Abstract
The Indian monsoons are a major seasonal climatic event over the Indian subcontinent, heralding the arrival of the wet season. Many features of life, biological and cultural, are intimately synchronized to this seasonality. In this paper, we show that the Indian monsoons might have played an important role in shaping the fruiting time and hence dispersal phenology of plant species in the subcontinent.
Keywords: India, monsoons, fruit dispersal, phenology, circular statistics, climate change
1. Introduction
The Indian monsoons, estimated to be about 15–20 million years old, are a major annual climatic event, bringing rainfall to the Indian subcontinent [1]. During the monsoons, there is a seasonal reversing of wind currents accompanied by corresponding changes in precipitation over the Indian land mass [2]. Based on the direction of wind currents, two distinct types of monsoon are recognized: Indian southwest (SW) and Indian northeast (NE) monsoons (figure 1). In the SW monsoon, moisture-laden winds from the Indian Ocean rush into the subcontinent and travel in a southwesterly direction up to the Himalayas, bringing in precipitation [3]. The SW monsoon, also called the summer monsoon, occurs between the months of June and September. During the NE monsoon, by contrast, cold wind flows from the Himalayas and the Indo-Gangetic Plains towards southern peninsular India, where it causes precipitation due to the moisture collected from the Bay of Bengal. The NE monsoon, also referred to as the winter or retreating monsoon, occurs between the months of October and December [4].
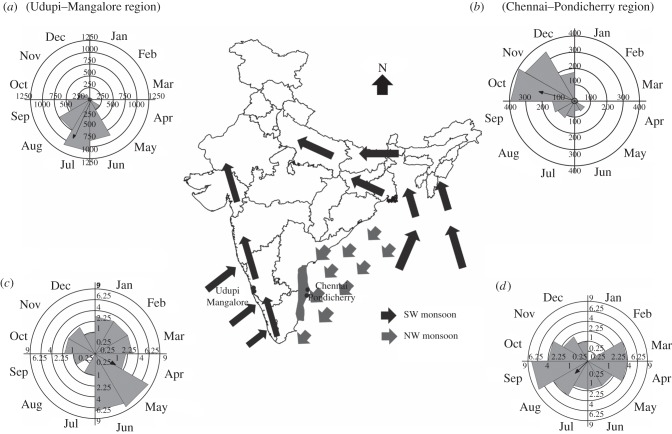
Figure 1.
Circular histograms, vectors and distributions showing the mean vector and distribution of (a) SW monsoon rainfall, (b) NE monsoon rainfall, (c) fruiting time of all species in the SW monsoon region, and (d) fruiting time of all species in NE monsoon region. Inset shows the path of the SW (dark arrows) and NE monsoons (light arrows) over India and the regions (Udupi–Mangalore and Chennai–Pondicherry) from which the data on fruiting time of species were sourced.
From an ecological perspective, the monsoons can be expected to have shaped the life-history strategies of plants and animals in the subcontinent, including the timing of critical events such as dispersal of fruits and seeds in plants and egg laying in birds [5,6]. Plants, for example, might be selected to synchronize their seed and fruit dispersal with the onset of the wet season [7] to maximize seed germination and seedling recruitment. Indeed, previous studies have shown that in many species, fruiting occurs just before the wet season such that seeds germinate and establish during the wet season when conditions are most favourable [8–13]. Among wind-dispersed species, winds that precede the wet season can be expected to disperse fruits to coincide with the onset of rainfall. Despite these observations, few studies have critically examined the temporal association of the monsoons and plant dispersal events [6,14].
In this paper, we hypothesize that the onset of the wet season is critical in determining the timing of fruit dispersal, and thus, peak fruit dispersal of plants occurring in regions receiving the SW monsoon should be different from that of plants occurring in regions receiving the NE monsoon. Specifically, we predict that for conspecific plants occurring in both regions, fruiting time (and hence dispersal) will peak before June for plants occurring in the SW monsoon path but will peak before October for those in the NE monsoon path. Using a set of common species occurring in both the SW and NE monsoon paths, we provide a test of the above prediction and show that the Indian monsoons might have played an important role in shaping fruiting time and thus the dispersal phenology of plants.
2. Material and methods
(a). Study sites and plant systems
We selected plants occurring in the Udupi–Mangalore (12.40°–13.65° N and 74.64°–75.65° E) and Chennai–Pondicherry regions (11.92°–13.50° N and 79.65°–80.33° E) in southern India that lie, respectively, in the paths of the Indian SW and Indian NE monsoons (figure 1). Based on the floristic information available for both sites, we selected 33 wind-dispersed species that are common to both the sites for which data on fruiting period were available. All species are native to India and included five shrubs, 11 climbers and 17 trees belonging to 13 families (see electronic supplementary material, table S1). Herbaceous species were excluded from analysis as these are ephemerals that tend to germinate after the rainfall and set seed at the end of the wet season.
