Abstract
Social insects deploy numerous strategies against pathogens including behavioural, biochemical and immunological responses. While past research has revealed that adult social insects can generate immunity, few studies have focused on the immune function during an insect's early life stages. We hypothesized that larvae of the black carpenter ant Camponotus pennsylvanicus vaccinated with heat-killed Serratia marcescens should be less susceptible to a challenge with an active and otherwise lethal dose of the bacterium. We compared the in vivo benefits of prior vaccination of young larvae relative to naive and ringer injected controls. Regardless of colony of origin, survival parameters of vaccinated individuals following a challenge were significantly higher than those of the other two treatments. Results support the hypothesis that ant larvae exhibit immune-priming. Based on these results, we can infer that brood care by workers does not eliminate the need for individual-level immunological responses. Focusing on these early stages of development within social insect colonies can start addressing the complex dynamics between physiological (individual level) and social (collective) immunity.
Keywords: social insects, Camponotus pennsylvanicus, individual versus social immunity
1. Introduction
Insects, in general, are known for their ability to respond immunologically against pathogens [1,2], and social insects are no exception. Indeed, the diversity and ecological dominance of social insects can, in part, be attributed to their efficient and diverse mechanisms of disease resistance [3]. Although immune defences are vital in solitary insects, immune investment against the backdrop of eusociality can result in the evolution of multi-layered immunological strategies. Through a combination of individual physiological defences, frequent behavioural interactions and potent glandular secretions, social insects can thrive in environments heavily colonized by pathogens [3–6].
Worker ants have immune defences at both the individual and communal levels ([7–13], but see [14]). Yet, our understanding of the onset of effective in vivo immunity is limited. Studies on the inception of immune function have focused mainly on honeybee (Apis mellifera) larvae which exhibit upregulation of immune-related genes, the highest haemocyte counts of any other developmental stage and an age-dependent resistance to disease ([15–18] and reference therein). These examples demonstrate that larvae are capable of mounting immune responses. Do similar patterns of immunogenicity exist in ant larvae? Is immune-priming a mechanism by which immature ant stages cope with possible pathogenic re-encounters? Here, we examine the in vivo effects of immune-priming in Camponotus pennsylvanicus larvae and discuss our results in light of individual versus social immunity (as defined by [7]).
2. Material and methods
(a). Colony collection and maintenance
Five colonies of C. pennsylvanicus were maintained as described in Hamilton et al. [8]. This species was selected as a test organism owing to its lack of a metapleural gland, which is responsible for the secretion of antibiotics [4]. Yet, this ant does excrete formic acid, recently reported as providing biochemical protection against fungi [13].
(b). Bacterial cultures
The ecologically relevant Serratia marcescens, an opportunistic pathogen, is known to infect ants [19]. Bacterial cultures were grown to a final concentration of 108 cells ml−1 following the protocols in the electronic supplemental material. Vaccines were created by boiling for 15 min. Active bacteria were identically grown, except for boiling.
(c). Injections and census
A total of 330 second and third instar larvae were divided into three treatments: (i) naive cold-immobilized, (ii) cold-immobilized injected with 0.3 μl of sterile Ringer solution and (iii) cold-immobilized vaccinated with 0.3 μl solution of a 108 cells ml−1 of heat-killed S. marcescens. Two replicates, each with 10 larvae per treatment, were established for four colonies; three replicates were set up for the fifth colony. The larvae were kept for three days inside a Petri dish (60 × 15 mm) lined with moist filter paper along with two workers. Presumably, larvae mounted an immune response during this time. Workers were provided with honey agar, water tubes and allowed to interact freely with the larvae. On the third day, all larvae were challenged by injecting 0.3 μl of a solution containing 102 cells ml−1 of live S. marcescens. On the challenge day, two additional subnests (composed of 10 naive untreated larvae and two workers) were also established (total n = 80). Their survival was used to determine death owing to separation from their parental nest. Larvae were monitored for 12 days and the number of live, dead or missing/cannibalized individuals recorded. For confirmation purposes, dead larvae were removed and transferred to a ‘morgue’, a Petri dish lined with moist filter paper maintained at 25°C until larvae turned bright pink (see electronic supplementary material, figure S1c).
