Abstract
Long-distance migrants may be particularly vulnerable to climate change on both wintering and breeding grounds. However, the relative importance of climatic variables at different stages of the annual cycle is poorly understood, even in well-studied Palaearctic migrant species. Using a national dataset spanning 46 years, we investigate the impact of wintering ground precipitation and breeding ground temperature on breeding phenology and clutch size of 19 UK migrants. Although both spring temperature and arid zone precipitation were significantly correlated with laying date, the former accounted for 3.5 times more inter-annual variation. Neither climate variable strongly affected clutch size. Thus, although carry-over effects had some impact, they were weaker drivers of reproductive traits than conditions on the breeding grounds.
Keywords: breeding success, migrant, climate change, carry-over effect, sub-Saharan Africa
1. Introduction
Many long-distance Palaearctic migrant birds have undergone recent dramatic population declines [1], and may be particularly vulnerable to impacts of climate change, as they are affected by weather conditions at various locations throughout their annual cycle. Climate on wintering grounds has been demonstrated to affect overwinter survival of some species [2,3], while changes in abundance of other species have been attributed to climate change on breeding grounds [4].
There is increasing awareness of the potential for linkages between breeding and wintering ground conditions to affect migrant populations [5]. Carry-over effects can occur if wintering ground climate influences the condition of birds when they come to depart on spring migration, potentially altering arrival time on breeding grounds and affecting reproductive success [6–8]. A recent analysis demonstrated a correlation between precipitation on West African wintering grounds and mean laying date in seven out of 11 migrant bird species [9] but did not account for breeding conditions. The relative importance of breeding and wintering ground climate in determining reproductive phenology and output across species therefore remains unknown.
We attempt to disentangle these effects using a dataset of UK nest records from 19 species of Afro-Palaearctic migrant birds over a 46-year period. We quantify the relative importance of precipitation on African wintering grounds (which drives vegetation growth and resource availability) and UK spring temperature on two reproductive traits likely to reflect female condition at the start of the breeding season: date of initiation of laying and clutch size.
2. Material and methods
We investigated 19 long-distance migrant species where all (17 species) or a significant proportion (two species) of the UK population winters south of the Sahara [10] and sufficient nest record data exist (table 1). Following the methods of Crick et al. [12], clutch size and first egg date (FED) were extracted from the British Trust for Ornithology's Nest Record Scheme dataset (1966–2011). Clutch size was the maximum number of eggs recorded in a given nest across all visits. FED was calculated by relating information about nest contents to the length of different stages of the nesting cycle (see electronic supplementary material, appendix S1).
Table 1.
Species used in analysis, with wintering zones derived from literature and recoveries of ringed birds and mean annual values and sample sizes for nesting parameters. Secondary wintering zones are given in parentheses where there is uncertainty over classification [11]. An unknown proportion of common chiffchaff and Eurasian blackcap winter north of the Sahara desert (northern).
| species | wintering zone | first egg date (days from 1 January) |
clutch size |
||
|---|---|---|---|---|---|
| mean (s.e.) | N | mean (s.e.) | N | ||
| European turtle dove Streptopelia turtur | humid (arid) | 167 (1.60) | 12.2 | 1.96 (0.011) | 11.3 |
| sand martin Riparia riparia | arid | 156 (3.90) | 33.7 | 4.30 (0.16) | 29.8 |
| barn swallow Hirundo rustica | southern | 169 (0.66) | 200 | 4.52 (0.011) | 454 |
| wood warbler Phylloscopus sibilatrix | humid | 143 (0.57) | 34.2 | 5.71 (0.054) | 18.3 |