The rationale of choosing only wind-dispersed species was based on the following assumptions: (i) because wind currents generally precede the wet season, wind-dispersed species, in contrast to species dispersed by other modes (e.g. animals), are expected to be more precisely cued to temporal differences in wind currents between the two regions; (ii) wind-dispersed species are expected to have a higher signal-to-noise ratio as these have only one dispersal vector compared with animal-dispersed species with a variety of vectors; and (iii) unlike animal-dispersed species, which can disperse fruits before and during the wet season, wind-dispersed species would be strongly selected to disperse their fruits before the onset of the wet season, for failure to do so can lead to inefficient dispersal due to wetting of their dispersal structures.
(b). Timing of dispersal
For each species, fruiting time was obtained from published records and floras of the respective regions [15,16] (see electronic supplementary material, table S1). Typically, the floras report a range of months over which the fruiting occurs. We took the mid-month of the reported range to determine peak fruiting time. For example, if a species is reported to fruit between the months of April to June, we took the mid-month, May as the period of peak fruiting. For cases in which a species was reported to fruit in the months of April and May, we took the earlier month as indicative of peak fruiting on the assumption that initiation of phenophase shift is a more appropriate indicator of phenology.
(c). Meteorological data
We obtained data on the average monthly precipitation (between 1982 and 2006; www.imd.gov.in; electronic supplementary material, figure S1) and average wind speed (between 2009 and 2013; www.windfinder.com) for the two regions, Udupi–Mangalore and the Chennai–Pondicherry (see electronic supplementary material, figure S2).
(d). Statistical analyses
The major aim of the analyses was to evaluate (i) whether the key meteorological data, namely monthly rainfall, differed significantly between the two regions receiving the SW and NE monsoons; (ii) whether the peak fruiting period of the species across the two regions differed significantly; and (iii) whether the shift in the peak fruiting period across the two regions was congruent with the shift in the onset of rainfall. Since all the data involve circular variables (monthly data on fruiting periods and meteorological data) where the beginning and end of their scales meet, we employed circular statistics to unravel the above questions [17].
The analysis was performed using Oriana v. 3.210 (Kovach Computing Services). The following parameters were computed for the three variables monthly wind speed, monthly rainfall and monthly frequency of species fruiting at each of the two (SW and NE monsoons) regions: circular mean and median vector, length of the mean vector and circular standard deviation with 95% and 99% confidence limits. We also performed a single-sample-distribution Rayleigh's test to determine whether the circular distribution of fruiting time was random or non-random. A two-sample Watson–Williams test (U) was performed to compare the mean vectors corresponding to the fruiting time between the SW and NE monsoon regions. We also determined whether the shift in the mean vector of fruiting time at the two regions was aligned with the expectation of our hypothesis, i.e. if the mean vector of fruiting in the SW and NE regions, respectively, precedes the mean vector of rainfall in the corresponding regions. Analysis was done separately for each plant habit (five shrubs, 11 climbers and 17 trees) and also by grouping the data for all three habits together.
3. Results
The two regions differed significantly with respect to their timing of average monthly rainfall. The mean vector for rainfall was 203° (July) for the region receiving the SW monsoon, while it was 285° (October) for that receiving the NE monsoon (table 1 and figure 1). Distribution of monthly rainfall was significantly non-random for both regions (Rayleigh test, p < 0.01). Compared with the NE monsoon (length of mean vector = 0.57), temporal spread of the SW monsoon was more compact (length of the mean vector = 0.71).
Table 1.
Circular statistics for rainfall and fruiting time of species in the two regions receiving the Indian SW and Indian NE monsoons.
