Abstract
Motion dazzle describes high-contrast patterns (e.g. zigzags on snakes and dazzle paint on World War I ships) that do not conceal an object, but inhibit an observer's perception of its motion. However, there is limited evidence for this phenomenon. Locusts have a pair of descending contralateral movement detector (DCMD) neurons which respond to predator-like looming objects and trigger escape responses. Within the network providing input to a DCMD, separate channels are excited when moving edges cause areas of the visual field to brighten or darken, respectively, and these stimuli interact antagonistically. When a looming square has an upper half and lower half that are both darker than background, it elicits a stronger DCMD response than the upper half does alone. However, when a looming square has a darker-than-background upper half and a brighter-than-background lower half, it elicits a weaker DCMD response than its upper half does alone. This effect allows high-contrast patterns to weaken and delay DCMD response parameters implicated in escape decisions, and is analogous to motion dazzle. However, the motion dazzle effect does not provide the best means of motion camouflage, because uniform bright squares, or low-contrast squares, elicit weaker DCMD responses than high-contrast, half dark, half bright squares.
Keywords: escape, motion camouflage, adaptive coloration, descending contralateral movement detector, lobula giant movement detector, looming motion
1. Introduction
Motion dazzle describes high-contrast patterns that do not conceal an object, but interfere with an observer's perception of its motion [1–4]. The only experimental evidence for motion dazzle is from studies of humans completing computer-simulated target-capture or speed-judgement tasks, with limited consensus among results [1–4]. Furthermore, the mechanistic basis of motion dazzle is unclear, although it might result from the integration of responses from neurons that view motion within limited receptive fields: if the moving edges of a pattern are viewed through such apertures, only the component of motion perpendicular to an edge is apparent [5].
Although investigators have focused on how dazzle patterns might protect moving prey from predator attack, such patterns might also disguise a predator's approach from its target prey. From the prey animal's viewpoint, a directly approaching predator is a looming stimulus, characterized by the symmetrical expansion of its edges over the viewer's eye. Locusts are important model organisms for understanding looming-detection and escape behaviour initiation. Each of a locust's compound eyes has a single lobula giant movement detector (LGMD) neuron which responds strongly to looming stimuli with a spike train that increases in frequency as a looming stimulus expands over the locust's eye [6,7]. The LGMD has a large dendritic fan, which receives retinotopically arranged excitatory inputs that respond to local brightening (ON stimulation) or darkening (OFF stimulation) at individual facets of the eye [8], as occurs when the edges of an object move over the eye. One explanation for looming-sensitivity is that lateral-inhibitory interactions between excitatory channels cause the LGMD to respond selectively to stimuli in which the extent of edge and speed of edge movement over the eye both increase [9,10]. An alternative explanation posits that the LGMD multiplies its excitatory input by feed-forward inhibitory inputs that it receives as a result of large-field luminance changes [11,12]. Each LGMD synapses with a single descending contralateral movement detector (DCMD) neuron, which faithfully reproduces the LGMD spike train and conveys it to the thoracic ganglia [13]. There, high DCMD spike rates are implicated in triggering emergency behavioural responses to looming threats [14].
An early investigation found that when ON and OFF stimuli were provided at the same time, they had antagonistic effects on the DCMD response [9]. One possibility is that this results from cross-pathway inhibition between separate ON and OFF channels that converge to form the excitatory inputs to the LGMD [8,9]. As patterns that dazzle humans contain high-contrast edges which would elicit ON and OFF stimuli during movement [1–3], I extend the early DCMD experiments to ask whether antagonistic interactions between ON and OFF stimuli provide a substrate for motion dazzle in the locust visual system.