| common chiffchaff. Phylloscopus collybita | arid/northern | 130 (0.97) | 54.5 | 5.46 (0.026) | 36.8 |
| willow warbler Phylloscopus trochilus | humid | 136 (0.50) | 84.4 | 5.93 (0.026) | 48.3 |
| Eurasian blackcap Sylvia atricapilla | arid/northern | 138 (0.92) | 39.8 | 4.55 (0.017) | 38.6 |
| garden warbler Sylvia borin | humid | 144 (0.69) | 22.1 | 4.35 (0.033) | 16.8 |
| lesser whitethroat Sylvia curruca | arid | 142 (0.75) | 8.65 | 4.61 (0.056) | 6.22 |
| common whitethroat Sylvia communis | arid | 147 (0.98) | 20.1 | 4.68 (0.019) | 31.0 |
| sedge warbler Acrocephalus schoenobaenus | arid | 148 (0.74) | 47.0 | 5.02 (0.025) | 34.9 |
| Eurasian reed warbler Acrocephalus scirpaceus | humid (arid) | 166 (0.79) | 198 | 3.89 (0.013) | 139 |
| spotted flycatcher Muscicapa striata | humid | 161 (0.43) | 70.4 | 4.24 (0.014) | 78.1 |
| pied flycatcher Ficedula hypoleuca | humid | 135 (0.62) | 430 | 6.69 (0.034) | 348 |
| common redstart Phoenicurus phoenicurus | arid | 137 (0.81) | 62.7 | 6.21 (0.034) | 49.0 |
| whinchat Saxicola rubetra | humid | 147 (0.52) | 26.9 | 5.55 (0.051) | 11.7 |
| wheatear Oenanthe oenanthe | arid | 136 (1.10) | 13.7 | 5.37 (0.071) | 11.2 |
| yellow wagtail Motacilla flava | arid | 147 (1.40) | 5.86 | 5.14 (0.066) | 5.38 |
| tree pipit Anthus trivialis | humid | 140 (0.79) | 19.9 | 4.84 (0.055) | 10.9 |
Nesting parameters were related to climatic conditions on species’ sub-Saharan wintering grounds defined using known winter African distribution and recoveries of ringed birds [11] into the following zones (see electronic supplementary material, appendix S2):
(i) Arid: 11° N–17° N, 17° W–13° E.
(ii) Humid: 4° N–8° N, 15° W–9° E.
(iii) Southern: 35° S–23° S, 17° E–33° E.
As most species that winter further south in Africa use the arid zone as a stopover point on northward migration [13], variables relating to this zone were tested across all species.
Precipitation on wintering grounds was calculated from dataset TS3.20 [14] as the sum of rainfall from May to October for West Africa (zones (i) and (ii)), and November to March for southern Africa (zone (iii)), the wet-seasons for these regions [15]. Species-specific spring temperature measures were calculated using the Central England Temperature dataset [16], as the mean temperature over the months during which 95% of laying attempts for each species have been recorded across all years, plus the month prior to this period [17]. Our results were robust to variation in the spring temperature window selected (see electronic supplementary material, appendix S3).
Separate linear mixed models were run for mean annual FED and clutch size using SAS v. 9.2 [18]. Both variables were normally distributed and estimates weighted by the inverse of their standard error, giving less weight to uncertain estimates. Predictor variables were spring temperature on breeding grounds and rainy season precipitation on wintering grounds. Species was included as a random effect, along with interactions between species and climate variables, to ensure that results were generalized across species. The species-specific results can be viewed in the electronic supplementary material, appendix S4. The proportion of variance explained by fixed effects was derived from changes in the variance components of the random effects.
3. Results
There was a significant advance in FED through time across all species from 1966 to 2011 (F1,815 = 287, p < 0.001; β =−0.21 days/year, 95% CI: −0.23, −0.19). Both arid zone precipitation of West Africa and UK spring temperature had significant negative effects on FED across all species (precipitation: F1,18 = 20.0, p < 0.001, β =−11.0 days m−1, 95% CI: −15.8, −6.16; temperature: F1,18 = 120, p < 0.001, β =−3.62 days/°C, 95% CI.: −4.27, −2.98). Spring temperature alone explained a much greater proportion of variance in FED (71%) than arid zone precipitation alone (21%; figure 1), accounting for a −0.12 day year −1 advance in FED. As the two climate variables were weakly correlated (r > 0.26 between arid zone precipitation and all measures of spring temperature), the addition of arid zone precipitation to the spring temperature model accounted for little additional variance (4%).
Figure 1.
