Abstract
When helping behaviour is costly, Hamiltonian logic implies that animals need to direct helpful acts towards kin, so that indirect fitness benefits justify the costs. We revisit inferences about nepotism and aggression in Hamilton's 1964 paper to argue that he overestimated the general significance of nepotism, but that other issues that he raised continue to suggest novel research agendas today. We now know that nepotism in eusocial insects is rare, because variation in genetic recognition cues is insufficient. A lower proportion of individuals breeding and larger clutch sizes selecting for a more uniform colony odour may explain this. Irreversible worker sterility can induce both the fiercest possible aggression and the highest likelihood of helping random distant kin, but these Hamiltonian contentions still await large-scale testing in social animals.
Keywords: clutch size, recognition, Gestalt, inclusive fitness, nepotism, unmatedness
1. Introduction
The second part of W. D. Hamilton's seminal 1964 paper [1] is about the evolution of social behaviour and social discrimination, topics which both stimulated large research programmes. Subsequent studies on vertebrates focused on the efficiency of kin-discrimination (reviewed in [2–4]), whereas social insect researchers primarily developed questions and tools for estimating relatedness [5]. However, later decades of kin recognition research led to the general consensus that eusocial insects are very good at binary discrimination between nest-mates (kin, at least on average; reviewed in [6,7]), but that within-colony discrimination according to degree of kin is all but absent [8,9], unlike vertebrate cooperative breeders that may have graded nepotism when it benefits the indirect fitness of group members [4,10]. Even in very small insect societies, we lack evidence that discrimination happens on the basis of kinship per se instead of belonging to the same nest [11,12].
Rather than elaborating on empirical work over a broad spectrum of recognition topics, we revisit foundational pieces of Hamiltonian logic [1] to briefly explore their relevance today. We focus on aspects of cost (c), both for helping and for discrimination. In Hamilton's rule (br > c), these costs are normally expressed in a personal reproduction currency, so that c in fact equals cro, where ro is (life-for-life) relatedness to own offspring (0.5 when parents are outbred). As long as all individuals can mate, this cro term, compared with the efficiency benefit (b) of raising non-offspring nest-mates of some average relatedness (rn), determines whether inclusive fitness is maximized by helping or dispersing. However, workers of obligatorily eusocial insects [13] never mate to disperse and breed elsewhere as they are destined to raise younger nest-mates of relatedness rn. So for anything else than indiscriminate altruism to nest-mates to be favoured the required Hamiltonian inequality becomes brn+ > crn rather than brn > cro, where rn+ is relatedness to a specifically targeted nest-mate of higher relatedness relative to an individual of average relatedness (rn).
2. Why it may almost never pay to be nicer to closer relatives in the eusocial domain?
In the section ‘Discrimination in social situations’ [1], we find Hamilton's summary statement that started most research programmes on kin recognition [2–4,6,7]:
The selective advantage of genes which make behaviour conditional in the right sense on the discrimination of factors which correlate with the relationship of the individual concerned is therefore obvious. … If he could learn to recognize those of his neighbours who really were close relatives and could devote his beneficial actions to them alone an advantage to inclusive fitness would at once appear’.(p. 21)
In the 1990s, Ratnieks [14] showed that kin-discrimination within colonies would rarely be accurate enough to be profitable, but that nest-mate recognition would be more stable over evolutionary time. His modelling elaborated on Crozier's paradox [15,16], the idea that recognition cue variation will become eroded in proportion to its nepotistic use to favour carriers of the same genes, unless it is maintained by another selection force [9]. Recent work by Holman et al. [17] indicates that an important general force might be inbreeding avoidance via disassortative mating, which can maintain negative frequency-dependent selection for genetic odour cues in spite of kin-discrimination. However, the hallmark of eusociality is that most individuals neither mate nor reproduce. Eusociality therefore entails a transition from reproductive skew among cooperative and facultatively eusocial breeders to sterility for most colony members. Such a transition to obligate eusociality is irreversible and this is significant when evaluating the likelihood of nepotism on either side of the transition [13].
