Abstract
We have previously shown that the range of prey sizes captured by co-occurring species of group-hunting social spiders correlates positively with their level of sociality. Here, we show that this pattern is probably caused by differences among species in colony size and the extent to which individuals participate in group hunting. We assess levels of participation for each species from the fraction of individuals responding to the struggling prey that partake as attackers and from the extent to which the number of attackers increases with colony size. Of two species that form equally large colonies, the one that captures on average larger prey engaged as attackers a significantly larger fraction of individuals that responded to struggling prey and also increased its number of attackers in larger colonies when presented with large prey items. Surprisingly, a third co-occurring species previously found to capture smaller insects than the other two exhibited the highest levels of participation. This species, however, typically forms small single-family colonies, thereby being limited in the size of insects it can capture. It is thus a combination of colony size and the extent of individual participation (or cooperation) that probably determines patterns of resource use in this community of co-occurring social predators.
Keywords: social, group hunting, cooperation, group size, participation
1. Introduction
Dietary differentiation helps facilitate coexistence among species. Among solitary predators this may occur via differences in body size and other morphological attributes [1,2]. Social predators, however, hunt in groups, with the size of prey captured probably determined by the size of the groups and the extent to which group members participate, or cooperate, in prey capture. To test this hypothesis, we investigated the group-hunting behaviour of three sympatric group-living spider species known to capture prey of different sizes and to display different levels of sociality [3]. In this community, the size of prey captured has been shown to correlate positively with a species’ level of sociality [3]. One species, Anelosimus baeza Agnarsson, which is considered to be subsocial as it lives in single-family groups, captures the smallest prey. Its colonies contain up to a few dozen young siblings hunting collectively prior to dispersing to live solitarily as adults. Another species, Anelosimus jabaquara Levi, captures medium-sized insects and is considered to be intermediate between subsocial and social as some females remain in the natal nest to adulthood to form multi-family groups, albeit with apparently limited levels of conspecific tolerance [4]. The final species, Anelosimus dubiosus Keyserling, captures the largest prey and is considered social as individuals remain together throughout their lives, showing higher degrees of conspecific tolerance and alloparental care [4]. We investigated the extent to which the size of the prey captured by these species was a reflection of the size of their colonies and of the extent to which individuals participated in prey capture, as larger colonies can potentially capture larger prey, but only if colony members actively cooperate in their capture.
We performed feeding trials on groups of varying size for each species using three size classes of prey. For each trial, we recorded the number of spiders responding to the prey, the average number of respondents attacking the prey at any time during the hunt versus the average number merely observing the hunt and the total number of respondents participating as attackers over the duration of the hunt. We found differences among species in the extent to which individuals participated in prey capture and in whether or not an increasing number of individuals participated as attackers as prey size increased. These differences, along with the spiders’ characteristic colony sizes, can explain the range of prey sizes they typically capture.
2. Material and methods
(a). Study species and habitat
The three species examined co-occur at Serra do Japi, a 354 km2 semi-protected forest in São Paulo, Brazil [5]. We located 16, 29 and 32 colonies of A. baeza, A. jabaquara and A. dubiosus, respectively, and estimated the population of each by visually inspecting them at dusk when spiders are most active at repairing their web [6]. We presented each colony with small (3.9 ± 0.16 mm), medium (8.0 ± 0.24 mm) and large (14.2 ± 0.37 mm) prey, roughly corresponding to one, two and three to four times the length of the largest spiders in the nests (older sub-adults in the subsocial species, adult females in the intermediate and social species). Prey consisted of live-captured flies and wasps that were propelled into the webs using filter-equipped straws. We first measured the magnitude of each colony's response by tracking all the spiders that emerged from their refuges and surrounded the prey (i.e. respondents). Of these respondents, we calculated the average number of spiders attacking the prey (i.e. biting or ensnaring) at any time during the hunt versus the average number merely observing the prey. By subtracting the latter from the former, we obtained a net average attackers-to-observers differential to assess the extent of individual participation in the hunt. Finally, we recorded the total number of attackers from each hunt to examine how overall participation varies with prey size and colony size.