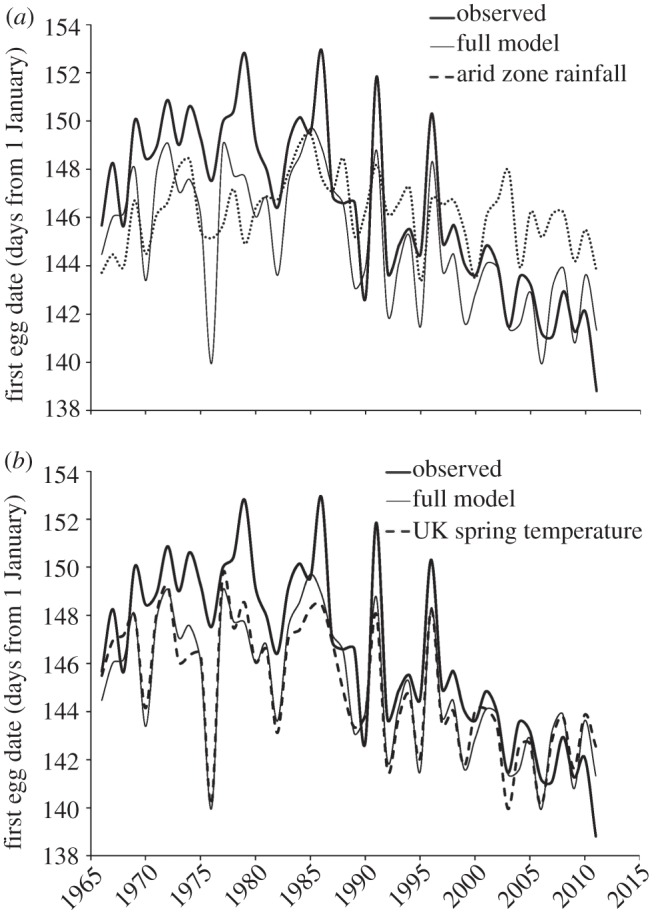
Observed and modelled changes in FED across 19 migrant species between 1966 and 2011. Plots shows predictions from models containing (a) arid zone precipitation or (b) UK spring temperature alone, plus a full model containing both terms that is identical across both panels.
There was a significant effect of species’ wintering zone on the relationship between arid zone rainfall and FED (wintering zone × arid zone rainfall interaction: F2,778 = 3.43, p = 0.03). Species that winter in the arid zone were more negatively affected by arid zone precipitation (β = −17.0 days m−1, 95% CI: −24.4, −9.7) than those that winter in the humid zone (β = −5.9 days m−1, 95% CI: −11.4, −0.5; contrast: F1,778 = 5.8, p = 0.017). Despite this, spring warming remained the main driver of FED in arid zone species (see electronic supplementary material, appendix S5). FED of the barn swallow Hirundo rustica, which winters in the southern zone, was also negatively affected by arid zone precipitation (see electronic supplementary material, appendix S4). Neither humid (F1,8 = 0.75, p > 0.4; β = −1.34 days m−1, 95% CI: −4.38, 1.69) nor southern zone (F1,42 = 0.6, p > 0.4; β =−7.08 days m−1, 95% CI: −25.1, 10.9) precipitation affected FED of species overwintering in these areas.
Although mean clutch size increased slightly with year (F1,769 = 3.9, p = 0.048; β = 0.0011 eggs year−1, 95% CI: −0.000011, 0.0021), it was not affected by either arid (F1,18 = 0.07, p > 0.7; β =−0.032 eggs m−1, 95% CI: −0.28, 0.21) or humid zone (F1,8 = 0.3, p > 0.6; β =−0.051 eggs m−1, 95% CI: −0.24, 0.14) precipitation. Southern zone precipitation negatively affected clutch size in barn swallows (F1,42 = 6.1, p = 0.02, β =−0.31 eggs m−1, 95% CI: −0.55, −0.063). Spring temperature in the UK showed a non-significant positive effect on clutch size across all species (F1,18 = 3.4, p = 0.08; β = 0.033 eggs °C−1, 95% CI: −0.0024, 0.069).
4. Discussion
Although precipitation in the arid zone of West Africa was correlated with long-distance migrant laying date, and especially for those migrants that winter in this region, we found little evidence that variation in such conditions had a major influence on FED across all species over 46 years (figure 1a). Breeding ground temperature explained 3.5× more inter-annual variation in laying date than wintering ground precipitation (figure 1b), and warming accounted for more than half of the observed FED advance [17].