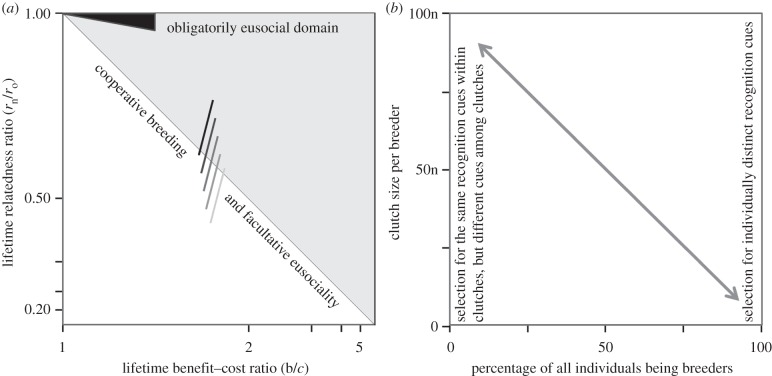
Increasing unmatedness implies that selection for mate-choice targets a decreasing proportion of individuals as reproductive division of labour increases. This is particularly so in the obligatorily eusocial Hymenoptera, where individuals either mate for life shortly after reaching sexual maturity or not at all [13,18], whereas vertebrate helpers that become breeders can mate later in life. In figure 1a, we have mapped the effective proportion of reproductively totipotent individuals (the fraction remaining in the white triangle: zero when eusociality is obligate and variable for cooperative and facultatively eusocial breeders) on the parameter space provided by Hamilton's rule. When the transition towards eusociality makes colony size increase, and only a single pair or few individuals breed, clutch sizes must necessarily increase and then disassortative outbreeding becomes easiest when all reproductives raised in a clutch have similar smells. This would almost inevitably induce selection for odour-mixing via trophallaxis or other mechanisms producing a unique colony Gestalt (figure 1b). In turn, this would make it hard for workers to identify more closely related sexuals when they provision them. Erosion of cue diversity would thus decrease the left-hand term in bn+rn+ > cnrn, so that nepotism would not pay off.
Figure 1.
Unmatedness, clutch size and the maintenance of recognition–cue diversity. (a) Heuristic diagram outlining how reproductive division of labour may erode recognition–cue diversity in spite of disassortative mating [17]. The log–log plot, derived from fig. 5a in [13], has a light-grey triangle where Hamilton's rule brn > cro is fulfilled (c is the efficiency of personal reproduction, b is the efficiency of raising nest-mates, ro is relatedness to offspring, rn is relatedness to immature nest-mates other than offspring) and a white triangle where Hamilton's rule is not satisfied over the lifespan of helpers. Being in the light-grey triangle makes the pursuit of indirect fitness more favourable than direct fitness and the opposite is true when being in the white triangle. Obligatorily eusocial Hymenoptera (ants, corbiculate bees, vespine wasps) have strict lifetime monogamous ancestors [13,18] so that rn/ro equalled one when workers became physically differentiated (the black triangle in the top-left), but this condition is not fulfilled in cooperative and facultatively eusocial breeders where individuals may change roles from helper to breeder later in life. In terms of lifetime inclusive fitness, individuals in such populations will always hover around the diagonal as helping never becomes fixed in the obligatorily eusocial sense. We have drawn hypothetical populations of cooperative or facultatively eusocial breeders as lines in different shades of grey, all crossing the diagonal but varying from very few individuals devoting their life to helping (lightest grey line) to most individuals being permanent helpers and very few managing to become breeders later in life. The likelihood of nepotistic discrimination should then be proportional to the relative number of individuals remaining in the white triangle. (b) The smaller the proportion of individuals in a population that breed (normally very low in obligatorily eusocial species and variable in cooperative and facultatively eusocial breeders), the larger the average clutch size of mating swarm reproductives produced per breeder (here assumed to be linearly proportional to multiples of n). Towards the right selection for disassortative mating should give individuals distinct odours, but towards the left odours should become the same within clutches and different among clutches.
This logic may explain why even in small halictid bee societies selection has not precluded the evolution of Gestalt odours [19]. It implies that with technology we can discriminate between patrilines within the same colony of honeybees [20], yellowjacket wasps [21], leaf-cutting ants [22] and matrilines of ponerine ants [23], even though the workers of these bee and ant species do not appear to use such differences for nepotism [8,22,23]. Finally, it is consistent with the transferability of recognition cues among Formica ant workers being positively correlated with their heritability [24]. Such transfers make it very difficult to discriminate between different degrees of kin within a colony, but they very efficiently mobilize the entire spectrum of available cues for obtaining a colony Gestalt that avoids inbreeding during nuptial flights.