3. Results
(a). Survival
Both colony of origin [Wald Statistic (WS) = 54.0, d.f. = 4, p ≤ 0.0001] and treatment (WS = 57.5, d.f. = 2, p ≤ 0.0001) were significant and independent predictors of larvae survival (Cox proportional regression, SPSS). After controlling for the effect of treatment, larvae from the different colonies exhibited between two and five times the likelihood of death relative to the colony with the highest survival (see electronic supplementary material, figure S2). Although larvae from these colonies differed in the magnitude of their susceptibility, insects from the vaccinated/challenged treatment consistently exhibited the highest survival relative to the other treatments (see electronic supplementary material, figure S3). After controlling for the effect of colony and relative to the vaccinated/challenged larvae, Ringer/challenged and naive/challenged larvae were three (WS = 35.1, d.f. = 1, p ≤ 0.0001) and four times (WS = 54.6, d.f. = 1, p ≤ 0.0001) more likely to die, respectively (table 1 and figure 1).
Table 1.
Survival parameters of C. pennsylvanicus larvae as a function of treatment. (n) Represents original sample size.
| susceptibility parameter (symbols explained correspond to the different treatments of figure 1) | treatment |
||||
|---|---|---|---|---|---|
| naive/challenged (squares) | Ringer/challenged (triangles) | vaccinated/challenged (inverted triangles) | p-value | unchallenged (open circles)¶ | |
| LT50 ± s.e.* | 4 ± 0.4a (n = 110) | 5 ± 0.2a (n = 110) | 8 ± 0.5b (n = 110) | overall log rank = 57.5, d.f. = 2 , p ≤ 0.0001† overall Breslow = 41.2, d.f. = 2, p ≤ 0.0001‡ |
>12c (n = 80) |
| % survival at 12 days post-challenge | 0 | 0 | 0 | n.a. | 100 |
| relative hazard (95%CI) | 4.0 (2.7–5.8)a | 3.0 (2.0–4.3)a | referenceb | overall χ2 = 102.8, d.f. = 6; p ≤ 0.0001 | n.a. |
*LT50 values followed by the same letter denote no significant differences in the pairwise comparisons of survival distributions after a Bonferroni correction.
†Log rank test compared differences in the survival distributions during the earlier parts of the census period.
‡Breslow test compared differences in the survival distributions during the later parts of the census period.
¶The LT50 estimate of unchallenged larvae extended beyond the census period. To generate a conservative analysis, the survival parameters of the unchallenged larvae were not included in the statistical analyses on the fifth column of this table.
Figure 1.
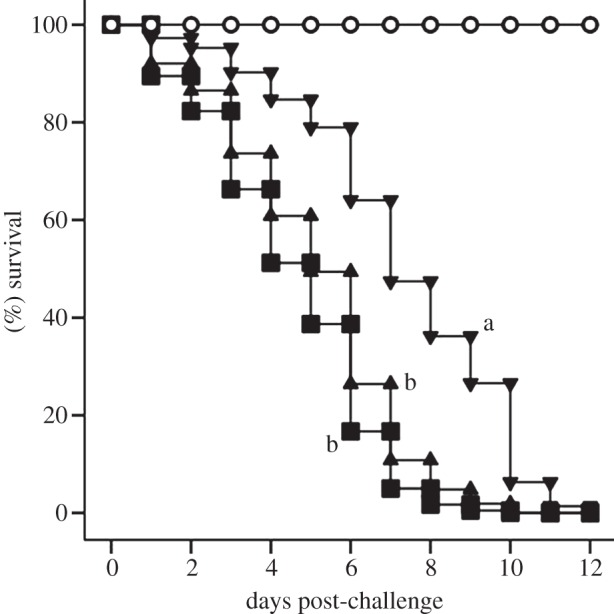
Time course of survival as a function of treatment. Open circles, naive/unchallenged, inverted triangles, vaccinated/challenged, triangles, Ringer/challenged and squares, naive/challenged larvae, all after controlling for the effect of colony of origin. Pairwise significant comparisons are indicated by different letters next to each survival curve. To be conservative, the survival data of the naive (never challenged) treatment were excluded from the statistical analysis. This figure should be interpreted together with parameters of table 1.
All challenged larvae, regardless of their original treatment and parental colony, perished by day 12 post-challenge (figure 1 and table 1; electronic supplementary material, figure S3). However, the time course of survival differed significantly, with vaccinated/challenged larvae exhibiting twice and 1.6 times longer median survival time (LT50) than the naive/challenged and the Ringers/challenged treatments, respectively (figure 1 and table 1; electronic supplementary material, figure S3). While controlling for the effect of parental colony, all naive/challenged and Ringer/challenged larvae died earlier than vaccinated/challenged larvae. Unchallenged naive larvae did not suffer any mortality (figure 1). No significant interactions between colony of origin and treatment were detected (WS = 7.5, d.f. = 11 (d.f. reduced due to linearly dependent covariates), p = 0.7). Taken together, these multiple survival parameters are consistent with young larvae exhibiting immune-priming.
