Abstract
The increase in the number of species with decreasing latitude is a striking pattern of global biodiversity. An important feature of studies of this pattern up to now has been the focus on species as the fundamental unit of interest, neglecting potential within-species ecological diversity. Here, we took a new perspective on this topic by measuring the degree to which individuals within populations differ in niche attributes across a latitudinal gradient (range: 54.01° S to 69.12° N). We show that 156 populations of 76 species across a wide range of vertebrate and invertebrate animal taxa contain more ecologically diverse assemblages of individuals towards lower latitudes. Our results add a new level of complexity to our understanding of global patterns of biodiversity and suggest the possibility that niche variation is partly responsible for the latitudinal gradients of species diversity.
Keywords: biodiversity, individual specialization, niche packing, niche variation
1. Introduction
Individuals can differ substantially in their ecological niches because of phenotypic differences between sexes (‘ecological sexual dimorphism’; [1]), age classes (‘ontogenetic niche shifts’; [2]) or readily distinguishable discrete morphs that are ecologically divergent (‘resource polymorphisms’; [3]). Even after accounting for these sources of variation, individuals can still differ in their niches, a phenomenon called ‘individual specialization’—an allusion to the fact that individuals have narrower niches and are, therefore, more specialized than the population as a whole [4]. The latter two forms of intraspecific niche variation are essentially the same phenomenon, representing two ends of a continuum from continuous to discrete niche variation. Importantly, in the last few years, ecologists have come to appreciate that niche variation is widespread [4,5] and can affect the ecological and evolutionary dynamics of populations and communities [6,7].
The latitudinal gradient of species diversity, in which the number of species increases towards the equator, is one of the most conspicuous ecological patterns [8,9]. An important feature of studies of this pattern up to now has been the focus on species as the fundamental unit of interest (but see [10,11]), neglecting potential within-species ecological diversity. Here, we took a new perspective on this topic by testing for the existence of a latitudinal gradient of within-species niche variation. If, as predicted by theory, niche variation decreases with the number of interspecific competitors [12], we should expect a gradient of increasing niche variation in the species-poor communities towards higher latitudes. In fact, some of the most striking examples of niche variation in natural populations result from competitive release in depauperate communities [13–15], suggesting that niche variation will tend to be stronger in less diverse communities. On the other hand, the potentially higher diversity of resources at lower latitudes—mainly driven by higher species diversity—should provide more ecological opportunity, which is expected to promote higher niche variation [16–19].
2. Material and methods
We reviewed the literature, searching for examples in which niche differences among individuals within-populations were quantified. We found 156 populations belonging to 76 animal species—71 of which measured individual variation in prey taxa consumed, one in microhabitat use, three in foraging behaviour and one in both prey taxa and microhabitat use—spanning a latitudinal gradient from 54.01° S to 69.12° N (electronic supplementary material, table S1). Measures of niche variation were directly collected from publications or calculated by us whenever raw ecological data were available. Geographical coordinates were directly taken from publications or, whenever they were not reported, we estimated them using the locality names provided in publications. We did not include data from experimental manipulations in our analysis, focusing on natural populations. Measures of niche variation were calculated at the population level. In order to avoid pseudo-replication, whenever two or more populations of the same species were analysed (or one single population was surveyed repeatedly across seasons or years), we calculated, for each species, a mean degree of niche variation across populations (or points in time) and estimated a geographical range centroid as the average of coordinates of reported localities (see electronic supplementary material, figure S1). It is possible that some of the populations assigned to a given species may actually be cryptic species [20], in which case our ‘species-level’ average would contain two or more closely related species. However, because we measured variation within populations and not between populations, our estimates of intraspecific variation should not be artificially inflated by interspecific variation.