3. When does it pay to be nasty to non-kin and nice to distant kin?
Hamilton was aware of the analogy between social insect colonies and metazoan bodies in which somatic cells readily kill themselves in defence of the germline [1]. As insect colonies are not clonal, he also realized that the extent of individual sterility should correlate with the degree of suicidal aggression. In the section ‘Colonies of social insects’ [1], we can read
The correlation of these (aggressive) characters with sterilization does seem to hold very well throughout the social Hymenoptera. Queens are always timid and reluctant to use their stings compared to workers. In Polistes, workers, unless very young, are more aggressive than auxiliaries, and auxiliaries more than the reigning queen. Races of honeybees in which laying workers occur more frequently or appear more readily when the hive becomes queenless are generally milder than the races where they are less prevalent. Polybiine wasps, pleiometrotic and lacking pronounced caste differences, are generally somewhat less fierce than vespines.(p. 39)
To the best of our knowledge, this suggestion has hardly been elaborated upon in spite of the intriguing generalizations that it may offer. Testing for a relationship between aggression and sterility across species would require adjustment for many confounding variables, but tests within species comparing the aggression of same-age workers with and without developed ovaries would seem very interesting.
It is important to realize that some relatedness has fundamentally different consequences than no relatedness at all. In Hamilton's section ‘Valuation of the welfare of relatives’ [1], we read
[…] the behaviour of a post-reproductive animal may be expected to be entirely altruistic, the smallest degree of relationship with the average neighbour being sufficient to favour the selection of a giving trait.(p. 21)
This refers to a solitary breeder for whom cro no longer exists, so that brn > cro is fulfilled for any brn > 0, favouring indiscriminate altruism towards random neighbours in genetically viscous populations. No non-human examples appear to have been documented, which is perhaps not quite surprising as animals rarely become post-reproductive in good health. However, in the obligatorily eusocial domain, this is equivalent to brn+ > crn, which is easier to satisfy when crn = 0 than in the within-colony nepotism scenario where crn > 0. This would apply to a colony of obligatorily sterile workers who have lost their mother queen without the possibility of replacing her, which should make them inclined to be altruistic to any distant relatives. Consistent with this idea, orphaned army ant workers are known to merge with a neighbouring colony in spite of distinct cuticular hydrocarbon profiles, as average relatedness among random neighbouring colonies is slightly positive because of reproduction by colony fission [25]. The ‘average’ is crucial here, as a generally adaptive behaviour does not preclude occasional non-adaptive mergers, such as some dwarf honeybees joining colonies of a closely related species, as long as colonies merge with the right other colonies most of the time [26].
4. Conclusion and implications
Although genetic variation allowing for kin-discrimination may be maintained in spite of Crozier's paradox [15,17], we hypothesize that the increasing unmatedness and larger clutch sizes that accompanied the loss of reproductive totipotency in the eusocial Hymenoptera shifted selection from favouring individuals with unique personal odours to clutches that entered mating swarms with the same odour (figure 1), a colony-level trait that precluded any form of eusocial nepotism. Rereading Hamilton's original paper, we conclude that his generalization of nepotism relied on the (as we now know incorrect) assumption of sufficient detectable intra-colonial variation for genetic recognition cues throughout all domains of social evolution. It now seems more likely that there is a gradient of nepotism, which is negatively correlated with reproductive division of labour, so that nepotism is most easily maintained in advanced primate (including human) groups, less so but still frequent in other vertebrate societies [4,10], but absent in the more organismal [27] insect societies. When high levels of organismality are reached [13,27,28], nepotism likely becomes an aberration rather than a kin-selected norm, analogous to cancer in metazoan bodies [29]. If it is to be maintained in obligatorily eusocial insects, it would seem most likely in cases of moderate secondary polygyny with high queen turnover, fluctuating relatedness, and substantial worker reproduction [30].
