Abstract
Bryophytes achieve substantial biomass and play several key functional roles in boreal forests that can influence how carbon (C) and nitrogen (N) cycling respond to atmospheric deposition of reactive nitrogen (Nr). They associate with cyanobacteria that fix atmospheric N2, and downregulation of this process may offset anthropogenic Nr inputs to boreal systems. Bryophytes also promote soil C accumulation by thermally insulating soils, and changes in their biomass influence soil C dynamics. Using a unique large-scale (0.1 ha forested plots), long-term experiment (16 years) in northern Sweden where we simulated anthropogenic Nr deposition, we measured the biomass and N2-fixation response of two bryophyte species, the feather mosses Hylocomium splendens and Pleurozium schreberi. Our data show that the biomass declined for both species; however, N2-fixation rates per unit mass and per unit area declined only for H. splendens. The low and high treatments resulted in a 29% and 54% reduction in total feather moss biomass, and a 58% and 97% reduction in total N2-fixation rate per unit area, respectively. These results help to quantify the sensitivity of feather moss biomass and N2 fixation to chronic Nr deposition, which is relevant for modelling ecosystem C and N balances in boreal ecosystems.
Keywords: atmospheric nitrogen deposition, bryophytes, cyanobacteria, diazotroph, feather moss, nitrogen fixation
1. Introduction
Since the industrial revolution, human activity has caused a three- to fivefold increase in global emissions of reactive nitrogen (Nr), which has subsequently resulted in elevated rates of atmospheric Nr deposition [1]. Productivity in some biomes, for instance boreal forests, is strongly limited by N availability, and there is great interest in understanding the impacts of N deposition in these regions [2,3]. As an example, there is substantial speculation that elevated Nr deposition rates may increase N availability to vascular plants, and thereby increase carbon (C) sequestration, although this remains controversial [2].
Bryophytes can achieve substantial biomass in boreal ecosystems and play several key functional roles that can strongly influence how ecosystem C dynamics respond to environmental change factors, for example atmospheric Nr deposition [4]. Several species of bryophytes, including feather mosses, are known to associate with cyanobacteria that fix appreciable quantities of N2 [5]. Downregulation of their N2-fixation activity has the potential to off-set atmospheric Nr inputs, and thereby ‘buffer’ the supply of N to primary production [5,6]. Additionally, bryophytes thermally insulate underlying soils [7] and produce litter that is highly recalcitrant [8], which collectively diminish soil C and N mineralization rates [4]. Changes in bryophyte biomass in response to Nr deposition therefore have the potential to cause soil C losses, while inversely coupling above-ground responses by influencing N mineralization and availability to vascular plants [4]. Understanding how bryophyte biomass and N2-fixation activity responds to Nr deposition is therefore a key aspect of understanding how ecosystem C balances will respond to Nr deposition [4].
In this study, we used a 16-year experimental set-up in the boreal forest of northern Sweden to investigate how two feather moss species, Pleurozium schreberi and Hylocomium splendens, respond to simulated atmospheric Nr deposition. We tested the following hypotheses: (i) that the biomass of each feather moss would decline in response to N addition, (ii) that the N2-fixation activity of each species per unit mass and per unit area would decline in response to N addition and (iii) that the two feather moss species would show differential responses to N addition, as has been shown in their response to some other environmental change factors [9]. Testing these hypotheses will greatly enhance our understanding of how boreal ecosystem will change in response to Nr deposition [4].
2. Material and methods
This study was conducted at Svartberget Experimental Forest (64°14′ N, 19°46′ E) in the central boreal zone, near Vindeln Sweden. The experimental site consists of an approximately 120-year-old closed canopy Norway spruce (Picea abies (L.) Karst.) forest. In 1996, we established a replicated randomized block design experiment at the site consisting of three levels of N addition (0, 12.5 and 50 kg ha−1 yr−1) applied to 0.1 ha plots (n = 6) every year directly after snow melt (i.e. May). The lowest N fertilization level of 12.5 kg N ha−1 yr−1 (subsequently referred to as the ‘low N treatment’) was chosen to represent upper level Nr deposition rates in the boreal biome [6], whereas the 50 kg ha−1 yr−1 (subsequently referred to as the ‘high N treatment’) was chosen because many other N addition experiments in boreal forests have applied similar rates. To our knowledge, this is the longest running experiment in a boreal forest environment where levels of N addition realistically simulating Nr deposition have been applied to replicated stands at a relatively large experimental scale (i.e. 0.1 ha plots).