| data | monsoons |
dispersal (all species) |
dispersal (only trees) |
|||
|---|---|---|---|---|---|---|
| SW | NE | SW | NE | SW | NE | |
| no. observations | 3906 | 1476 | 33 | 33 | 17 | 17 |
| data grouped | yes | yes | yes | yes | yes | yes |
| group width (and no. groups) | 30° (12) | 30° (12) | 30° (12) | 30° (12) | 30° (12) | 30° (12) |
| mean vector (μ) | 203.47° | 284.98° | 89.08° | 220.58° | 111.34° | 190.67° |
| mean group | July | Oct | Mar | Aug | Apr | July |
| length of mean vector (r) | 0.71 | 0.57 | 0.361 | 0.291 | 0.736 | 0.288 |
| concentration | 2.09 | 1.39 | 0.775 | 0.608 | 2.252 | 0.602 |
| circular variance | 0.29 | 0.43 | 0.639 | 0.709 | 0.264 | 0.712 |
| circular standard deviation | 47.13° | 60.82° | 81.77° | 90.32° | 44.86° | 90.38° |
| one sample test | ||||||
| Rayleigh test (Z) | 1985.32 | 478.33 | 4.304 | 2.7945 | 9.209 | 1.412 |
| Rayleigh test (p) | <1×10–12 | <1×10–12 | 0.012 | 0.06 | 2.20×10−5 | 0.247 |
Fruiting time of all species (shrubs, climbers and trees) in both regions was non-randomly distributed around the year (Rayleigh test; p < 0.01). Species in the region receiving the SW monsoon predominantly fruit in March just before the onset of the SW monsoon (mean vector = 89°, table 1 and figure 1). On the other hand, for the same set of species that occur in the region receiving the NE monsoon, the predominant fruiting period is in August, which is a month later than the onset of SW monsoon but a month earlier than the NE monsoon (mean vector = 221°, table 1 and figure 1). The shift in mean vectors corresponding to monthly fruiting period between the two regions was statistically significant (Watson–Williams U-test, p = 0.000; table 2). Furthermore, the direction of the shift was along the lines predicted, in concordance with the expectation that species should adjust peak fruiting time to precede the onset of rainfall (figure 1).
Table 2.
Watson–Williams U-test for fruiting time between regions receiving SW and NE monsoons.
| Watson–Williams U-test | climbers | shrubs | trees | all species |
|---|---|---|---|---|
| overall kappa | 0.582 | 1.235 | 1.186 | 0.688 |
| overall R | 5.523 | 7.268 | 14.200 | 11.926 |
| U | 0.717 | 1.312 | 6.980 | 15.051 |
| p = | 0.407 | 0.285 | 0.013 | 0.000 |
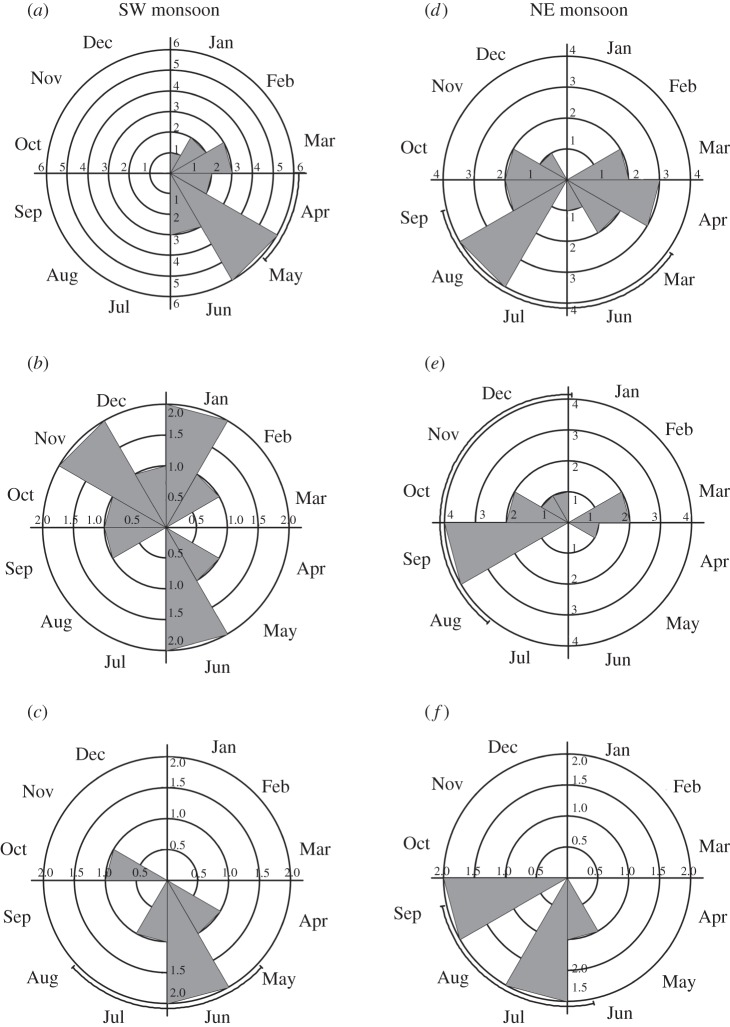
A similar pattern of shift in fruiting time between the two regions was also evident when the analysis was done separately for only trees (n = 17 species; table 1). The mean vector of fruiting time of trees in the SW monsoon region was 111° (April) compared with 191° (July) for trees in the NE monsoon region (Watson–Williams U-test, p = 0.013; table 2). There was, however, no significant shift in fruiting periods between the two regions for shrubs and climbers analysed individually (figure 2 and table 2).