Revisiting Hamilton's original text on helper sterility and aggression [1] reveals that obvious questions remain to be broadly investigated. For example, can aggression differences between castes be experimentally manipulated by altering the likelihood of sterility? A recent study suggests that this is the case in leaf-cutting ants [31]. Or conversely, does the likelihood of sterility decrease when relatedness incentives for personal reproduction increase? A recent study indicates that the answer is affirmative for honeybees [32]. Finally, might the occasional merging of orphaned and queen-right army ant colonies in viscous natural populations just be an example of a more common phenomenon? Similar to army ants, invasive ant workers seem unable to produce males from worker laid eggs and always appear to have genetically viscous populations when they become unicolonial [33]. It seems we still have a long way to go until we will have exhausted the inspirational observations reported in Hamilton's classic 1964 paper [1], a contribution that forever changed the way in which we understand social evolution.
Acknowledgements
We thank Luke Holman and Francis Ratnieks for discussion and comments during the preparation of the manuscript.
Funding statement
We were supported by the Danish National Research Foundation (DNRF57).
References
- 1.Hamilton WD. 1964. Genetical evolution of social behaviour 2. J. Theor. Biol. 7, 17–52 (doi:10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 2.Holmes WG. 2004. The early history of Hamiltonian-based research on kin recognition. Ann. Zool. Fennici 41, 691–711 [Google Scholar]
- 3.Komdeur J, Richardson DS, Hatchwell B. 2008. Kin-recognition mechanisms in cooperative breeding systems: ecological causes and behavioral consequences of variation. In Ecology of social evolution (eds Korb J, Heinze J.), pp. 175–193 [Google Scholar]
- 4.Cornwallis CK, West SA, Griffin AS. 2009. Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal. J. Evol. Biol. 22, 2445–2457 (doi:10.1111/j.1420-9101.2009.01853.x) [DOI] [PubMed] [Google Scholar]
- 5.Crozier RH, Pamilo P. 1996. Evolution of social insects colonies: sex allocation and kin selection, p. 306 Oxford, UK: Oxford University [Google Scholar]
- 6.Lenoir A, Fresneau D, Errard C, Hefetz A. 1999. Individuality and colonial identity in ants: the emergence of the social representation concept. In Information processing in social insects (eds Detrain C, Deneubourg JL, Pasteels JM.), pp. 219–237 Birkhauser [Google Scholar]
- 7.Van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons (eds Blomquist GJ, Bagnères AG.), pp. 222–243 Cambridge, UK: Cambridge University Press [Google Scholar]
- 8.Keller L. 1997. Indiscriminate altruism: unduly nice parents and siblings. Trend Ecol. Evol. 12, 99–103 (doi:10.1016/S0169-5347(96)10065-3) [DOI] [PubMed] [Google Scholar]
- 9.Rousset F, Roze D. 2007. Constraints on the origin and maintenance of genetic kin recognition. Evolution 61, 2320–2330 (doi:10.1111/j.1558-5646.2007.00191.x) [DOI] [PubMed] [Google Scholar]
- 10.Griffin AS, West SA. 2003. Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636 (doi:10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- 11.Buckle GR, Greenberg L. 1981. Nestmate recognition in sweat bees (Lasioglossum zephyrum): does an individual recognize its own odor or only odors of its nestmates. Anim. Behav. 29, 802–829 (doi:10.1016/S0003-3472(81)80014-0) [Google Scholar]
- 12.Gamboa GJ. 1988. Sister, aunt niece, and cousin recognition by social wasps. Behav. Genet. 18, 409–423 (doi:10.1007/BF01065511) [DOI] [PubMed] [Google Scholar]
- 13.Boomsma JJ. 2013. Beyond promiscuity: mate-choice commitments in social breeding. Phil. Trans. R. Soc. B 368, 20120050 (doi:10.1098/rstb.2012.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratnieks FLW. 1991. The evolution of genetic odor-cue diversity in social Hymenoptera. Am. Nat. 137, 202–226 (doi:10.1086/285154) [Google Scholar]
