Abstract
Avian embryos undergo extremely rapid development over a relatively short period of time, and so are likely to suffer high levels of oxidative damage unless this is mitigated by sufficient maternal allocation of appropriate antioxidants. At a species level, it is therefore predicted that antioxidants should be allocated to eggs according to the rate of embryonic growth, such that eggs containing embryos that grow faster are furnished with higher antioxidant levels, independent of egg size. We tested this prediction for three potentially important classes of dietary-derived yolk antioxidants: carotenoids, vitamin E and vitamin A. Across species, we found positive relationships between embryonic growth rate and total yolk levels of each of the three antioxidant classes. Moreover, there were consistent differences in antioxidant provision between pairs of species that share a common initial egg mass yet have differing rates of embryonic growth, such that the eggs of the faster-developing species have higher levels of carotenoids and vitamin E. These results may explain the marked interspecific variation in antioxidant provision and provide evidence for the role that these antioxidants play during embryonic development.
Keywords: oxidative stress, avian embryos, carotenoids, egg provisioning
1. Introduction
Avian embryos undergo extremely rapid development over a relatively short period of time. Prenatal growth is directly associated with the production of reactive oxidative metabolites and free radicals, which are by-products of normal metabolism and which can cause extensive damage to DNA, proteins and lipids (i.e. oxidative stress; [1]). Rapidly growing embryos are therefore likely to suffer high levels of oxidative damage unless this is mitigated by sufficient levels of appropriate antioxidants [2,3].
Dietary-derived antioxidants, such as carotenoids and vitamins E and A, deposited in egg yolk may reduce oxidative stress in developing embryos by deactivating oxidative metabolites and free radicals [2]. Vitamin E, particularly α-tocopherol, is considered to be one of the most effective dietary-derived antioxidants [2,4] and is therefore thought to be particularly important during growth [5]. Indeed, maternal supplementation with vitamin E has been shown to reduce oxidative stress in hatchlings [6]. In addition, carotenoids and vitamin A are also known to have antioxidant effects in vitro and in vivo [7], and the antioxidant properties of carotenoids in particular have repeatedly been shown to be important during growth and development [8], although the antioxidant role of carotenoids is not without controversy and may be less important than often assumed [9]. Maternal allocation of these antioxidants to egg yolk may therefore constitute a strategy to minimize oxidative damage to developing embryos [10].
However, growth rates, and hence potentially antioxidant requirements, vary markedly between bird species. Although eggs of the same initial mass produce hatchlings of equal weights, i.e. hatchling mass is always approximately 70% of the initial egg mass, irrespective of phylogeny or developmental maturity [11], the rate of development is influenced by the duration of incubation, which is phylogenetically constrained [12]. As a result, eggs of the same initial mass can produce hatchlings that have grown at considerably different rates: the 50 g egg of a mallard (Anas platyrhynchos), for example, develops over an incubation period of approximately 26 days compared with approximately 53 days for the 50 g egg of the phoenix petrel (Pterodroma alba) [12,13]. At a species level, it is therefore predicted that antioxidants should be allocated according to the rate of embryonic growth, such that eggs containing embryos that grow faster are furnished with higher antioxidant levels. This leads to two specific predictions: first, there will be a positive relationship between embryonic growth rate and the quantity of antioxidants allocated to egg yolk. Second, for any two species that share a common initial egg mass, yet have differing rates of embryonic growth, the eggs of the faster-developing species will have higher levels of antioxidant provision.
2. Material and methods
Data on yolk concentrations of total carotenoids, vitamin A and vitamin E (calculated as the summed concentrations of δ-, γ-, and α-tocopherol) obtained from Biard et al. [14] were cross referenced with egg and yolk mass data, and data on developmental mode for a variety of bird species collated from the literature [15]. Data for both antioxidant concentration and egg components were available for a total of 39 species from 12 orders (figure 1), although sample sizes were slightly reduced for analyses including vitamin E (n = 37) and vitamin A (n = 32). Total yolk levels of each antioxidant (μg) were calculated by multiplying the reported concentration (μg g−1) by the mean mass of the yolk (g) for each species. We focus on the total available antioxidants, rather than their concentration, as this represents the antioxidants available to the embryo during growth. Embryonic growth rate (g day–1) was assumed to correspond to the mass of the hatchling (g), which is 70% of the initial egg mass [11], divided by the incubation period for that species (days; see electronic supplementary materials, table S1). Total antioxidant and growth rate data were normalized using a log transformation prior to analysis.