Constraints on the ability of long-distance migrants to adapt to warming on the breeding grounds may increase their vulnerability to climate change [19,20]. Although Sahelian rainfall affects the timing of migrant arrival in Europe [6,7], our result suggests that this is not the main determinant of breeding phenology. Migrants nest earlier in warmer springs, although it remains unclear whether this advance is sufficient to avoid negative population consequences of warming. This adjustment may be achieved through covarying temperature effects on migratory speed across Europe [21]; for example, spring (April–June) temperatures in Spain and England are correlated (1960–2000, r = 0.60, p < 0.0001). Alternatively, as many migratory species are income breeders [22], using food resources from the breeding grounds for egg production, low temperatures on arrival may limit food availability and delay laying.
Clutch size across all species was weakly correlated with spring temperature and unrelated to African precipitation. This tentatively reinforces the importance of breeding season weather over carry-over effects in determining female breeding decisions. Several hypotheses may account for the lack of carry-over effect on clutch size. Wintering ground precipitation may not affect clutch size if it does not strongly influence arrival phenology. Secondly, after unfavourable winters, individuals may prioritize investment in reproduction over self-maintenance, resulting in carry-over effects on survival [23], which were not examined. Thirdly, any increase in the survival of low-quality females during favourable winters may increase the proportion of small clutches laid, masking any potential carry-over benefit for fitter individuals [24]. Finally, females experiencing favourable climatic conditions may elect to nest earlier rather than lay more eggs.
Humid and southern zone precipitation had little impact on breeding parameters. Arid region savannah vegetation is likely to be most responsive to precipitation, affecting resource availability. The arid zone is also used by the highest number of species and shows the greatest inter-annual fluctuations in precipitation, increasing our analytical power. The apparent negative effect of southern zone precipitation on barn swallow clutch size was an exception; wet winters also reduce survival rates in this species, potentially by reducing foraging conditions [25].
To conclude, we have demonstrated that despite the potential for impacts of climate change on wintering grounds to carry over and affect reproductive decisions [26], when averaged across a range of long-distance migrants the magnitude of such effects appears small relative to the influence of climate on breeding grounds. However, if future climate change degrades overwintering conditions more than those on breeding grounds, then carry-over effects could become more important through time.
Data accessibility
The annual estimates of laying date and clutch size used in this paper can be viewed at http://www.bto.org/about-birds/birdtrends and are available from the BTO.
Funding statement
We are grateful to the volunteer contributors to the NRS, which is funded by the JNCC/BTO partnership on behalf of the Country Agencies.
References
- 1.Sanderson FJ, Donald PF, Pain DJ, Burfield IJ, van Bommel FPJ. 2006. Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 131, 93–105 (doi:10.1016/j.biocon.2006.02.008) [Google Scholar]
- 2.Peach W, Baillie S, Underhill L. 1991. Survival of British sedge warblers Acrocephalus schoenobaenus in relation to west African rainfall. Ibis 133, 300–305 (doi:10.1111/j.1474-919X.1991.tb04573.x) [Google Scholar]
- 3.Baillie SR, Peach WJ. 1992. Population limitation in Palaearctic-African migrant passerines. Ibis 134, 120–132 (doi:10.1111/j.1474-919X.1992.tb04742.x) [Google Scholar]
- 4.Both C, Van Turnhout CAM, Bijlsma RG, Siepel H, Van Strien AJ, Foppen RPB. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. B 277, 1259–1266 (doi:10.1098/rspb.2009.1525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small-Lorenz SL, Culp LA, Ryder TB, Will TC, Marra PP. 2013. A blind spot in climate change vulnerability assessments. Nat. Clim. Change 3, 91–93 (doi:10.1038/nclimate1810) [Google Scholar]