(b). Cannibalism and confirmation rates
Average cannibalism across the five colonies did not differ significantly when comparing naive/challenged, Ringer/challenged and vaccinated/challenged treatments (figure 2a). In sharp contrast, unchallenged naive larvae suffered no cannibalism. Hence, cannibalism was preferentially directed towards animals that were bacterially infected and/or had their cuticle punctured (F3,16 = 8.1; p = 0.002; ANOVA; figure 2a). The average number of dead larvae positively confirming bacterial infection did not differ significantly across the three challenged treatments (F2,12 = 0.4; p = 0.6; ANOVA; figure 2b).
Figure 2.
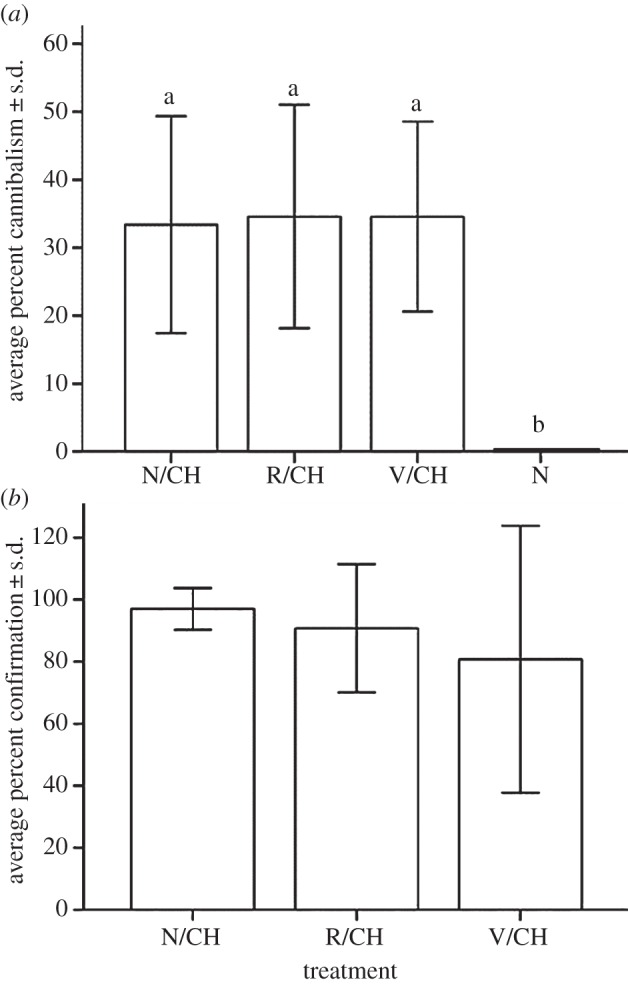
(a) Average percent cannibalism ± s.d. and (b) average number of dead larvae ± s.d. positively confirming S. marcescens infection. In (a), significant post hoc pairwise comparisons (Tukey's HSD) are indicated with different letters above the bars. These differences suggest that cannibalism may serve as a form of hygienic behaviour by workers, although further studies are needed to test the adaptive value of cannibalism in the face of pathogenic risks. In (b), the calculation of the percent confirmation excludes cannibalized individuals. Naive (never challenged) larvae had zero mortality and therefore, no confirmation data were available.