In 2011, the biomass of the two most abundant feather mosses at the site (P. schreberi and H. splendens) was estimated by measuring the shoot density per area of each species and the mean shoot mass for each species in each of the 0.1 ha treatment plots (n = 6). The biomass of each moss species per hectare was then estimated by scaling up the number of shoots to a per-hectare basis and multiplying this value by mean shoot mass. N2-fixation rates of the mosses were estimated using the acetylene reduction method [10]. Moss N2-fixation rates were measured five times between May and October.
Data were analysed using analysis of variance (ANOVA), with N treatment and species as fixed factors. When significant differences were detected, post hoc Student–Neuman–Keuls tests were conducted to evaluate pairwise differences. When necessary, data were transformed (log(x + 1)) to satisfy parametric assumptions. All data were analysed using SPSS (v. 20.0). Detailed methodology and supporting data are provided as the electronic supplementary material, appendices S1 and S2.
3. Results
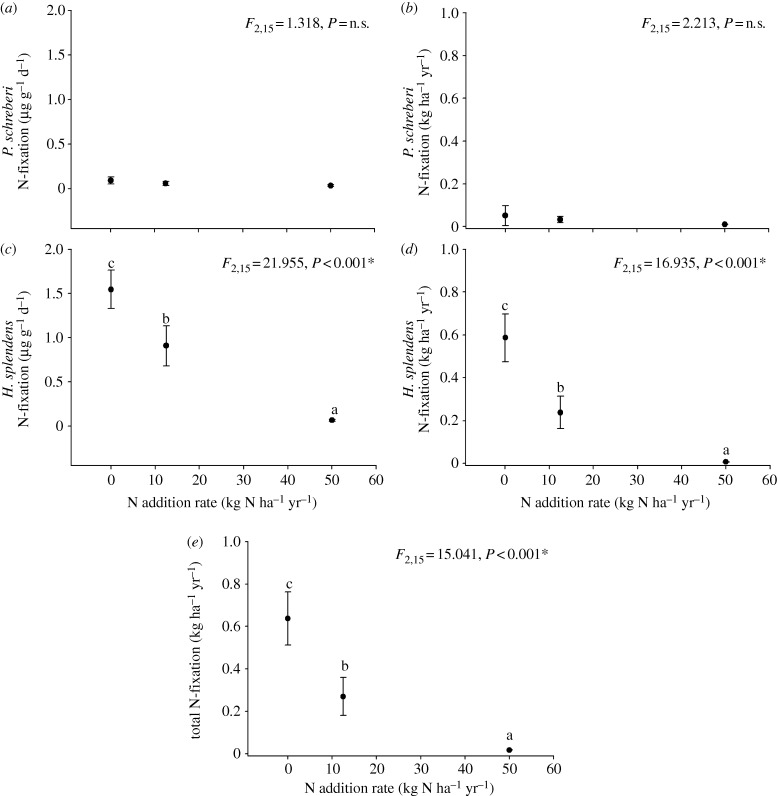
Hylocomium splendens showed a significantly lower mass per unit area but higher N2-fixation rate per unit mass and per unit area compared with P. schreberi (see electronic supplementary material, table S1; table 1 and figure 1). For both moss species, tissue N concentrations remained unchanged in response to the low N treatment but were significantly elevated in response to the high N treatment (table 1). The biomass of both species significantly declined in the low N treatment relative to the control, and in the high- relative to the low-N treatment (table 1); however, the significant interactive effect between moss species and N treatment showed that H. splendens biomass was significantly more sensitive to N addition than P. schreberi (see electronic supplementary material, table S1; table 1 and figure 1).
Table 1.
