection is identical under direct selection (DS) on queens or kin selection (KS) on workers when queens are singly mated (KS-single). However, when queens are multiply mated, beneficial alleles (s > 0) fix at lower rates and deleterious alleles (s < 0) fix at higher rates, when they are subjected to KS in workers (KS-multiple). (b,c) Regions in which antagonistic selection results in fixation of the queen-favoured a allele, the worker-favoured A allele or polymorphism. Solid lines and dashed lines delineate regions for singly and multiply mated queens, respectively. sQ and sW represent the strength of selection on performance against alleles A and a in queens and workers, respectively. In (b), all possible combinations of selection coefficients are shown, whereas panel (c) expands the region with more realistic, weaker selection. (Online version in colour.)
This prediction was tested using data in two social insects, the fire ant Solenopsis invicta and the honeybee Apis mellifera [9] (figure 1a,b). The queens of these two species mate different numbers of times; fire ant queens mate once, whereas honeybee queens mate multiply. Thus, the rates of evolution of queen- and worker-biased genes were predicted to be different in the honeybee but similar in the fire ant. Queen- and worker-biased genes did evolve at significantly different rates in the honeybee and non-significantly different rates in the fire ant, consistent with expectations [9]. Thus, these studies began to link molecular and genome evolution with theoretical predictions of kin selection.
3. Caste-antagonistic pleiotropy in social genomes
Extending Hamilton's ideas to genome evolution requires the development of theory to predict how kin selection affects patterns of genetic variation. Of particular relevance for highly social species, such as social insects, is the case of antagonistic selection between castes [10], which occurs when distinct castes have different phenotypic optima for the same trait (cf. [11]). If this trait is controlled by the same gene(s) in both castes, then alleles favoured in one caste may be disfavoured in another. For example, wing muscle development may be beneficial for queen ants, which partake in mating flights, but is unlikely to be beneficial for worker ants, which do not fly. Thus, an allele that increases wing muscle development would potentially be subject to antagonistic selection across castes.
We determined the outcome of antagonistic selection arising from caste-antagonistic pleiotropy in haplodiploid social insects. Here, we present the special case where gene effects were additive at a single locus that affected caste ‘performance’, which in turn affected colony fitness (details in the electronic supplementary material). In this model, queens reproduced and were subject to direct selection, whereas workers were incapable of reproduction and subject exclusively to kin selection. We assumed that allele a was favoured in queens and allele A was favoured in workers. We then determined the combinations of selection coefficients in which either allele was fixed or both alleles were maintained as a polymorphism. Our interest was in understanding whether kin selection in workers was overwhelmed by direct selection acting in the opposite direction in queens, and whether caste-antagonistic pleiotropy was likely to lead to detectable genetic patterns within the genomes of social species.
We found that the region of the parameter space in which the queen-favoured allele fixed was the same size as the region in which the worker-favoured allele fixed, but only when queens mated once (figure 2b,c). By contrast, when queens mated with multiple males, the region of the parameter space in which the worker-favoured allele fixed was substantially smaller, and the region where the queen-favoured allele fixed was substantially larger (figure 2b,c). These differences arose because kin selection operating on workers was weaker than direct selection operating on queens when queens mated many times. Regardless, in both cases, the region of the parameter space allowing polymorphism was limited, especially when selection coefficients were realistically small (figure 2c).
4. Outlook: kin selection and social insect genomics
The revolution in social insect genomics [12–14] now allows rates of evolution and levels of polymorphism to be determined for all loci across multiple genomes. Thus, population genetic predictions arising from kin selection theory can be tested using newly emerging genomic data.
The model presented here makes three predictions. First, antagonistic selection across castes is unlikely to maintain polymorphism. Consequently, loci affecting traits in multiple castes are not expected to show high levels of polymorphism compared with other loci. Overall, this suggests that factors other than caste-antagonistic pleiotropy may be responsible for the maintenance of genetic polymorphism in social species.