(b). Analysis
For a between-species comparison of respondents and net average attackers-to-observers differential, we constructed a generalized linear mixed-effects model with a Poisson error distribution and a linear mixed-effects model with a normal error distribution, respectively, both of which included as factors log10 colony size, species and prey-size class, as well as their two- and three-way interactions. To test within-species variation in overall participation for particular prey sizes, we constructed separate mixed-effects ANCOVAs for each species examining the interaction of log10 colony-size and prey-size class on the log10[total number of attackers + 1]. In all models, colony identity was a random factor to account for each colony being tested with three prey items. Species differences were determined using Tukey–Kramer tests. All analyses were performed in R (v. 3.0.2).
3. Results
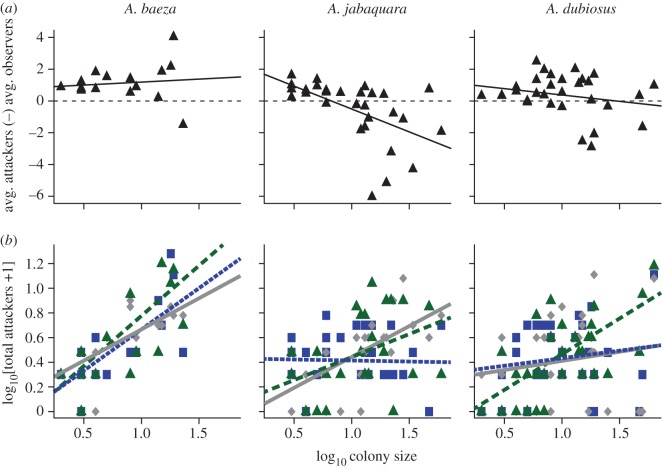
While all species employed a similar number of respondents for all prey sizes (figure 1a), the subsocial A. baeza maintained the highest net average attackers-to-observers differential, followed by the social A. dubiosus, and then the intermediate social–subsocial A. jabaquara (figure 1b). Moreover, while the subsocial and social species showed no correlation between colony size and the net average attackers-to-observers differential, the intermediate social–subsocial A. jabaquara had a significant negative correlation (figure 2a). That is, even though A. jabaquara increased the number of respondents with increasing colony size, most of these additional respondents did not actively engage the prey. Finally, we found that the subsocial A. baeza increased its total number of attackers as colony size increased, for all prey sizes, while the two more social species had significant interactions between colony-size and prey-size class: in the intermediate social–subsocial A. jabaquara, there was an increased number of attackers for both large and medium prey, while for the more social A. dubiosus this increase was present for large prey only (figure 2b). See the electronic supplementary material for complete statistical analyses.
Figure 1.
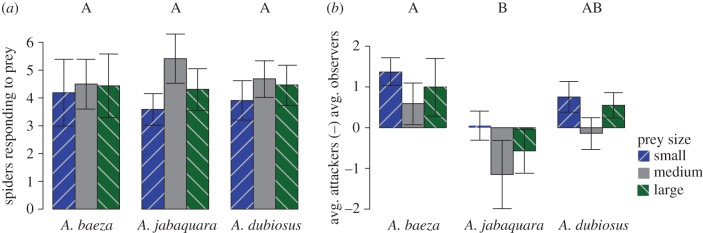
(a) Mean ± s.e. number of respondents for each species and prey size class. There were significant differences among prey sizes (F2,142 = 4.75, p = 0.01), but not among species (F2,71 = 1.68, p = 0.19) or the species–prey-size interaction (F4,142 = 0.07, p = 0.59). (b) Mean ± s.e. net average attackers-to-observers differential for each species and prey size class. There were significant differences among species (F2,71 = 4.84, p = 0.01) and prey sizes (F2,142 = 3.05, p = 0.05), but not for the interaction between species and prey size (F4,142 = 0.07, p = 0.99). Species with dissimilar letters above their bars are deemed significantly different via Tukey–Kramer tests.
Figure 2.