Figure 1.
Assumed phylogenetic relationship between the avian species used in the present analyses, as derived from the phylogenetic analysis reported by Hackett et al. [16].
The relationships between embryo growth rate and yolk antioxidant levels were tested using both general linear models and, to account for autocorrelation from phylogenetic non-independence, phylogenetic generalized least-squares models (PGLMs) [17,18]. In both types of model, developmental mode (altricial, semi-altricial, semiprecocial or precocial) was controlled for by including it as a fixed factor. For the PGLMs, we used a phylogeny of birds resolved to the species level [12] based on the phylogeny produced by Hackett et al. [16]. All analyses were conducted in R 2.15.2 [19].
A bootstrap randomization procedure [20] was used to test whether there were differences in antioxidant allocation and yolk mass between pairs of species that have approximately equal initial egg masses yet differ in embryonic growth rate. Specifically, we randomly paired species that differed in egg mass by less than a given percentage (here, we tested all whole percentages between 5 and 15%) and calculated the difference in total antioxidant levels or yolk mass between the species of each pair having the fastest growth rate and the species with the slowest growth rate. This process was repeated 103 times. Under the assumption that mean differences should be greater than 0 if species with the fastest growth rate also allocate higher levels of total antioxidants to egg yolk, or have the largest yolks, bootstrap p-values were obtained by calculating the proportion of difference values less than or equal to 0 [20]. Results are presented for a 10% difference in egg mass, although they are qualitatively identical for all egg mass differences between 5 and 15%.
3. Results
Over all species, embryonic growth rate significantly predicted the total amount of carotenoids (GLM: F1,34 = 57.10, p < 0.001; PGLM controlling for phylogenetic non-independence: F1,34 = 45.85, p < 0.001), vitamin E (F1,32 = 138.14, p < 0.001; F1,32 = 70.50, p < 0.001) and vitamin A (F1,27 = 65.65, p < 0.001; F1,27 = 17.88, p < 0.001) allocated to the yolk. Hence, faster-growing embryos developed in eggs furnished with higher total levels of all three antioxidants (figure 2), regardless of their developmental strategy. These results are qualitatively identical if egg mass is included as a covariate, with growth rate significantly predicting antioxidant allocation in all cases (all p < 0.02; see electronic supplementary materials, tables S2–S7).
Figure 2.
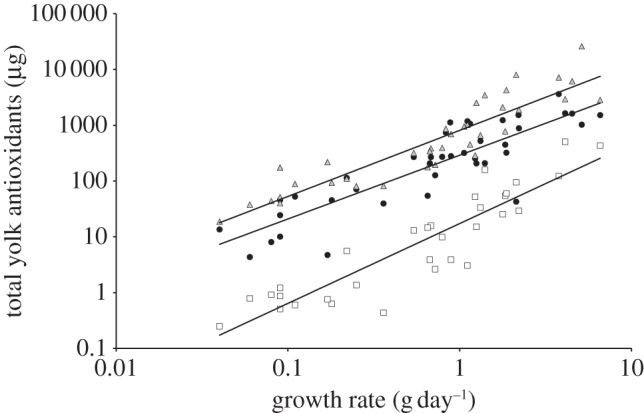
Relationships between embryonic growth rate and the total quantity of three classes of antioxidant: carotenoids (circles), vitamin E (triangles) and vitamin A (squares), allocated to egg yolk. Each data point represents a species’ average, and least-square regression lines are shown.