- 15.Crozier RH. 1987. Genetic aspects of kin recognition: concepts, models, and synthesis. In Kin recognition in animals (eds Fletcher DJC, Michener CD.), pp. 55–73 Chichester, UK: Wiley [Google Scholar]
- 16.Tsutsui ND. 2004. Scents of self: the expression component of self/nonself recognition systems. Ann. Zool. Fennici 41, 713–727 [Google Scholar]
- 17.Holman L, van Zweden JS, Linksvayer TA, d'Ettorre P. 2013. Crozier's paradox revisited: maintenance of genetic recognition systems by disassortative mating. 13, 211 (doi:10.1186/1471-2148-13-211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boomsma JJ. 2007. Kin selection versus sexual selection: why the ends do not meet? Curr. Biol. 17, R673–R683 (doi:10.1016/j.cub.2007.06.033) [DOI] [PubMed] [Google Scholar]
- 19.Soro A, Ayasse M, Zobel MU, Paxton RJ. 2011. Kin discriminators in the eusocial sweat bee Lasioglossum malachurum: the reliability of cuticular and Dufour's gland odours. Behav. Ecol. Sociobiol. 65, 641–653 (doi:10.1007/s00265-010-1066-1) [Google Scholar]
- 20.Arnold G, et al. 1996. Kin recognition in honeybees. Nature 379, 498 (doi:10.1038/379498a0) [Google Scholar]
- 21.Goodisman MAD, Kovacs JL, Hoffman EA. 2007. Lack of conflict during queen production in the social wasp Vespula maculifrons. Mol. Ecol. 16, 2589–2595 (doi:10.1111/j.1365-294X.2007.03316.x) [DOI] [PubMed] [Google Scholar]
- 22.Nehring V, Evison SEF, Santorelli LA, d'Ettorre P, Hughes WOH. 2011. Kin-informative recognition cues in ants. Proc. R. Soc. B 278, 1942–1948 (doi:10.1098/rspb.2010.2295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helantera H, Aehle O, Roux M, Heinze J, d'Ettorre P. 2013. Family-based guilds in the ant Pachycondyla inversa. Biol. Lett. 9, 20130125 (doi:10.1098/rsbl.2013.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Zweden JS, Brask JB, Christensen JH, Boomsma JJ, Linksvayer TA, d'Ettorre P. 2010. Blending of heritable recognition cues among ant nestmates creates distinct colony gestalt odours but prevents within-colony nepotism. J. Evol. Biol. 23, 1498–1508 (doi:10.1111/j.1420-9101.2010.02020.x) [DOI] [PubMed] [Google Scholar]
- 25.Kronauer DJC, Schoning C, d'Ettorre P, Boomsma JJ. 2010. Colony fusion and worker reproduction after queen loss in army ants. Proc. R. Soc. B 277, 755–763 (doi:10.1098/rspb.2009.1591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wongvilas S, Deowanish S, Lim J, Xie VRD, Griffith OW, Oldroyd BP. 2010. Interspecific and conspecific colony mergers in the dwarf honey bees Apis andreniformis and A. florea. Insect Soc. 57, 251–255 (doi:10.1007/s00040-010-0080-7) [Google Scholar]
- 27.Queller DC, Strassmann JE. 2009. Beyond society: the evolution of organismality. Phil. Trans. R. Soc. B 364, 3143–3155 (doi:10.1098/rstb.2009.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boomsma JJ. 2009. Lifetime monogamy and the evolution of eusociality. Phil. Trans. R. Soc. B 364, 3191–3207 (doi:10.1098/rstb.2009.0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin SJ, Beekman M, Wossler TC, Ratnieks FLW. 2002. Parasitic Cape honeybee workers, Apis mellifera capensis, evade policing. Nature 415, 163–165 (doi:10.1038/nature714) [DOI] [PubMed] [Google Scholar]
- 30.Ozan M, Helanterä H, Sundström L. 2013. The value of oviposition timing, queen presence and kinship in a social insect. Proc. R. Soc. B 280, 20131231 (doi:10.1098/rspb.2013.1231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nehring V, Boomsma JJ, d'Ettorre P. 2012. Wingless virgin queens assume helper roles in Acromyrmex leaf-cutting ants. Curr. Biol. 22, R671–R673 (doi:10.1016/j.cub.2012.06.038) [DOI] [PubMed] [Google Scholar]
- 32.Woyciechowski M, Kuszewska K. 2012. Swarming generates rebel workers in honeybees. Curr. Biol. 22, 707–711 (doi:10.1016/j.cub.2012.02.063) [DOI] [PubMed] [Google Scholar]
- 33.Helantera H, Strassmann JE, Carrillo J, Queller DC. 2009. Unicolonial ants: where do they come from, what are they and where are they going? Trends Ecol. Evol. 24, 341–349 (doi:10.1016/j.tree.2009.01.013) [DOI] [PubMed] [Google Scholar]