Second, the model predicts that the evolution of antagonistic alleles that are favoured in workers, but disfavoured in queens, is impeded by multiple mating by queens. Such worker-beneficial alleles are strong candidates for alleles that would lead to distinct worker phenotypes. Consequently, these results suggest that phenotypic differentiation between the queen and worker castes, or within the worker caste, could have evolved more easily in species with singly mated queens, and that multiple mating hinders the evolution of caste differences.
Interestingly, currently available empirical data suggest that hymenopteran social insects that have genetically diverse colonies (e.g. are headed by multiply mated queens) have more phenotypically diverse workers [15]. If phenotypic differentiation in workers is due to selection in workers, then available data are inconsistent with our theoretical expectations, suggesting that caste-antagonistic pleiotropy is not a pervasive force. However, caste-antagonistic pleiotropy may have been important in the early evolution of sociality, when castes were first evolving and gene expression patterns in proto-queens and workers were similar. Subsequently, caste-antagonistic pleiotropy could have been resolved through the evolution of differential expression of genes between castes [16–19], allowing worker phenotypic differences to result from genes under selection (e.g. expressed) in workers only.
Third, our model predicts that adaptive evolution of genes that function in both queens and workers is more likely to be due to fixation of alleles that give queen-favoured phenotypes in species with multiply mated queens. By contrast, adaptive alleles are expected to be just as likely to give worker-favoured as queen-favoured phenotypes in species with singly mated queens. If adaptive evolution in social species is driven primarily by selection on performance of workers, then genes that function in both queens and workers should show higher rates of adaptive evolution in species with singly mated, rather than multiply mated, queens. Conversely, if adaptive evolution is driven by selection on queen performance, the opposite pattern is predicted. Thus, the patterns of molecular evolution may give insight into whether selection acts primarily on worker or queen performance.
In conclusion, models for interpreting genomic data have great potential for testing kin selection theory by determining how social evolution affects molecular evolution (e.g. [20–27]). Moreover, these investigations provide further inspiration for the development of new theory aimed at generating predictions regarding how genes should evolve under direct and kin selection. With several large-scale sequencing projects in progress, the genomes of social animals offer a natural playground for data and theory to come together in testing kin selection theory and providing insight into the evolution of social behaviours.
References
- 1.Hamilton WD. 1996. Narrow roads of gene land: evolution of social behavior. New York, NY: W. H. Freeman & Co [Google Scholar]
- 2.West SA, Griffin AS, Gardner A. 2007. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 20, 415–432 (doi:10.1111/j.1420-9101.2006.01258.x) [DOI] [PubMed] [Google Scholar]
- 3.Queller DC, Strassmann JE. 1998. Kin selection and social insects. Bioscience 48, 165–175 (doi:10.2307/1313262) [Google Scholar]
- 4.Abbot P, et al. 2011. Inclusive fitness theory and eusociality. Nature 471, E1–E4 (doi:10.1038/nature09831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West SA, Griffin AS, Gardner A, Diggle SP. 2006. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 4, 597–607 (doi:10.1038/nrmicro1461) [DOI] [PubMed] [Google Scholar]
- 6.Dudley SA, File AL. 2007. Kin recognition in an annual plant. Biol. Lett. 3, 435–438 (doi:10.1098/rsbl.2007.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs JL, Mueller UG, Wilcox TP, Bull JJ. 2004. The evolution of cooperation. Q. Rev. Biol. 79, 135–160 (doi:10.1086/383541) [DOI] [PubMed] [Google Scholar]