4. Discussion
Most work on the immunity of the social Hymenoptera has focused on measuring one or a few parameters related to the immune function of workers: haemocyte counts, protein and fat content, phenoloxidase (PO) activity, encapsulation rates and the presence of antimicrobial peptides through zone of inhibition assays ([15], and references therein). Seldom are these parameters accompanied with in vivo assays to test whether prior exposure to pathogen-associated molecular patterns results in reduced susceptibility. Recently, with the advent of the honeybee genome project (http://www.ncbi.nlm.nih.gov/assembly/254398/) and transcriptomic, proteomic and metabolomic approaches, the identification and quantification of relative expression of immune genes in bee larvae has begun ([18], and references therein). Comparatively, few studies have systematically investigated the onset of immune protection in other social insects, including ants. Yet, determining the developmental stage during which social insects start exhibiting immunological defences, some of which are analogous to the vertebrate adaptive immune systems [20], may shed light on the relation and potential trade-offs between inherent individual-level immune responses and the insect's social milieu. Previous studies have reported on the effect of age on immune maturation in several honeybee and ant species [13–16]. Unfortunately, given the confounding effects of division of labour, its associated pathogenic risks and immune senescence on disease susceptibility, the selective pressures and factor(s) fashioning immune responses of young versus old workers remain elusive. This is particularly true in species where age and risk-prone activities are coupled in time and space. Thus, pulling apart the independent effects of chronological age, pathogenic risks and immune senescence on the maturation and function of the immune system of workers becomes difficult. A case in point is the heightened PO activity of older Acromyrmex octospinosus workers relative to their younger counterparts [21]. Is the elevated PO in older foraging individuals due to heightened pathogenic pressures? Or is it a by-product of worker senescence, where the ability to store PO in the inactive non-toxic form (i.e. as Pro-PO) is compromised by old age? [12]. By studying the immune function of ant larvae, we can begin decoupling immune function maturation from its associated task-specific pathogenic risks.
Social Hymenoptera larvae depend on older siblings for their nutrition and hygiene. Because the costs of mounting an immune response during the immature stages may outweigh the benefits [22], particularly if meticulous grooming by workers reduces infection risks, it is reasonable to assume that brood care by workers may have emancipated larvae from energetically costly immune defences. Our results do not support such a contention. Young vaccinated/challenged larvae have the longest median survival time and exhibit delayed mortality. Further in vivo results are required to pinpoint if such survival benefits are influenced by the cellular and/or humoural compartments of the immune system and whether older larvae are better able to cope with immune insults. This is the first in vivo demonstration that young ant larvae benefit from rudimentary immune-priming. A more sophisticated (mature-like) response would have probably resulted in a higher proportion of vaccinated/challenged larvae surviving beyond 12 days. As larvae age, this observed basic immune system is likely to progressively mature to an optimal level before immune senescence in foragers sets in [21].
The social context in which these larvae are reared does not appear to eliminate the need for individual immune responses. This is interesting in light of research showing that honeybees have one-third fewer immune-related genes than Drosophila [23,24]. Admittedly, this comparison requires caution given the phylogenetic distance between these two insect groups, but together with the fact that honeybee Toll pathway genes have signatures of relaxed selective constraints [25], these results point towards the possibility of eusociality making innate immune defences superfluous [25]. In the case of C. pennsylvanicus, neither brood care (with its hygienic and biochemical protection benefits [13]) nor eusociality appear to undermine individual physiological immunity. On the contrary, individual and social immunity are probably complementing each other [8]. When dealing with disease resistance strategies of any social insects, it is imperative to focus on both the individual and collective behavioural, biochemical and physiological responses. Such perspective can foster a better understanding of the ecological dominance and vast geographical distribution of social insects that exploit microbial-rich environments.
Acknowledgements
R.B.R. conceived and designed the experiment, analysed the data and wrote the manuscript. T.M. and C.M. performed the experiments and maintained ant colonies. The authors appreciate the comments and suggestions from anonymous reviewers.
Funding statement
This research was partly funded by an NSF REU grant to R.B.R. (DEB 0447316).