For comparisons of species pairs that had approximately equal initial egg masses, yet differed in embryonic growth rate, total levels of carotenoids and vitamin E, but not vitamin A, were all significantly higher in the species of the pair that had the faster growth rate (mean ± s.d. differences, carotenoids: 170.8 ± 33.5 µg, bootstrap p < 0.001; vitamin E: 212.0 ± 28.5 µg, p < 0.001; vitamin A: p > 0.05). Differences in total antioxidants do not result from differences in yolk mass between pairs of species growing at high and low rates (mean ± s.d. difference in yolk mass, 0.09 ± 0.24 g; p = 0.361).
4. Discussion
It has long been known that egg composition varies markedly between bird species [21–25], and recent evidence demonstrates that this holds for specific components of the egg, for instance antioxidants [14]. One possible explanation for the observed interspecific variation in antioxidant provision is that allocation of these antioxidants to egg yolk acts to minimize oxidative damage to rapidly developing embryos [10]. It was therefore predicted that, at a species level, antioxidants should be allocated to egg yolk according to the rate of embryonic growth, such that the eggs containing embryos that grow faster are furnished with higher antioxidant levels.
The results of this study confirm this prediction by showing positive relationships between growth rate and total yolk levels of three potentially important classes of antioxidants (with and without controlling for phylogenetic non-independence between species). In addition, the results directly demonstrate higher levels of carotenoids and vitamin E in the eggs of species with faster-growing embryos, after egg mass was controlled. As an example, the Sooty Tern (Onychoprion fuscatus) has an initial egg mass of 36.7 g and a growth rate of 0.89 g day−1, and it allocates 279.8 µg of carotenoids to the yolk. By contrast, the Eurasian coot (Fulica atra), which has an initial egg mass of 36.5 g and a 25% faster growth rate of 1.11 g day−1, allocates markedly more carotenoids (a total of 1180.2 µg) to its yolk. Similar relationships existed, on average, for all pairs of comparable species. It is interesting that the best support for our hypothesis comes from analyses involving total carotenoids and vitamin E. Given that these two classes of antioxidant appear to act synergistically [2,8], females may need to exert tight control over their relative allocation in order to ensure effective antioxidant protection.
Owing to their likely scarcity in natural diets [2,26], evidence suggests that the allocation of carotenoids (and potentially other antioxidants) is costly for females [27]. This may explain why we found such a tight linkage between embryo growth rates and antioxidant allocation: while under-allocation may be detrimental for developing embryos, over-allocation (which, if it occurred, would weaken the relationship with growth rate) may incur significant costs for females. Our results also highlight the potential importance of carotenoids for developing embryos, which may reflect the elevated requirement for antioxidants during this, if not other [9], life-history stages.
Acknowledgements
We thank Geoff Birchard, Carl Soulsbury and Marcello Ruta for helpful discussions, and two anonymous referees for comments which greatly enhanced the final paper.
References
- 1.von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. 1999. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. Lond. B 266, 1–12 (doi:10.1098/rspb.1999.0597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surai PF. 2002. Natural antioxidants in avian nutrition and reproduction. Nottingham, UK: Nottingham University Press [Google Scholar]
- 3.Catoni C, Peters A, Schaefer MH. 2008. Life history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim. Behav. 76 1107–1119 (doi:10.1016/j.anbehav.2008.05.027) [Google Scholar]
- 4.Klasing KC. 1998. Nutritional modulation of resistance to infectious diseases. Poult. Sci. 77, 1119–1125 [DOI] [PubMed] [Google Scholar]