We used the V index of within-population niche variation, which varies from 0 to 1 and assumes higher decimal values as individuals become more heterogeneous [21] (see the electronic supplementary material for details). We also used Roughgarden's [22] total niche width (TNW) to quantify the niche width of populations, which is the Shannon index of diversity applied to the population's distribution of resource use. TNW can be partitioned into a within-individual component (WIC) of niche width—the average individual niche width—and a between-individual component (BIC) of niche width—the variation between-individuals’ niche positions—so that TNW = WIC + BIC. Niche variation can also be measured by the ratio WIC/TNW [12,22]. In 12 cases in which this measure was reported and we had no raw data with which we could calculate the V index, we built a function to convert WIC/TNW into V (see the electronic supplementary material for details). We separately regressed V, TNW, WIC and BIC on absolute latitude. In the case of V, we ran an additional model including TNW as a covariate to account for the fact that V measures will tend to be higher as population diet breadth increases (see the electronic supplementary material for details). Because we sampled a wide range of taxa (see electronic supplementary material, table S1), we also ran mixed-effects models, including ‘taxon’ as a random effect to account for any taxonomic structure in our data. Measures of niche variation were calculated in the program IndSpec v. 1.0 [23] and regressions were performed in SYSTAT13.
3. Results
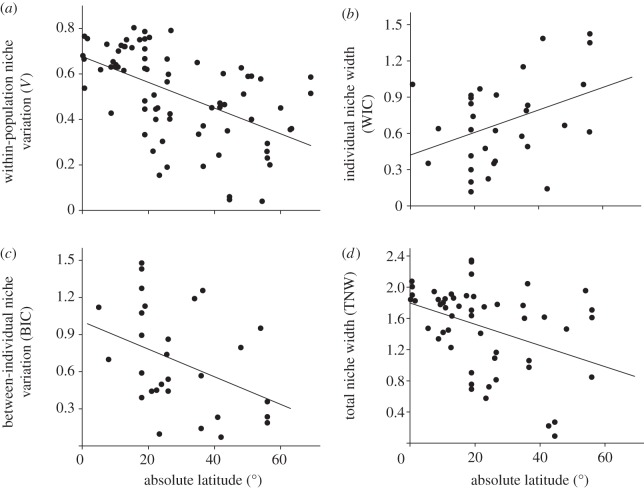
We found a negative relationship between the degree of within-population niche variation and latitude (figure 1a)—the model including TNW as a covariate yielded qualitatively similar results (table 1). We found that individual niches became increasingly narrower towards lower latitudes (figure 1b). This decrease in absolute individual niche width was offset by higher levels of interindividual niche variation (figure 1c), leading to the broadening of population niches (figure 1d). An analysis focusing on the subset of case studies measuring individual variation in prey taxa yielded essentially the same results (see electronic supplementary material, table S2). The models including ‘taxon’ as a random effect showed qualitatively similar results (see electronic supplementary material, table S3), but the relationship between WIC and latitude was no longer significant. This was probably a result of low power owing to the relatively small sample size and the inclusion of the random ‘taxon’ term.
Figure 1.
Relationship between latitude and niche variation. Dots represent species averaged across 1–15 populations. (a) The V index of within-population niche variation; higher values indicate that populations contain more ecologically diverse assemblages of individuals. (b) The within-individual component (WIC) of niche width representing the average niche width of individuals within populations, (c) the between-individual component (BIC) measuring the variation among individuals’ niche positions and (d) the TNW of the population; TNW = WIC + BIC.
Table 1.
Model results of regression analysis relating niche components and latitude.
| dependent variable | factor | slope | s.e. | t | p | F | d.f. | r2 | p |
|---|---|---|---|---|---|---|---|---|---|
| V | latitude | −0.006 | 0.001 | −5.390 | <0.001 | 29.051 | 1,73 | 0.29 | <0.001 |
| Va | 42.742 | 2,48 | 0.64 | <0.001 | |||||
| latitude | −0.005 | 0.001 | −4.086 | <0.001 | |||||
| TNW | 0.207 | 0.035 | 5.834 | <0.001 | |||||
| WIC | latitude | 0.009 | 0.004 | 2.222 | 0.034 | 4.938 | 1,29 | 0.15 | 0.034 |
| BIC | latitude | −0.011 | 0.005 | −2.387 | 0.024 | 5.696 | 1,29 | 0.16 | 0.024 |
| TNW | latitude | −0.014 | 0.005 | −3.009 | 0.004 | 9.055 | 1,50 | 0.15 | 0.004 |
aThe interaction term latitude × TNW was not significant (p = 0.774) and was removed from the model; V, WIC, BIC and TNW as in figure 1.