Figure 2.
Circular histograms, vectors and distributions showing the mean vectors and distributions of fruiting times of (a) trees, (b) climbers and (c) shrubs in the SW (left) and of (d) trees, (e) climbers and (f) shrubs in the NE (right) monsoon region.
4. Discussion
Dispersal phenology is an important life-history component of plants [7], and the timing of dispersal is expected to be shaped by factors that maximize the lifetime fitness of the organism. One such factor is the arrival of the wet season. Species would be selected to optimize their timing of dispersal to long-stabilized seasonality of the wet seasons in their local habitats. While previous studies have demonstrated an association between dispersal events and onset of the wet season [9,13,18], few have documented adaptive phenological shifts in relation to climate change [19,20].
In this study of the two contrasting Indian monsoons that bring rainfall at two distinct periods of the year, we show that for a common set of wind-dispersed species, mean fruiting time is associated with the arrival of the wet season, independent of region. Thus, species occurring in the SW monsoon region fruit in March, before the onset of the SW monsoon in July, and the same species occurring in the NE monsoon region fruit in August, before the onset of the NE monsoon in October. The concordance of fruiting time with the arrival of the wet season is stronger in trees than in climbers and shrubs. While it is not immediately clear why such differences should prevail, it is possible that selection for dispersal phenology could be weaker in shrubs and climbers as they may have evolved more plastic responses consequent to their life-history strategies. Indeed, trees often have a single peak of fruit production, whereas shrubs and climbers tend to have two or more fruiting peaks [21,22].
Considering that the Indian monsoons stretch back around 15–20 Myr [1], it is possible that differences in fruiting time among populations of different regions reflect adaptive genetic differences. These observed shifts in phenology could also be a consequence of phenotypic plasticity, which itself might have been selected for. Reciprocal transplant experiments as well as hybridization studies between conspecific populations from contrasting regions (SW and NE monsoons) may throw more light on the nature of factors controlling the shift in fruiting time of these species.
Finally, it would be interesting to address how patterns of dispersal phenology as shaped by long-term climatological variables such as the Indian monsoons respond to both expected and current climate change over the Indian subcontinent. Lal et al. [23] have argued that date of onset of summer monsoon over India could become more variable in future owing to climate change. Under these conditions, it might be difficult for dispersal phenologies tied to monsoons to track an increasingly variable climate [24,25]. A recent study, for example, has shown that long-established phenologies can be disrupted by climate change [26]. It would, therefore, be interesting to follow the dispersal phenologies of some of the species addressed here with reference to future climate change in the Indian subcontinent.
Acknowledgement
We would like to thank Dr Peter Raven and Dr N. V. Joshi for useful comments and discussion, and the reviewers for their constructive criticism.