2. Material and methods
(a). Looming stimuli
Visual stimuli were programmed using Visionegg software [15] on an Intel Pentium 4-equipped PC with a PNY (Parsippany, NJ, USA) Nvidia Geforce 6200 graphics card and were displayed on an Iiyama (Tokyo, Japan) Visionmaster pro 454 CRT monitor with 200 Hz frame rate. The monitor's display measured 357 × 267 mm and had a resolution of 640 × 480 pixels. Locusts viewed the monitor monocularly from a distance of 0.07 m, where the display subtended 137 × 125°, and an individual pixel in the centre of the display subtended 0.46 × 0.46°. Stimuli were 80 × 80 mm squares that loomed directly towards the centre of a locust's compound eye at 4 m s−1, beginning their approach at a simulated distance of 10.07 m, and ending at 0.07 m from the eye (there subtending approx. 60°). Squares comprised equal-sized upper and lower halves with varying luminance. Background luminance was 29.0 cd m−2, and stimulus luminances varied from 1.4 to 102.6 cd m−2 (see the electronic supplementary material, S1). Stimuli are described by their contrast relative to the background, calculated by: Lobject–Lbackground/Lbackground [9]. Positive-contrast stimuli are brighter than background, negative-contrast stimuli are darker than background and zero-contrast stimuli are indistinguishable from background.
(b). Electrophysiology
Experiments were conducted on adult Schistocerca gregaria Forskål supplied by Blades Biological (Edenbridge, Kent, UK). DCMD activity was recorded extracellularly using 50 μm copper wires inserted through holes pierced in the ventral neck sclerite (see the electronic supplementary material, S1; [16]). Each locust received three presentations of each stimulus, delivered in pseudorandom order and separated by a 2.5 min interval. Mean response measurements across the three presentations of a stimulus were used for analysis.
(c). Statistical analysis
DCMD responses were analysed using one- and two-factor repeated-measures ANOVA or repeated-measures multiple linear regression (see the electronic supplementary material, S1), conducted using IBM (Armonk, NY, USA) SPSS Statistics v. 19.0. In repeated-measures ANOVA, sphericity was assessed using Maunchly's test and evaluation of epsilon, and corrected using the Greenhouse–Geisser correction. A priori simple contrasts tested for augmentation or reduction of DCMD response versus a control stimulus. Least significant difference (LSD) tests were used post hoc.
3. Results and discussion
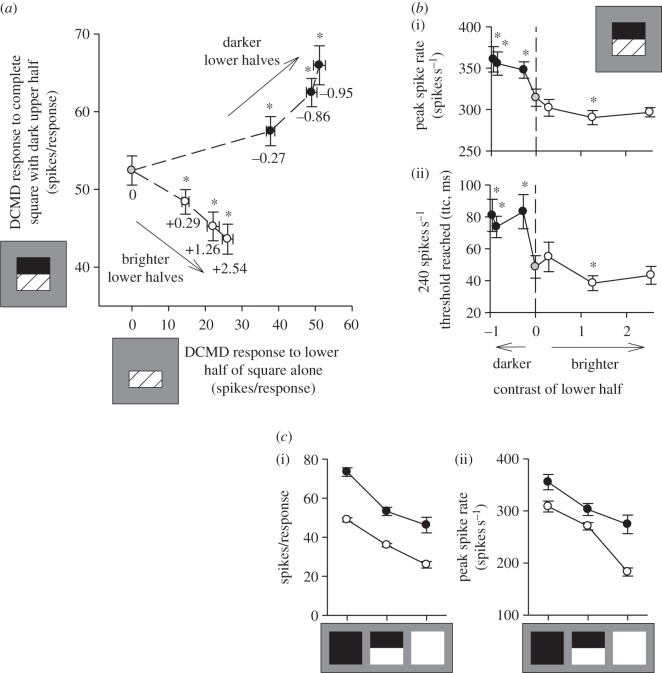
Figure 1a illustrates the antagonistic effect of ON and OFF stimulation on the DCMD response to a looming square. Increasingly darker-than-background square lower halves (negative contrasts, black circles) and increasingly brighter-than-background square lower halves (positive contrasts, open circles), both elicited increasingly strong DCMD responses when they loomed without a discernible upper half (x-axis). However, when delivered in combination with a dark upper half, dark lower halves progressively augmented the response caused by the upper half alone (grey circle), while bright lower halves progressively reduced it (y-axis; F2.24,20.17 = 123.730, p < 0.001). Therefore, there was an antagonistic effect on the DCMD response when the patterning of a looming stimulus caused its expanding edges to elicit both ON and OFF stimulation to different areas of the eye (see also [9]). The antagonistic effect of integrating these potentially conflicting local sensations resembles current explanations for motion dazzle in humans [2,17].