4. Discussion
Our results can be summarized as follows: the degree of within-population niche variation increases towards lower latitudes because individual niches become increasingly narrower and more disparate, with a concomitant expansion of the population niche (figure 2). If we assume that there is no gradient in WIC (see electronic supplementary material, table S3), latitudinal gradients in niche variation are primarily driven by changes in BIC. Regardless of the mechanism underlying changes in the degree of niche variation (both changes in WIC and BIC or only the latter), this pattern indicates that not only are there more species at lower latitudes, but also their populations contain more ecologically diverse assemblages of individuals. Our results suggest that the high diversity of resources (ecological opportunity; [5]) in the tropical region promotes higher intraspecific niche variation. Importantly, this effect is apparently more important than interspecific competition—which should increase with species diversity and constrain niche variation [12]—in determining the levels of intraspecific niche variation at a global scale.
Figure 2.
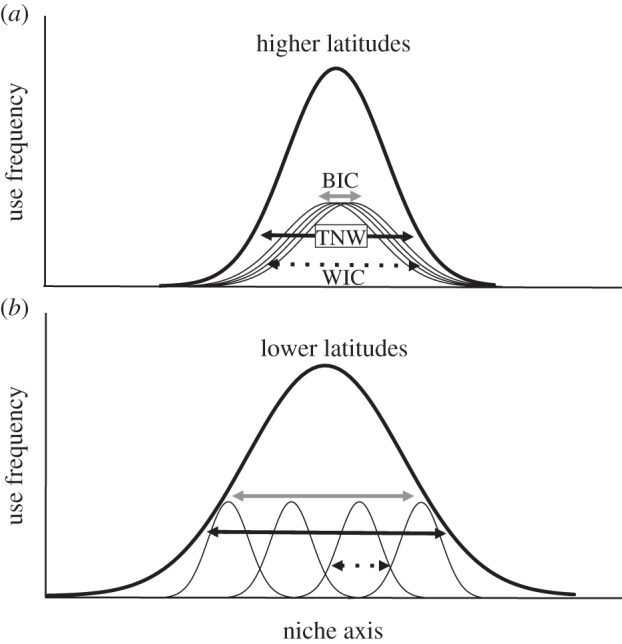
Conceptual representation showing the increase in niche variation from (a) higher to (b) lower latitudes. Curves represent the population (thick curves) and individual (thin curves) use frequency along an arbitrary niche axis. As we move from higher to lower latitudes, individual niches become narrower (smaller WIC) and more disparate (higher BIC). The decrease in WIC is offset by the increase in BIC, so that there is an expansion of the population niche, TNW (WIC, BIC and TNW as in figure 1).
The patterns described here are in stark contrast with predictions from classic ‘niche packing’ theories [24,25], according to which species should have narrower niches in species-rich communities. Moreover, our results suggest that within-species niche variation may actually be a driver of species diversity. First, within-population niche variation is predicted to facilitate speciation [26–28]. In line with this prediction, clades of amphibians and fishes in which resource polymorphisms have evolved are consistently more diverse than clades showing no such niche variation [29]. Second, within-species niche and life-history variation can increase population persistence and act as a buffer against extinction, as suggested by recent studies on insects, amphibians, lizards, snakes and mammals [30–33]. Finally, recent ‘individual variation’ theory has highlighted the importance of individual variation in allowing species coexistence, and therefore promoting species diversity [34,35]. This theory has been proposed as an alternative to ‘niche’ and ‘neutral’ theories of biodiversity [36] and predicts a positive relationship between niche variation and species diversity, as reported here. Our results, therefore, suggest the possibility that niche variation is partly responsible for the latitudinal gradients of species diversity.