References
- 1.Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551 (doi:10.1146/annurev.ento.50.071803.130420). [DOI] [PubMed] [Google Scholar]
- 2.Tsakas S, Marmaras VJ. 2010. Insect immunity and its signalling: an overview. Invert. Surviv. J. 7, 228–238 [Google Scholar]
- 3.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Schluns H, Crozier RH. 2009. Molecular and chemical immune defenses in ants (Hymenoptera: Formicidae). Myrmecol. News 12, 237–249 [Google Scholar]
- 5.Rosengaus RB, Traniello JFA, Bulmer MS. 2011. Ecology, behavior and evolution of disease resistance in termites. In Biology of termites: a modern synthesis (eds Bignell DE, Roisin Y, Lo N.), pp. 165–192 Berlin, Germany: Springer [Google Scholar]
- 6.Boomsma JJ, Schmid-Hempel P, Hughes WOH. 2005. Life histories and parasite pressure across the major groups of social insects. In Insect evolutionary ecology (eds Fellowes MDE, Holloway GJ, Rolff J.), pp. 139–175 Wallingford, UK: CABI Publishing [Google Scholar]
- 7.Cremer S, Armitage S, Schmid-Hempel P. 2007. Social immunity. Curr. Biol. 17, 693–702 (doi:10.1016/j.cub.2007.06.008) [DOI] [PubMed] [Google Scholar]
- 8.Hamilton C, Lejeune B, Rosengaus R. 2010. Trophallaxis and prophylaxis: social immunity in the carpenter ant Campanotus pennsylvanicus. Biol. Lett. 7, 89–92 (doi:10.1098/rsbl.2010.0466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ugelvig L, Cremer S. 2007. Social prophylaxis: group interaction promotes collective immunity in ant colonies. Curr. Biol. 17, 1–6 (doi:10.1016/j.cub.2007.10.029) [DOI] [PubMed] [Google Scholar]
- 10.Konrad K, et al. 2012. Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol. 10, e1001300 (doi:10.1371/journal.pbio.1001300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker TN, Hughes WHO. 2009. Adaptive social immunity in leaf-cutting ants. Biol. Lett. 5, 446–448 (doi:10.1098/rsbl.2009.0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helft F, Tirard C, Doums C. 2012. Effects of division of labour on immunity in workers of the ant Cataglyphis cursor. Insectes Soc. 59, 333–340 (doi:10.1007/s00040-012-0225-y) [Google Scholar]
- 13.Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, Cremer S. 2013. Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr. Biol. 23, 76–82 (doi:10.1016/j.cub.2012.11.034) [DOI] [PubMed] [Google Scholar]
- 14.Reber A, Chapuisat M. 2012. No evidence for immune priming in ants exposed to a fungal pathogen. PLoS ONE 7, e35372 (doi:10.1371/journal.pone.0035372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans JD. 2004. Transcriptional immune responses by honey bee larvae during invasion by the bacterial pathogen, Paenibacilus larvae. J. Invertebr. Pathol. 85, 105–111 (doi:10.1016/j.jip.2004.02.004) [DOI] [PubMed] [Google Scholar]
- 16.Wilson-Rich N, Dres ST, Starks PT. 2008. The ontogeny of immunity: development of innate immune strength in the honey bee (Apis mellifera). J. Insect Physiol. 54, 1392–1399 (doi:10.1016/j.jinsphys.2008.07.016) [DOI] [PubMed] [Google Scholar]
- 17.Schmid MR, Brockmann A, Pirk CWW, Stanley DW, Tautz J. 2008. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 54, 439–444 (doi:10.1016/j.jinsphys.2007.11.002) [DOI] [PubMed] [Google Scholar]
- 18.Gregorc A, Evans JD, Scharf M, Ellis JD. 2012. Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect Physiol. 58, 1042–1049 (doi:10.1016/j.jinsphys.2012.03.015) [DOI] [PubMed] [Google Scholar]
- 19.Sirviö A, Pamilo P. 2010. Multiple endosymbionts in populations of the ant Formica cinerea. BMC Evol. Biol. 10, 335 (doi:10.1186/1471-2148-10-335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz J, Armitage SAO. 2006. Alternative adaptive immunity in invertebrates. Trends Immunol. 27, 493–496 (doi:10.1016/j.it.2006.09.001) [DOI] [PubMed] [Google Scholar]
- 21.Armitage SA, Boomsma JJ. 2010. The effects of age and social interactions on innate immunity in a leaf-cutting ant. J. Insect Physiol. 56, 780–787 (doi:10.1016/j.jinsphys.2010.01.009) [DOI] [PubMed] [Google Scholar]
- 22.Pursall ER, Rolff J. 2011. Immune responses accelerate ageing: proof-of-principle in an insect model. PLoS ONE 6, e19972 (doi:10.1371/journal.pone.0019972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans JD, et al. 2006. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 15, 645–656 (doi:10.1111/j.1365-2583.2006.00682.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felix TM, Hughes KA, Stone EA, Drnevich JM, Leips J. 2012. Age-specific variation in immune response in Drosophila melanogaster has a genetic basis. Genetics 191, 989–1002 (doi:10.1534/genetics.112.140640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harpur BA, Zayed A. 2013. Accelerated evolution of innate immunity proteins in social insects: adaptive evolution or relaxed constraint? Mol. Biol. Evol. 30, 1665–1674 (doi:10.1093/molbev/mst061) [DOI] [PubMed] [Google Scholar]