Figure 1.
Motion dazzle in locusts. (a) DCMD spike numbers elicited by looming squares. Responses to squares with zero-contrast, indiscernible upper halves and lower halves of varying contrast are shown on the x-axis (lower half contrasts stated below data points). Darker-than-background (negative contrasts, black circles) and brighter-than-background lower halves (positive contrasts, open circles), both elicited increasingly strong DCMD responses as their degree of contrast against background increased. DCMD responses to looming squares with dark, −0.95 contrast upper halves and lower halves of varying contrast are shown on the y-axis. The response to such a square with a zero-contrast, indiscernible lower half (the response to the upper half alone, grey circle), was progressively augmented by dark lower halves of increasing contrast, but reduced by bright lower halves of increasing contrast. (b) DCMD peak spike rate (i) and the time to collision (ttc) at which a 240 spikes s−1 threshold was reached (ii), in response to looming squares with dark upper halves and lower halves of varying contrast. The DCMD response to the dark upper half alone (zero-contrast lower half; dashed lines and grey circles) was augmented by addition of a dark lower half (black circles), but reduced or unaffected by addition of a bright lower half (open circles). (c) DCMD spike number (i) and peak spike rate (ii), in response to looming squares with varying pattern and contrast. DCMD responses were weaker to low-contrast (−0.27 and +0.29, open circles) than high-contrast stimuli (−0.95 and +2.54, black circles). Bright/dark combination squares elicited weaker DCMD responses than dark squares, but generally stronger responses than bright squares. (a,b) Means ± s.e.m, n = 10 locusts; (c) means ± s.e.m, n = 9 locusts from a separate experiment. Asterisks: responses that differed significantly from that to the looming upper half alone (simple contrasts, p < 0.05).
I next investigated whether this motion dazzle effect affected spike train parameters implicated in triggering a locust's escape (see the electronic supplementary material, S2). In flying locusts, stimuli that elicit higher DCMD spike rates also elicit higher probabilities of emergency glide behaviour [14,16]. In standing locusts, the DCMD response reaching an approximately 240 spikes s−1 threshold is associated with the onset of hindleg muscle co-contraction which stores energy to power a jump, and where co-contraction is delayed, jumps tend not to occur [18]. Both peak spike rate and the time to collision at which a 240 spikes s−1 threshold was reached were significantly augmented by addition of a darker-than-background square lower half to a darker-than-background square upper half, but reduced or unchanged by addition of a brighter-than-background lower half to a darker-than-background upper half (figure 1b; spike rate: F1.96,17.62 = 14.428, p < 0.001; 240 spikes s−1 threshold: F3.41,30.65 = 9.392, p < 0.001). The stepped appearance of the curves in figure 1b underline that a specific response decrement is attributable to the interaction of bright and dark stimulus edges, rather than simply the declining mean luminance of the whole stimulus. Multiple linear regression indicated that both mean stimulus luminance and the sign of lower half contrast had significant effects on DCMD spike counts elicited by complete square stimuli (mean luminance: Wald 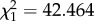 , p < 0.001; contrast sign: Wald
, p < 0.001; contrast sign: Wald 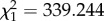 , p < 0.001). The sign of lower half contrast but not mean stimulus luminance significantly affected peak spike rate (mean luminance: Wald
, p < 0.001). The sign of lower half contrast but not mean stimulus luminance significantly affected peak spike rate (mean luminance: Wald 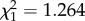 , p = 0.261; contrast sign: Wald
, p = 0.261; contrast sign: Wald 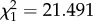 , p < 0.001), and the time before collision at which a 240 spikes s−1 threshold was reached (mean luminance: Wald
, p < 0.001), and the time before collision at which a 240 spikes s−1 threshold was reached (mean luminance: Wald 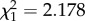 , p = 0.140; contrast sign: Wald
, p = 0.140; contrast sign: Wald 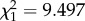 , p = 0.002). Thus, antagonism between ON and OFF stimuli caused a small but significant decrement to behaviourally relevant DCMD response parameters which would impede or delay a locust's escape, meaning that high-contrast dazzle patterns could advantage a predator in catching a locust.