(a) Net average attackers-to-observers differential as a function of log10 colony size (CS). Of the three species, only the intermediate social–subsocial A. jabaquara has a significant correlation: A. baeza differential = 0.38 × CS + 0.81, r2 = 0.06, p = 0.67; A. jabaquara differential = −2.88 × CS + 2.37, r2 = 0.27, p ≤ 0.01; A. dubiosus differential = −0.80 × CS + 1.17, r2 = 0.02, p = 0.20. Each point represents one colony's response, averaged across all three prey sizes. Dashed line indicates an equal differential of attackers to observers. (b) Log10[total number of attackers + 1] for each species as a function of log10 colony size for the three prey sizes. There was no significant interaction between prey size and colony size for A. baeza (F2,42 = 0.52, p = 0.60), but there was for A. jabaquara (F2,81 = 4.74, p = 0.01) and A. dubiosus (F2,90 = 3.50, p = 0.03).
4. Discussion
While Guevara et al. [3] found that these co-occurring species captured different sizes of prey, here we have shown that both colony size and the extent of participation among colony members may play a role in determining this pattern. When species have similar group sizes, the species in which more colony members participate should be able to capture larger prey. Thus, the more social A. dubiosus not only maintained a higher net average attackers-to-observers differential than the less social A. jabaquara, but its larger colonies had more individuals participate only when encountering the largest prey. High levels of participation, however, cannot make up for smaller group sizes. Thus, even though the least social species, A. baeza, displayed the highest levels of participation among colony members, as a species it captures the smallest prey, probably because its colonies are not only significantly smaller than those of the other two species, but also because a large fraction of its population consists of solitary individuals who cannot subdue large prey [3]. Thus, populations of this species as a whole capture smaller prey on average.
We also found that the two more social species, A. jabaquara and A. dubiosus, do not passively filter aerial prey, but instead exhibit increased colony member participation for particular sizes of prey, perhaps to better exploit the most appropriate resources for their colony sizes. Yip et al. [7] found that growing spider colonies face a scaling predicament as the three-dimensional volume of the refuge area increases more quickly than the two-dimensional surface of the prey-capture web, resulting in less capture area per individual. The spiders make up for the fewer insects caught per capita by capturing increasingly large ones as colony size increases [7]. Thus, species that form large colonies are only found in lowland tropical and subtropical areas, where there is a much greater abundance of large insects than at higher elevations or latitudes [8,9].
Serra do Japi is a uniquely situated mid-elevation and mid-latitude habitat that is suitable for Anelosimus species of different levels of sociality [5]. Whether the dietary differentiation observed here is coincidental, or the product of mechanisms to prevent competition among these species, such as non-random species assemblage or character displacement, is yet to be determined. Perhaps selection on different behavioural strategies bestows each species with a particular range of colony sizes and a certain degree of cooperative behaviour, which in turn lead to dietary differentiation. Similar means of dietary differentiation may characterize other communities with otherwise potentially competing social predators [10]. Further studies of other social communities and of communities of other social predators are needed, however, before more definite conclusions can be drawn.
The elevated levels of participation displayed by group-living A. baeza juveniles might seem contradictory to their solitary adult lifestyle, but as adults, these spiders must be self-sufficient to survive and reproduce. Thus, one might expect strong selection against passivity, as group-living juveniles with a tendency to hesitate to attack might not survive as adults. By contrast, individuals living in permanent multi-family colonies probably face relaxed selection for aggressive behaviour, as passive individuals can take advantage of prey captured by their more aggressive nest-mates. Reduced aggression within social species has already been documented in the genus Anelosimus: females of less social species protect their egg-sacs more aggressively than females from more social cooperatively breeding species [11], and a closely related species shows a positive correlation between group size and the frequency of individuals displaying a passive behavioural phenotype [12].
Empirical and theoretical studies of group-hunting predators tend to focus on group size as the variable expected to correlate with prey capture success and the size of prey caught [13,14]. Here, we show that whether the hunting potential of a given group size is realized will depend on the behaviour of its members. Thus, studies of group-hunting predators may need to be revisited with an eye to the need for assessing the degree to which hunting party members do (or do not) participate in the hunt.