Acknowledgements
We thank C. Hammerschlag-Peyer, I. Rosa, C. Roswell and T. A. Schriever for providing data, and L. Culot for helping with data analysis. Dan Bolnick and two anonymous reviewers made useful suggestions on earlier versions of the manuscript.
Funding statement
This research was supported by FAPESP (M.S.A.) and CNPq (R.C.).
References
- 1.Shine R. 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q. Rev. Biol. 64, 419–461 (doi:10.1086/416458) [DOI] [PubMed] [Google Scholar]
- 2.Polis GA. 1984. Age structure component of niche width and intra-specific resource partiotioning: can age groups function as ecological species? Am. Nat. 123, 541–564 (doi:10.1086/284221) [Google Scholar]
- 3.Skúlason S, Smith TB. 1995. Resource polymorphisms in vertebrates. Trends Ecol. Evol. 10, 366–370 (doi:10.1016/S0169-5347(00)89135-1) [DOI] [PubMed] [Google Scholar]
- 4.Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. 2003. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 161, 1–28 (doi:10.1086/343878) [DOI] [PubMed] [Google Scholar]
- 5.Araújo MS, Bolnick DI, Layman CA. 2011. The ecological causes of individual specialisation. Ecol. Lett. 14, 948–958 (doi:10.1111/j.1461-0248.2011.01662.x) [DOI] [PubMed] [Google Scholar]
- 6.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192 (doi:10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198 (doi:10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaston KJ. 2000. Global patterns in biodiversity. Nature 405, 220–227 (doi:10.1038/35012228) [DOI] [PubMed] [Google Scholar]
- 9.Mittelbach GG, et al. 2007. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (doi:10.1111/j.1461-0248.2007.01020.x) [DOI] [PubMed] [Google Scholar]
- 10.Martin PR, McKay JK. 2004. Latitudinal variation in genetic divergence of populations and the potential for future speciation. Evolution 58, 938–945 (doi:10.1111/j.0014-3820.2004.tb00428.x) [DOI] [PubMed] [Google Scholar]
- 11.Eo SH, Wares JP, Carroll JP. 2008. Population divergence in plant species reflects latitudinal biodiversity gradients. Biol. Lett. 4, 382–384 (doi:10.1098/rsbl.2008.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roughgarden J. 1974. Niche width: biogeographic patterns among Anolis lizard populations. Am. Nat. 108, 429–442 (doi:10.1086/282924) [Google Scholar]
- 13.Robinson BW, Wilson DS, Margosian AS, Lotito PT. 1993. Ecological and morphological differentiation of pumpkinseed sunfish in lakes without bluegill sunfish. Evol. Ecol. 7, 451–464 (doi:10.1007/BF01237641) [Google Scholar]
- 14.Ebenman B, Nilsson SG. 1982. Components of niche width in a territorial bird species: habitat utilization in males and females of the chaffinch (Fringilla coelebs) on islands and mainland. Am. Nat. 119, 331–344 (doi:10.1086/283913) [Google Scholar]
- 15.Schluter D. 1995. Adaptive radiation in sticklebacks: trade-offs in feeding performance and growth. Ecology 76, 82–90 (doi:10.2307/1940633) [Google Scholar]
- 16.Layman CA, Quattrochi JP, Peyer CM, Allgeier JE. 2007. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 10, 937–944 (doi:10.1111/j.1461-0248.2007.01087.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darimont CT, Paquet PC, Reimchen TE. 2009. Landscape heterogeneity and marine subsidy generate extensive intrapopulation niche diversity in a large terrestrial vertebrate. J. Anim. Ecol. 78, 126–133 (doi:10.1111/j.1365-2656.2008.01473.x) [DOI] [PubMed] [Google Scholar]
- 18.Herrera LGM, Korine MC, Fleming TH, Arad Z. 2008. Dietary implications of intrapopulation variation in nitrogen isotope composition of an Old World fruit bat. J. Mammal. 89, 1184–1190 (doi:10.1644/07-MAMM-A-263.1) [Google Scholar]