References
- 1.Sanyal P, Sinha R. 2010. Evolution of the Indian summer monsoon: synthesis of continental records. In Monsoon evolution and tectonics—climate linkage in Asia (eds Clift PD, Tada R, Zheng H.), pp. 342, 151–179 London, UK: Geological Society, Special Publications [Google Scholar]
- 2.Shankar D, Vinayachandran PN, Unnikrishnan AS. 2002. The monsoon currents in the north Indian Ocean. Progr. Oceanogr. 52, 63–120 (doi:10.1016/S0079-6611(02)00024-1) [Google Scholar]
- 3.Ramage C. 1971. Monsoon meteorology. International Geophysics Series, vol. 15, pp. 296 San Diego, CA: Academic Press [Google Scholar]
- 4.Gadgil S. 2003. The Indian monsoon and its variability. Annu. Rev. Earth Planet. Sci. 31, 429–467 (doi:10.1146/annurev.earth.31.100901.141251) [Google Scholar]
- 5.Pramod P, Yom-Tov Y. 2000. Breeding season and clutch size of Indian passerines. Ibis 142, 75–81 (doi:10.1111/j.1474-919X.2000.tb07686.x) [Google Scholar]
- 6.Murali KS, Sukumar R. 1994. Reproductive phenology of a tropical dry forest in Mudumalai, southern India. J. Ecol. 82, 759–767 (doi:10.2307/2261441) [Google Scholar]
- 7.Howe HF, Smallwood J. 1982. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–208 (doi:10.1146/annurev.es.13.110182.001221) [Google Scholar]
- 8.Corlett RT, LaFrankie JV. 1998. Potential impacts of climate change on tropical Asian forests through an influence on phenology. Climate Change 39, 439–453 (doi:10.1023/A:1005328124567) [Google Scholar]
- 9.Smythe N. 1970. Relationships between fruiting seasons and seed dispersal methods in a Neotropical forest. Am. Nat. 104, 25–35 (doi:10.1086/282638) [Google Scholar]
- 10.Jackson JF. 1981. Seed size as a correlate of temporal and spatial patterns of seed fall in a neotropical forest. Biotropica 13, 121–130 (doi:10.2307/2387714) [Google Scholar]
- 11.Auffenberg W. 1988. Gray's monitor lizard, p. 419 Gainesville, FL: University of Florida Press [Google Scholar]
- 12.Opler PA, Baker HG, Frankie GW. 1975. Reproductive biology of some Costa Rican Cordia species (Boraginaceae). Biotropica 7, 234–247 (doi:10.2307/2989736) [Google Scholar]
- 13.Frankie GW, Baker HG, Opler PA. 1974. Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Costa Rica. J. Ecol. 62, 881–919 (doi:10.2307/2258961) [Google Scholar]
- 14.Bhat DM. 1992. Phenology of tree species of tropical moist forest of Uttara Kannada district, Karnataka, India. J. Biosci. 17, 325–352 (doi:10.1007/BF02703158) [Google Scholar]
- 15.Bhat KG. 2003. Flora of Udupi. Udupi, India: Indian Naturalist [Google Scholar]
- 16.Das AA, Jayakanthan M, Muthukumaran J, Chandrasekar S, Balachandran N, Kichenamourthy S, Punetha A, Sundar D. 2012. Flora of Pondicherry region. See http://web.iitd.ac.in/~sundar/floradb/ (accessed on 12 June 2012)
- 17.Batschelet E. 1981. Circular statistics in biology. New York, NY: Academic Press [Google Scholar]
- 18.Wilkander T. 1984. Mecanismos de dispersión de diasporas de una selva decídua en Venezuela. Biotropica 16, 276–283 (doi:10.2307/2387936) [Google Scholar]
- 19.Menzel A, et al. 2006. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 (doi:10.1111/j.1365-2486.2006.01193.x) [Google Scholar]
- 20.Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. 2007. Shifting plant phenology in response to global change. Trends Ecol. Evol. 22, 357–365 (doi:10.1016/j.tree.2007.04.003) [DOI] [PubMed] [Google Scholar]
- 21.Opler PA, Frankie GW, Baker HG. 1980. Comparative phenological studies of treelet and shrub species in tropical wet and dry forest in the lowlands of Costa Rica. J. Ecol. 68, 167–88 (doi:10.2307/2259250) [Google Scholar]
- 22.Ralhan PK, Khanna RK, Singh SP, Singh JS. 1985. Certain phenological characters of the shrub layer of Kumaun Himalayan forests. Vegetatio 63, 113–120 (doi:10.1007/BF00044061) [Google Scholar]
- 23.Lal M, et al. 2001. Future climate change: implications for Indian summer monsoon and its variability. Curr. Sci. 81, 1196–1207 [Google Scholar]
- 24.Ibáñez I, Primack RB, Miller-Rushing AJ, Ellwood E, Higuchi H, Lee SD, Kobori H, Silander JA. 2010. Forecasting phenology under global warming. Phil. Trans. R. Soc. B 365, 3247–3260 (doi:10.1098/rstb.2010.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panchen ZA, Primack RB, Aniśko T, Lyons RE. 2012. Herbarium specimens, photographs, and field observations show Philadelphia area plants are responding to climate change. Am. J. Bot. 99, 751–756 (doi:10.3732/ajb.1100198) [DOI] [PubMed] [Google Scholar]
- 26.Ovaskainen O, Skorokhodova S, Yakovleva M, Sukhovb A, Kutenkov A, Kutenkova N, Shcherbakov A, Meyke E, Delgado MM. 2013. Community-level phenological response to climate change. Proc. Natl Acad. Sci. USA 110, 13 434–13 439 (doi:10.1073/pnas.1305533110) [DOI] [PMC free article] [PubMed] [Google Scholar]