The mean (±s.e.) nitrogen content (%) and mass (Mg ha−1) of P. schreberi and H. splendens in response to 16 years of experimental N addition treatments (0, 12.5 and 50 kg N ha−1 yr−1). Data were compared using ANOVA, and significant post hoc differences are indicated by different letters within each row (a, b or c).
| 0 kg N ha−1 yr−1 | 12.5 kg N ha−1 yr−1 | 50 kg N ha−1 yr−1 | F-value | p-value | |
|---|---|---|---|---|---|
| N content (%) | |||||
| P. schreberi | 1.33 (0.08)a | 1.16 (0.04)a | 2.27 (0.09)b | 66.643 | <0.001 |
| H. splendens | 1.36 (0.05)a | 1.33 (0.069)a | 2.39 (0.08)b | 70.687 | <0.001 |
| mass (Mg ha−1) | |||||
| P. schreberia | 2.55 (0.33)c | 2.17 (0.32)b | 1.50 (0.13)a | 4.425 | 0.031 |
| H. splendens | 1.69 (0.16)c | 1.11 (0.10)b | 0.51 (0.05)a | 26.145 | <0.001 |
| totala | 4.25 (0.40)b | 3.27 (0.38)ab | 1.97 (0.12)a | 14.871 | <0.001 |
aData were transformed (log (x + 1)) to meet parametric assumptions prior to statistical analysis.
Figure 1.
The mean (±s.e.) N2-fixation rate (a,b) per unit mass, (c,d) per unit area of P. schreberi and H. splendens and (e) total N2-fixation rate per unit area in response to 16 years of experimental N addition treatments (0, 12.5 and 50 kg N ha−1 yr−1). Data were compared using ANOVA, and post hoc differences are indicated by different letters within each subpanel (a, b or c). An asterisk next to the p-value indicates that data were transformed (log (x + 1)) prior to analysis.
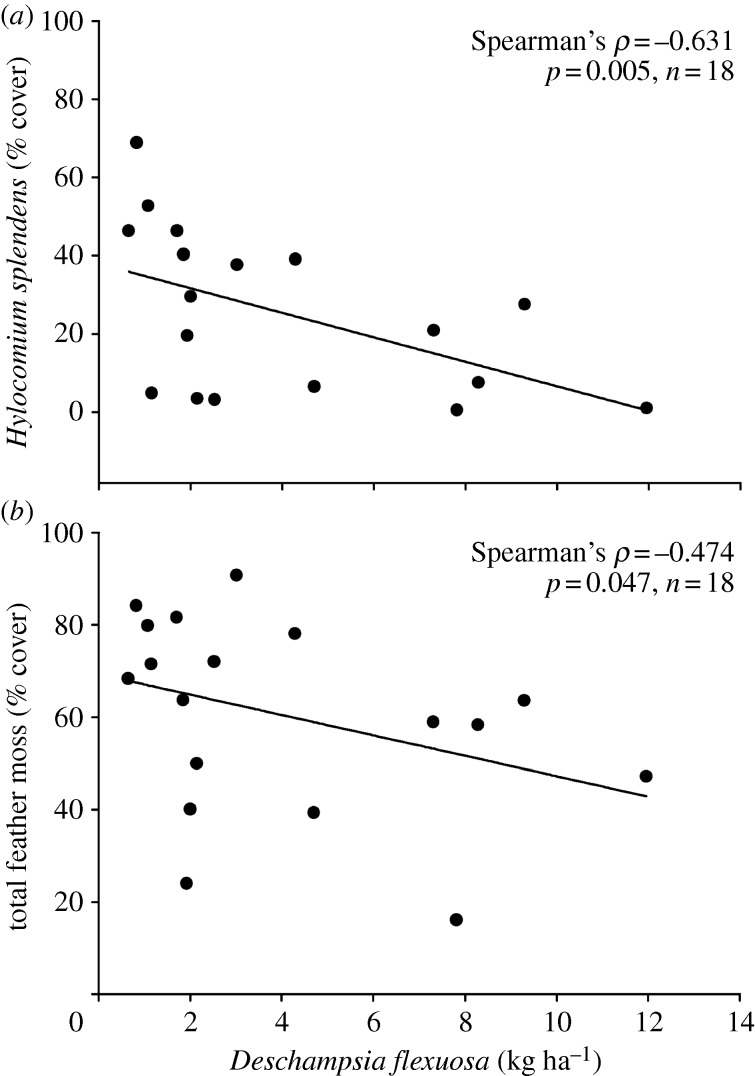
The two moss species differed substantially in their N2-fixation rates per unit mass and per unit area (see electronic supplementary material, table S1; figure 1), with rates of H. splendens an order of magnitude higher than P. schreberi (figure 1a–d). Hylocomium splendens N2 fixation per unit mass and per unit area significantly declined in the low N treatment relative to the control, and in the high- relative to the low-N treatment (see electronic supplementary material, table S1; figure 1b,d), whereas P. schreberi was unresponsive. The response of H. splendens N2-fixation rates resulted in a corresponding decline in total N2 fixation per unit area (figure 1e). Per cent cover of H. splendens and total feather moss cover were negatively correlated with the biomass of the grass species Deschampsia flexuosa (figure 2).