- 5.de Ayala RM, Martinelli R, Saino N. 2006. Vitamin E supplementation enhances growth and condition of nestling barn swallows (Hirundo rustica). Behav. Ecol. Sociobiol. 60, 619–630 (doi:10.1007/s00265-006-0206-0) [Google Scholar]
- 6.Lin Y-F, Tsai H-L, Lee Y-C, Chang S-J. 2005. Maternal vitamin E supplementation affects the antioxidant capability and oxidative status of hatching chicks. J. Nutr. 135, 2457–2461 [DOI] [PubMed] [Google Scholar]
- 7.Kiokias S, Gordon MH. 2004. Antioxidant properties of carotenoids in vitro and in vivo. Food Rev. Int. 20, 99–121 (doi:10.1081/FRI-120037155) [Google Scholar]
- 8.Orledge JM, Blount JD, Hoodless AN, Pike TW, Royle NJ. 2012. Synergistic effects of supplementation of dietary antioxidants during growth on adult phenotype in ring-necked pheasants, Phasianus colchicus. Funct. Ecol. 26, 254–264 (doi:10.1111/j.1365-2435.2011.01932.x) [Google Scholar]
- 9.Costantini D, Møller AP. 2008. Carotenoids are minor antioxidants for birds. Funct. Ecol. 22, 367–370 (doi:10.1111/j.1365-2435.2007.01366.x) [Google Scholar]
- 10.Blount JD, Surai PF, Nager RG, Houston DC, Møller AP, Trewby ML, Kennedy MW. 2002. Carotenoids and egg quality in the lesser black-backed gull Larus fuscus: a supplemental feeding study of maternal effects. Proc. R. Soc. Lond. B 269, 29–36 (doi:10.1098/rspb.2001.1840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeming DC, Birchard GF. 2007. Allometry of egg and hatchling mass in birds and reptiles: roles of developmental maturity, eggshell structure and phylogeny. J. Zool. 271, 78–87 (doi:10.1111/j.1469-7998.2006.00219.x) [Google Scholar]
- 12.Deeming DC, Birchard GF, Crafer R, Eady PE. 2006. Egg mass and incubation period allometry in birds and reptiles: the effects of phylogeny. J. Zool. 270, 209–218 (doi:10.1111/j.1469-7998.2006.00131.x) [Google Scholar]
- 13.Whittow GC. 1997. Factors affecting egg mass loss in the phoenix petrel on Christmas Island. J. Field Ornithol. 68, 376–381 [Google Scholar]
- 14.Biard C, Gil D, Karadaş F, Saino N, Spottiswoode CN, Surai PF, Møller AP. 2009. Maternal effects mediated by antioxidants and the evolution of carotenoid-based signals in birds. Am. Nat. 174, 696–708 (doi:10.1086/606021) [DOI] [PubMed] [Google Scholar]
- 15.Deeming DC. 2007. Allometry of mass and composition in bird eggs: effects of phylogeny and hatchling maturity. Avian Poult. Biol. Rev. 18, 71–86 (doi:10.3184/147020607X289022) [Google Scholar]
- 16.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 (doi:10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 17.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 18.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 19.R Core Development Team. 2012. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 20.Chernick MR. 1999. Bootstrap methods: a practitioner's guide. New York, NY: John Wiley & Sons [Google Scholar]
- 21.Romanoff AL, Romanoff AJ. 1949. The avian egg. New York, NY: John Wiley & Sons [Google Scholar]
- 22.Carey C, Rahn H, Parisi P. 1980. Calories, water, lipid and yolk in avian eggs. Condor 82, 335–343 (doi:10.2307/1367405) [Google Scholar]
- 23.Sotherland PR, Rahn H. 1987. On the composition of bird eggs. Condor 89, 48–65 (doi:10.2307/1368759) [Google Scholar]
- 24.Vleck CM, Vleck D. 1987. Metabolism and energetics of avian embryos. J. Exp. Zool. 1(Suppl. 1), 111–125 [PubMed] [Google Scholar]
- 25.Vleck CM, Bucher TL. 1998. Energy metabolism, gas exchange, and ventilation. In Avian growth and development: evolution within the altricial–precocial spectrum (eds Strack JM, Ricklefs RE.), pp. 89–116 Oxford, UK: Oxford University Press [Google Scholar]
- 26.Olson VA, Owens IPF. 1998. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 13, 510–514 (doi:10.1016/S0169-5347(98)01484-0) [DOI] [PubMed] [Google Scholar]
- 27.Blount JD. 2004. Carotenoids and life-history evolution in animals. Arch. Biochem. Biophys. 430, 10–15 (doi:10.1016/j.abb.2004.03.039) [DOI] [PubMed] [Google Scholar]