- 6.Saino N, Szép T, Romano M, Rubolini D, Spina F, Møller AP. 2004. Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecol. Lett. 7, 21–25 (doi:10.1046/j.1461-0248.2003.00553.x) [Google Scholar]
- 7.Saino N, Rubolini D, Jonzén N, Ergon T, Montemaggiori A, Stenseth N, Spina F. 2007. Temperature and rainfall anomalies in Africa predict timing of spring migration in trans-Saharan migratory birds. Clim. Res. 35, 123–134 (doi:10.3354/cr00719) [Google Scholar]
- 8.Studds CE, Marra PP. 2011. Rainfall-induced changes in food availability modify the spring departure programme of a migratory bird. Proc. R. Soc. B 278, 3437–3443 (doi:10.1098/rspb.2011.0332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwarts L, Bijlsma RG, van der Kamp J, Wymenga E. 2009. Carry-over effects of Sahel drought on reproduction. In Living on the edge: wetlands and birds in a changing Sahel, pp. 472–479 Zeist, The Netherlands: KNNV Publishing [Google Scholar]
- 10.Wernham C, Toms M, Marchant J, Clark J, Baillie S. 2002. The migration atlas: movements of the birds of Britain and Ireland. London, UK: A&C Black [Google Scholar]
- 11.Ockendon N, Hewson CM, Johnston A, Atkinson PW. 2012. Declines in British-breeding populations of Afro-Palaearctic migrant birds are linked to bioclimatic wintering zone in Africa, possibly via constraints on arrival time advancement. Bird Study 59, 111–125 (doi:10.1080/00063657.2011.645798) [Google Scholar]
- 12.Crick HQP, Baillie SR, Leech DI. 2003. The UK nest record scheme: its value for science and conservation. Bird Study 50, 254–270 (doi:10.1080/00063650309461318) [Google Scholar]
- 13.Jones PJ. 1995. Migration strategies of Palaearctic passerines in Africa. Isr. J. Zool. 41, 393–406 [Google Scholar]
- 14.University of East Anglia Climatic Research Unit (CRU) [Phil Jones, Ian Harris] 2008. CRU Time Series (TS) high resolution gridded datasets. NCAS British Atmospheric Data Centre See http://badc.nerc.ac.uk/view/badc.nerc.ac.uk__ATOM__dataent_1256223773328276
- 15.Nicholson SE, Some B, Kone B. 2000. An analysis of recent rainfall conditions in West Africa, including the rainy seasons of the 1997 El Niño and the 1998 La Niña years. J. Clim. 13, 2628–2640 (doi:10.1175/1520-0442(2000)013<2628:AAORRC>2.0.CO;2) [Google Scholar]
- 16.Parker DE, Legg TP, Folland CK. 1992. A new daily central England temperature series, 1772–1991. Int. J. Clim. 12, 317–342 (doi:10.1002/joc.3370120402) [Google Scholar]
- 17.Crick HQP, Sparks TH. 1999. Climate change related to egg-laying trends. Nature 399, 423 (doi:10.1038/20839) [Google Scholar]
- 18.SAS Institute 2008. The SAS system for Windows. Release 9.2. Cary, NC: SAS Institute [Google Scholar]
- 19.Both C, Visser ME. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 (doi:10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- 20.Møller AP, Rubolini D, Lehikoinen E. 2008. Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc. Natl Acad. Sci. USA 105, 16 195–16 200 (doi:10.1073/pnas.0803825105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Both C. 2010. Flexibility of timing of avian migration to climate change masked by environmental constraints en route. Curr. Biol. 20, 243–248 (doi:10.1016/j.cub.2009.11.074) [DOI] [PubMed] [Google Scholar]
- 22.Silverin B. 1981. Reproductive effort, as expressed in body and organ weights, in the pied flycatcher. Ornis Scand. 12, 133–139 (doi:10.2307/3676040) [Google Scholar]
- 23.Le Bohec C, et al. 2008. King penguin population threatened by Southern Ocean warming. Proc. Natl Acad. Sci. USA 105, 2493–2497 (doi:10.1073/pnas.0712031105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laaksonen T, Ahola M, Eeva T, Väisänen RA, Lehikoinen E. 2006. Climate change, migratory connectivity and changes in laying date and clutch size of the pied flycatcher. Oikos 114, 277–290 (doi:10.1111/j.2006.0030-1299.14652.x) [Google Scholar]
- 25.Møller AP. 1989. Population dynamics of a declining swallow Hirundo rustica population. J. Anim. Ecol. 58, 1051–1063 (doi:10.2307/5141) [Google Scholar]
- 26.Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliffe LM. 2004. Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc. R. Soc. Lond. B 271, 59–64 (doi:10.1098/rspb.2003.2569) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The annual estimates of laying date and clutch size used in this paper can be viewed at http://www.bto.org/about-birds/birdtrends and are available from the BTO.