Figure 2.
The correlation between D. flexuosa biomass and percent cover of (a) H. splendens and (b) total feather mosses.
4. Discussion
Our aim was to determine how the biomass and N2-fixation activity of two widely occurring boreal moss species responded to chronic N addition, which has implications for understanding how boreal N and C cycles respond to atmospheric Nr deposition. In support of our first and third hypotheses the data clearly show that the biomass of both species significantly declined in response to even the low N addition treatment; however, the decline in H. splendens biomass was more severe than that of P. schreberi at both N addition levels. A likely mechanism contributing to this moss decline was an increase in vascular plant biomass, which is likely to have resulted in greater shading or soil water loss via transpiration, thereby affecting the moss growing environment [11,12]. In support of this mechanism, per cent cover of H. splendens and all feather mosses combined were negatively correlated with the biomass of D. flexuosa (figure 2a), a grass species that responded positively to the N addition treatments.
In support of our second and third hypotheses, N2-fixation activity per unit mass and per unit area declined in response to N addition. This negative response occurred only for H. splendens, which accounted for a majority (approx. 90%) of N2 fixation at the experimental site. Previous studies have shown that N2-fixation rates of P. schreberi can be equal to or higher than H. splendens [9,13]; however, several studies have suggested that cyanobacteria associated with H. splendens achieve higher N2-fixation rates in darker and colder environments relative to those associated with P. schreberi [9,14]. Given that our study site was established in a closed canopy spruce forest, with an inherently dark and cool understory environment, underlying physiological differences in cyanobacterial communities associated with the two feather mosses are likely to explain their different N2-fixation rates [15].
These results have implications for understanding how C and N cycles respond to atmospheric Nr deposition in boreal forests. Bryophyte tissues have been shown to decompose up to fivefold more slowly than vascular plant tissues [8]; furthermore, bryophytes have been shown to reduce mean soil temperatures during the growing season by 2–3°C and maximum soil temperatures by 4°C [7,16]. The significant reduction in moss biomass found in our study may reduce C sequestration rates into boreal soils, which may in part offset any increases in C sequestration in vascular plant biomass, thereby influencing ecosystem C balances in the boreal region [4]. In addition to the impacts of bryophytes on soil C, quantifying changes in their N2-fixation activity in response to atmospheric Nr inputs is relevant for understanding how ecosystem N budgets are altered [17]. Our data clearly show that N2-fixation activity sharply declines in response to N addition. However; our low N treatment simulated upper level Nr deposition rates in the boreal region, and decreased N2-fixation rates offset only a minor quantity of these annual additions at this level. These results suggest that changes in feather moss N2-fixation rates in response to upper level Nr deposition rates (approx. 12 kg N ha−1 yr−1) in the boreal region are likely to have a minor impact on ecosystem N balances, a finding which is highly relevant for modelling the impacts of Nr deposition on ecosystem N and C balances [6].
Funding statement
The project was supported with funds to M.G. from the Swedish research council FORMAS and the TC4F program, and to A.N. from the NiCAF program funded by FORMAS.