, p = 0.002). Thus, antagonism between ON and OFF stimuli caused a small but significant decrement to behaviourally relevant DCMD response parameters which would impede or delay a locust's escape, meaning that high-contrast dazzle patterns could advantage a predator in catching a locust.
The above results demonstrate the existence of motion dazzle, but do not probe its relative effectiveness in camouflaging motion. Figure 1c compares DCMD responses to dark squares, bright squares and bright/dark combination squares in high- and low-contrast configurations. The DCMD is known to respond more strongly to dark than bright looms and to respond more strongly with increasing stimulus contrast [6]. DCMD responses were significantly weaker in response to low-contrast (open circles) compared with high-contrast stimuli (black circles; spike count: F1,8 = 232.057, p < 0.001; spike rate: F1,8 = 85.877, p < 0.001; cf. [2]). DCMD responses to combination squares were weaker than those to dark squares, but still stronger than those to bright squares, except in terms of peak spike rate in the high-contrast configuration (stimulus effect—spike count: F1.13,9.07 = 78.727, p < 0.001; spike rate: F1.25,10.01 = 77.894, p < 0.001; interaction—spike count: F1.12,8.99 = 3.638, p = 0.086; spike rate: F1.27,10.17 = 8.910, p = 0.010; post hoc LSD tests, p < 0.05). Furthermore, the low-contrast combination square had a mean luminance approximately equal to its background, but a uniform stimulus of this luminance (providing virtually no contrast) would elicit a negligible DCMD response [6]. Studies in other contexts also indicate that motion dazzle may not be the most effective form of motion camouflage. For humans, contrasting patterns make translating targets difficult to capture, but low-contrast uniform targets are harder still to capture [1,2]. Cuttlefish (which actively control their coloration) reduce high-contrast pattern elements when moving [19]. Nevertheless, my results demonstrate that dazzle patterns can impede looming motion perception, and such motion dazzle effects might benefit organisms when other pressures select for high-contrast markings [2]. The well-studied visual systems and tractable behaviour of insects make them excellent models for the further investigation of motion dazzle.
Data accessibility
Data available from the Dryad Digital Repository (doi:10.5061/dryad.kb5cb) [20].