- 8.Linksvayer TA, Wade MJ. 2009. Genes with social effects are expected to harbor more sequence variation within and between species. Evolution 63, 1685–1696 (doi:10.1111/j.1558-5646.2009.00670.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall DW, Goodisman MAD. 2012. The effects of kin selection on rates of molecular evolution in social insects. Evolution 66, 2080–2093 (doi:10.1111/j.1558-5646.2012.01602.x) [DOI] [PubMed] [Google Scholar]
- 10.Kovacs JL, Hoffman EA, Marriner SM, Goodisman MAD. 2010. Detecting selection on morphological traits in social insect castes: the case of the social wasp Vespula maculifrons. Biol. J. Linnean Soc. 101, 93–102 (doi:10.1111/j.1095-8312.2010.01495.x) [Google Scholar]
- 11.Bonduriansky R, Chenoweth SF. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288 (doi:10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 12.Robinson GE, Fernald RD, Clayton DF. 2008. Genes and social behavior. Science 322, 896–900 (doi:10.1126/science.1159277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson GE, et al. 2011. Creating a buzz about insect genomes. Science 331, 1386 (doi:10.1126/science.331.6023.1386) [DOI] [PubMed] [Google Scholar]
- 14.Foster KR. 2011. The sociobiology of molecular systems. Nat. Rev. Genet. 12, 193–203 (doi:10.1038/nrg2903) [DOI] [PubMed] [Google Scholar]
- 15.Fjerdingstad EJ, Crozier RH. 2006. The evolution of worker caste diversity in social insects. Am. Nat. 167, 390–400 (doi:10.1086/499545) [DOI] [PubMed] [Google Scholar]
- 16.Mank JE, Wedell N, Hosken DJ. 2013. Polyandry and sex-specific gene expression. Phil. Trans. R. Soc. B 368, 20120047 (doi:10.1098/rstb.2012.0047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth AL, et al. 2007. Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 318, 441–444 (doi:10.1126/science.1146647) [DOI] [PubMed] [Google Scholar]
- 18.Goodisman MAD, Kovacs JL, Hunt BG. 2008. Functional genetics and genomics in ants (Hymenoptera: Formicidae): the interplay of genes and social life. Myrmecol. News 11, 107–117 [Google Scholar]
- 19.Smith CR, Toth AL, Suarez AV, Robinson GE. 2008. Genetic and genomic analyses of the division of labour in insect societies. Nat. Rev. Genet. 9, 735–748 (doi:10.1038/nrg2429) [DOI] [PubMed] [Google Scholar]
- 20.Toth AL, Robinson GE. 2010. Evo-devo and the evolution of social behavior: brain gene expression analyses in social insects. In Cold Spring Harbor Symposia on Quantitative Biology, Vol. LXXIV, pp. 1–8 New York, NY: Cold Spring Harbor Laboratory Press; [DOI] [PubMed] [Google Scholar]
- 21.Van Dyken JD, Wade MJ. 2012. Detecting the molecular signature of social conflict: theory and a test with bacterial quorum sensing genes. Am. Nat. 179, 436–450 (doi:10.1086/664609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sucgang R, et al. 2011. Comparative genomics of the social amoebae Dictyostelium discoideum and Dictyostelium purpureum. Genome Biol. 12, Artn R20 (doi:10.1186/gb-2011-12-2-r20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischman BJ, Woodard SH, Robinson GE. 2011. Molecular evolutionary analyses of insect societies. Proc. Natl Acad. Sci. USA 108, 10 847–10 854 (doi:10.1073/pnas.1100301108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt BG, Wyder S, Elango N, Werren JH, Zdobnov EM, Yi SV, Goodisman MAD. 2010. Sociality Is linked to rates of protein evolution in a highly social insect. Mol. Biol. Evol. 27, 497–500 (doi:10.1093/molbev/msp225) [DOI] [PubMed] [Google Scholar]
- 25.Hunt BG, Ometto L, Wurm Y, Shoemaker D, Yi SV, Keller L, Goodisman MAD. 2011. Relaxed selection is a precursor to the evolution of phenotypic plasticity. Proc. Natl Acad. Sci. USA 108, 15 936–15 941 (doi:10.1073/pnas.1104825108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bromham L, Leys R. 2005. Sociality and the rate of molecular evolution. Mol. Biol. Evol. 22, 1393–1402 (doi:10.1093/molbev/msi133) [DOI] [PubMed] [Google Scholar]
- 27.Viljakainen L, Evans JD, Hasselmann M, Rueppell O, Tingek S, Pamilo P. 2009. Rapid evolution of immune proteins in social insects. Mol. Biol. Evol. 26, 1791–1801 (doi:10.1093/molbev/msp086) [DOI] [PubMed] [Google Scholar]