Acknowledgements
The authors thank João Vasconcellos-Neto for significant logistical assistance, and Suzana Diniz, Ruth Sharpe and Maxence Salomon for assistance with data collection. They also thank the Prefecture of Jundiai, São Paulo, for research permits.
Funding statement
The authors thank NSERC Discovery and the James S. McDonnell Foundation grants to L.A. for funding.
References
- 1.Gittleman JL. 1985. Carnivore body size: ecological and taxonomic correlates. Oecologia 67, 540–554 (doi:10.1007/BF00790026) [DOI] [PubMed] [Google Scholar]
- 2.Cohen JE, Pimm SL, Yodzis P, Saldana J. 1993. Body sizes of animal predators and animal prey in food webs. J. Anim. Ecol. 62, 67–78 (doi:10.2307/5483) [Google Scholar]
- 3.Guevara J, Gonzaga MO, Vasconcellos-Neto J, Avilés L. 2011. Sociality and resource use: insights from a community of social spiders in Brazil. Behav. Ecol. 22, 630–638 (doi:10.1093/beheco/arr022) [Google Scholar]
- 4.Marques ESA, Vasconcelos-Neto J, de Mello MB. 1998. Life history and social behavior of Anelosimus jabaquara and Anelosimus dubiosus (Araneae, Theridiidae). J. Arachnol. 26, 227–237 [Google Scholar]
- 5.Purcell J, Vasconcellos-Neto J, Gonzaga MO, Fletcher JA, Avilés L. 2012. Spatio-temporal differentiation and sociality in spiders. PLoS ONE 7, e34592 (doi:10.1371/journal.pone.0034592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agnarsson I. 2006. A revision of the New World eximius lineage of Anelosimus (Araneae, Theridiidae) and a phylogenetic analysis using worldwide exemplars . Zool. J. Linnean Soc. Lond. 146, 453–593 (doi:10.1111/j.1096-3642.2006.00213.x) [Google Scholar]
- 7.Yip EC, Powers KS, Avilés L. 2008. Cooperative capture of large prey solves scaling challenge faced by spider societies. Proc. Natl Acad. Sci. USA 105, 11 818–11 822 (doi:10.1073/pnas.0710603105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powers KS, Avilés L. 2007. The role of prey size and abundance in the geographical distribution of spider sociality. J. Anim. Ecol. 76, 995–1003 (doi:10.1111/j.1365-2656.2007.01267.x) [DOI] [PubMed] [Google Scholar]
- 9.Avilés L, Agnarsson I, Salazar PA, Purcell J, Iturralde G, Yip EC, Powers KS, Bukowski TC. 2007. Altitudinal patterns of spider sociality and the biology of a new mid-elevation social Anelosimus species in Ecuador. Am. Nat. 170, 783–792 (doi:10.1086/521965) [DOI] [PubMed] [Google Scholar]
- 10.Hayward MW, Kerley GIH. 2008. Prey preferences and dietary overlap amongst Africa's large predators. S. Afr. J. Wildl. Res. 38, 93–108 (doi:10.3957/0379-4369-38.2.93) [Google Scholar]
- 11.Samuk KM, LeDue EE, Avilés L. 2012. Sister clade comparisons reveal reduced maternal care behavior in social cobweb spiders. Behav. Ecol. 23, 35–43 (doi:10.1093/beheco/arr146) [Google Scholar]
- 12.Pruitt JN, Iturralde G, Avilés L, Riechert SE. 2011. Amazonian social spiders share similar within-colony behavioural variation and behavioural syndromes . Anim. Behav. 82, 1449–1455 (doi:10.1016/j.anbehav.2011.09.030) [Google Scholar]
- 13.Creel S, Creel NM. 1995. Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 50, 1325–1339 (doi:10.1016/0003-3472(95)80048-4) [Google Scholar]
- 14.Caraco T, Wolf LL. 1975. Ecological determinants of group sizes of foraging lions. Am. Nat. 109, 343–352 (doi:10.2307/2459698) [Google Scholar]