References
- 1.Stevens M, Yule DH, Ruxton GD. 2008. Dazzle coloration and prey movement. Proc. R. Soc. B 275, 2639–2643 (doi:10.1098/rspb.2008.0877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens M, Searle WTL, Seymour JE, Marshall KLA, Ruxton GD. 2011. Motion dazzle and camouflage as distinct anti-predator defenses. BMC Biol. 9, 81 (doi:10.1186/1741-7007-9-81) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott-Samuel NE, Baddeley R, Palmer CE, Cuthill IC. 2011. Dazzle camouflage affects speed perception. PLoS ONE 6, e20233 (doi:10.1371/journal.pone.0020233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Helversen B, Schooler LJ, Czienskowski U. 2013. Are stripes beneficial? Dazzle camouflage influences perceived speed and hit rates. PLoS ONE 8, e61173 (doi:10.1371/journal.pone.0061173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adelson EH, Movshon JA. 1982. Phenomenal coherence of moving visual patterns. Nature 300, 523–525 (doi:10.1038/300523a0) [DOI] [PubMed] [Google Scholar]
- 6.Rind FC, Simmons PJ. 1992. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. I. Selective responses to approaching objects. J Neurophysiol. 68, 1654–1666 [DOI] [PubMed] [Google Scholar]
- 7.Hatsopoulos N, Gabbiani F, Laurent G. 1995. Elementary computation of object approach by a wide-field visual neuron. Science 270, 1000–1003 (doi:10.1126/science.270.5238.1000) [DOI] [PubMed] [Google Scholar]
- 8.O'Shea M, Rowell CHF. 1976. Neuronal basis of a sensory analyzer, the acridid movement detector system. II. Response decrement, convergence, and the nature of the excitatory afferents to the fan-like dendrites of the LGMD. J. Exp. Biol. 65, 289–308 [DOI] [PubMed] [Google Scholar]
- 9.Simmons PJ, Rind FC. 1992. Orthopteran DCMD neuron: a reevaluation of responses to moving objects. II. Critical cues for detecting approaching objects. J. Neurophysiol. 68, 1667–1682 [DOI] [PubMed] [Google Scholar]
- 10.Rind FC, Bramwell DI. 1996. Neural network based on the input organisation of an identified neuron signaling impending collision. J. Neurophysiol. 75, 967–984 [DOI] [PubMed] [Google Scholar]
- 11.Gabbiani F, Krapp HG, Koch C, Laurent G. 2002. Multiplicative computation in a visual neuron sensitive to looming. Nature 420, 320–324 (doi:10.1038/nature01190) [DOI] [PubMed] [Google Scholar]
- 12.Rowell CHF, O'Shea M, Williams JLD. 1977. Neuronal basis of a sensory analyzer, the acridid movement detector system. IV. The preference for small field stimuli. J. Exp. Biol. 68, 157–185 [DOI] [PubMed] [Google Scholar]
- 13.O'Shea M, Rowell CHF, Williams JLD. 1974. The anatomy of a locust visual interneurone: the descending contralateral movement detector. J. Exp. Biol. 60, 1–12 [Google Scholar]
- 14.Simmons PJ, Rind FC, Santer RD. 2010. Escapes with and without preparation: the neuroethology of visual startle in locusts. J. Insect Physiol. 56, 876–883 (doi:10.1016/j.jinsphys.2010.04.015) [DOI] [PubMed] [Google Scholar]
- 15.Straw AD. 2008. Vision Egg: an open-source library for realtime stimulus generation. Front. Neuroinform. 2, 4 (doi:10.3389/neuro.11.004.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santer RD, Rind FC, Simmons PJ. 2012. Predator versus prey: locust looming-detector neuron and behavioural responses to stimuli representing attacking bird predators. PLoS ONE 7, e50146 (doi:10.1371/journal.pone.0050146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troscianko T, Benton CP, Lovell PG, Tolhurst DJ, Pizlo Z. 2009. Camouflage and visual perception. Phil. Trans. R. Soc. B 364, 449–461 (doi:10.1098/rstb.2008.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fotowat H, Harrison RR, Gabbiani F. 2011. Multiplexing of motor information in the discharge of a collision detecting neuron during escape behaviors. Neuron 69, 147–158 (doi:10.1016/j.neuron.2010.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zylinski S, Osorio D, Shohet AJ. 2009. Cuttlefish camouflage: context-dependent body pattern use during motion. Proc. R. Soc. B 276, 3963–3969 (doi:10.1098/rspb.2009.1083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santer RD. 2013. Data from: motion dazzle: a locust's eye view. Dryad Digital Repository. (doi:10.5061/dryad.kb5cb) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository (doi:10.5061/dryad.kb5cb) [20].