References
- 1.Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (doi:10.1126/science.1136674) [DOI] [PubMed] [Google Scholar]
- 2.Schlesinger WH. 2009. On the fate of anthropogenic nitrogen. Proc. Natl Acad. Sci. USA 106, 203–208 (doi:10.1073/pnas.0810193105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnani F, et al. 2007. The human footprint in the carbon cycle of temperate and boreal forests. Nature 447, 848–850 (doi:10.1038/nature05847) [DOI] [PubMed] [Google Scholar]
- 4.Lindo Z, Nilsson M-C, Gundale MJ. 2013. Bryophyte–cyanobacteria associations as regulators of the northern latitude carbon balance in response to global change. Glob. Change Biol. 19, 2022–2035 (doi:10.1111/gcb.12175) [DOI] [PubMed] [Google Scholar]
- 5.DeLuca TH, Zackrisson O, Nilsson M-C, Sellstedt A. 2002. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419, 917–920 (doi:10.1038/nature01051) [DOI] [PubMed] [Google Scholar]
- 6.Gundale MJ, Deluca TH, Nordin A. 2011. Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob. Change Biol. 17, 2743–2753 (doi:10.1111/j.1365-2486.2011.02407.x) [Google Scholar]
- 7.Startsev NA, Lieffers VJ, McNabb DH. 2007. Effects of feathermoss removal, thinning and fertilization on lodgepole pine growth, soil microclimate and stand nitrogen dynamics. For. Ecol. Manage. 240, 79–86 (doi:10.1016/j.foreco.2006.12.010) [Google Scholar]
- 8.Lang SI, Cornelissen JHC, Klahn T, van Logtestijn RSP, Broekman R, Schweikert W, Aerts R. 2009. An experimental comparison of chemical traits and litter decomposition rates in a diverse range of subarctic bryophyte, lichen and vascular plant species. J. Ecol. 97, 886–900 (doi:10.1111/j.1365-2745.2009.01538.x) [Google Scholar]
- 9.Gundale MJ, Nilsson M, Bansal S, Jaderlund A. 2012. The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol. 194, 453–463 (doi:10.1111/j.1469-8137.2012.04071.x) [DOI] [PubMed] [Google Scholar]
- 10.Gundale MJ, Gustafsson H, Nilsson MC. 2009. The sensitivity of nitrogen fixation by a feathermoss–cyanobacteria association to litter and moisture variability in young and old boreal forests. Can. J. For. Res. 39, 2542–2549 (doi:10.1139/x09-160) [Google Scholar]
- 11.van der Wal R, Pearce ISK, Brooker RW. 2005. Mosses and the struggle for light in a nitrogen-polluted world. Oecologia 142, 159–168 (doi:10.1007/s00442-004-1706-0) [DOI] [PubMed] [Google Scholar]
- 12.Nordin A, Strengbom J, Forsum A, Ericson L. 2009. Complex biotic interactions drive long-term vegetation change in a nitrogen enriched boreal forest. Ecosystems 12, 1204–1211 (doi:10.1007/s10021-009-9287-8). [Google Scholar]
- 13.Gundale MJ, Wardle DA, Nilsson MC. 2010. Vascular plant removal effects on biological N fixation vary across a boreal forest island gradient. Ecology 91, 1704–1714 (doi:10.1890/09-0709.1) [DOI] [PubMed] [Google Scholar]
- 14.Gentili F, Nilsson MC, Zackrisson O, DeLuca TH, Sellstedt A. 2005. Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. J. Exp. Bot. 56, 3121–3127 (doi:10.1093/jxb/eri309) [DOI] [PubMed] [Google Scholar]
- 15.Zackrisson O, DeLuca TH, Gentili F, Sellstedt A, Jäderlund A. 2009. Nitrogen fixation in mixed Hylocomium splendens moss communities. Oecologia 160, 309–319 (doi:10.1007/s00442-009-1299-8) [DOI] [PubMed] [Google Scholar]
- 16.Gornall JL, Jonsdottir IS, Woodin SJ, Van der Wal R. 2007. Arctic mosses govern below-ground environment and ecosystem processes. Oecologia 153, 931–941 (doi:10.1007/s00442-007-0785-0). [DOI] [PubMed] [Google Scholar]
- 17.Ackermann K, Zackrisson O, Rousk J, Jones DL, DeLuca TH. 2012. N2 fixation in feather mosses is a sensitive indicator of N deposition in boreal forests. Ecosystems 15, 986–998 (doi:10.1007/s10021-012-9562-y) [Google Scholar]