- 19.Murray IW, Wolf BO. 2013. Desert tortoise (Gopherus agassizii) dietary specialization decreases across a precipitation gradient. PLoS ONE 8, e66505 (doi:10.1371/journal.pone.0066505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfenninger M, Schwenk K. 2007. Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evol. Biol. 7, 121 (doi:10.1186/1471-2148-7-121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolnick DI, Svanback R, Araujo MS, Persson L. 2007. Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc. Natl Acad. Sci. USA 104, 10 075–10 079 (doi:10.1073/pnas.0703743104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roughgarden J. 1979. Theory of population genetics and evolutionary ecology: an introduction. New York, NY: Macmillan [Google Scholar]
- 23.Bolnick DI, Yang LH, Fordyce JA, Davis JM, Svanbäck R. 2002. Measuring individual-level resource specialization. Ecology 83, 2936–2941 (doi:10.1890/0012-9658(2002)083[2936:MILRS]2.0.CO;2) [Google Scholar]
- 24.MacArthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385 (doi:10.1086/282505) [Google Scholar]
- 25.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 26.Knudsen R, Klemetsen A, Amundsen PA, Hermansen B. 2006. Incipient speciation through niche expansion: an example from the Arctic charr in a subarctic lake. Proc. R. Soc. B 273, 2291–2298 (doi:10.1098/rspb.2006.3582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knudsen R, Primicerio R, Amundsen PA, Klemetsen A. 2010. Temporal stability of individual feeding specialization may promote speciation. J. Anim. Ecol. 79, 161–168 (doi:10.1111/j.1365-2656.2009.01625.x) [DOI] [PubMed] [Google Scholar]
- 28.Siwertsson A, Knudsen R, Præbel K, Adams C, Newton J, Amundsen P-A. 2013. Discrete foraging niches promote ecological, phenotypic, and genetic divergence in sympatric whitefish (Coregonus lavaretus). Evol. Ecol. 27, 547–564 (doi:10.1007/s10682-012-9607-x) [Google Scholar]
- 29.Pfennig DW, McGee M. 2010. Resource polyphenism increases species richness: a test of the hypothesis. Phil. Trans. R. Soc. B 365, 577–591 (doi:10.1098/rstb.2009.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsman A, Wennersten L, Karlsson M, Caesar S. 2012. Variation in founder groups promotes establishment success in the wild. Proc. R. Soc. B 279, 2800–2806 (doi:10.1098/rspb.2012.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González-Suárez M, Revilla E. 2013. Variability in life-history and ecological traits is a buffer against extinction in mammals. Ecol. Lett. 16, 242–251 (doi:10.1111/ele.12035) [DOI] [PubMed] [Google Scholar]
- 32.Forsman A, Hagman M. 2009. Association of coloration mode with population declines and endangerment in Australian frogs. Conserv. Biol. 23, 1535–1543 (doi:10.1111/j.1523-1739.2009.01244.x) [DOI] [PubMed] [Google Scholar]
- 33.Forsman A, Åberg V. 2008. Associations of variable coloration with niche breadth and conservation status among Australian reptiles. Ecology 89, 1201–1207 (doi:10.1890/07-1670.1) [DOI] [PubMed] [Google Scholar]
- 34.Clark JS. 2010. Individuals and the variation needed for high species diversity in forest trees. Science 327, 1129–1132 (doi:10.1126/science.1183506) [DOI] [PubMed] [Google Scholar]
- 35.Clark JS, Dietze M, Chakraborty S, Agarwal PK, Ibanez I, LaDeau S, Wolosin M. 2007. Resolving the biodiversity paradox. Ecol. Lett. 10, 647–659 (doi:10.1111/j.1461-0248.2007.01041.x) [DOI] [PubMed] [Google Scholar]
- 36.Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J. 2012. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252 (doi:10.1016/j.tree.2011.11.014) [DOI] [PubMed] [Google Scholar]