Abstract
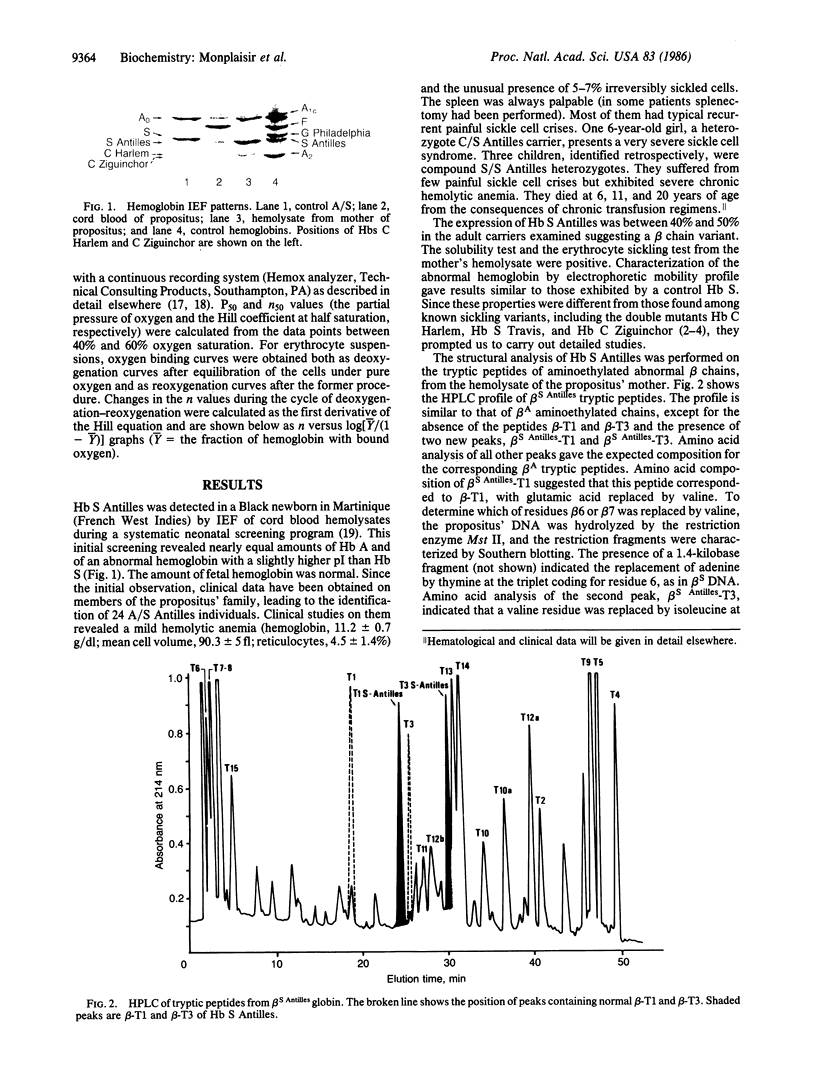
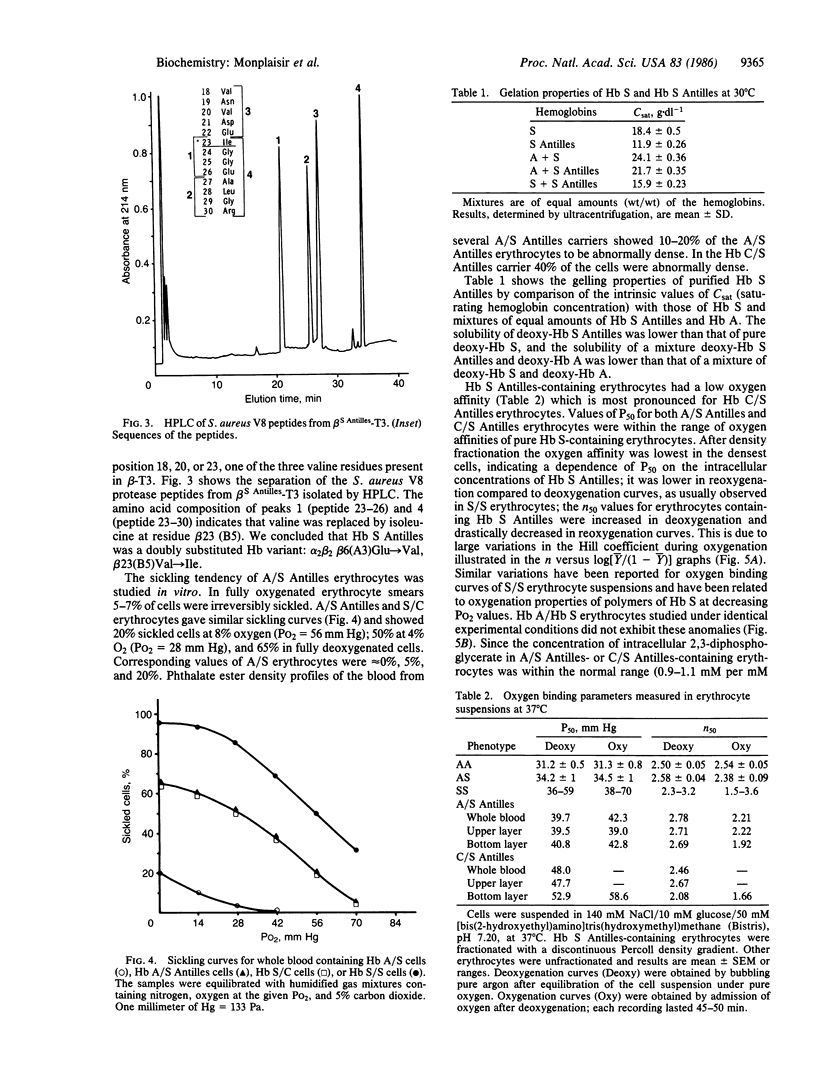
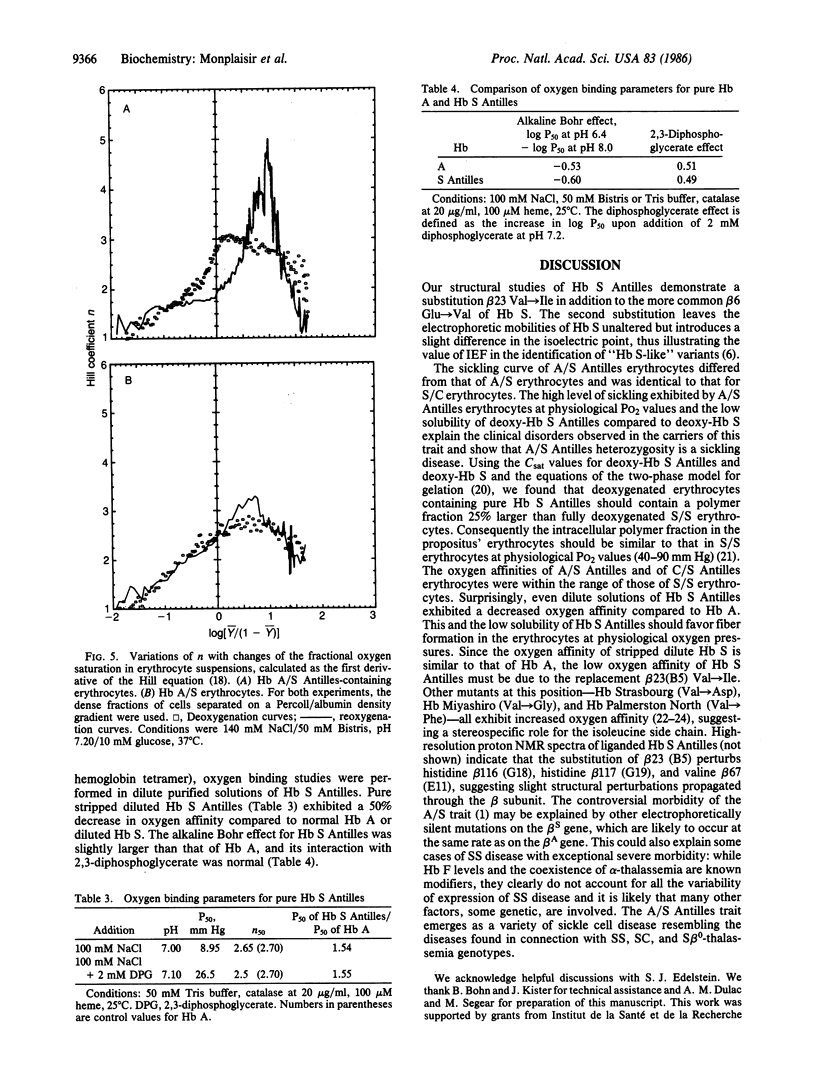
We have found a sickling variant, Hb S Antilles, alpha 2 beta 2(6 Glu----Val, 23 Val----Ile), that has the same electrophoretic mobility as Hb S but a distinct isoelectric focus and produces sickling in the carriers of the Hb A/S Antilles trait. The carriers' erythrocytes tend to sickle at O2 partial pressures similar to those that induce sickling in Hb S/C disease. Pure deoxy-Hb S Antilles is 50% as soluble as deoxy-Hb S (saturating concentration = 11 g X dl-1 compared to 18.4 for Hb S). Dilute solutions of pure Hb S Antilles have a lower oxygen affinity than those of Hb A or Hb S (partial pressure for 50% binding is 9 mm Hg compared to 5.5 mm Hg for Hb A or S at pH 7.00). A/S Antilles erythrocytes have a much lower oxygen affinity than A/S cells; this is further decreased in dense cells fractionated on a Percoll density gradient. Their oxygen equilibrium curves had anomalous shapes like those of S/S cells. Fiber formation in the erythrocytes of Hb S Antilles carriers is clearly due to its low solubility and oxygen affinity, showing that heterozygosity for this hemoglobin presents another sickle cell syndrome and suggesting that Hb S heterozygotes who exhibit symptoms of sickle cell disease should be carefully screened for double mutations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E. C., Reese A., Stallings M., Huisman T. H. Separation of human hemoglobins by DEAE-cellulose chromatography using glycine-KCN-NaC1 developers. Hemoglobin. 1976;1(1):27–44. doi: 10.3109/03630267609031020. [DOI] [PubMed] [Google Scholar]
- Barwick R. C., Schneider R. G. The computer-assisted differentiation of hemoglobin variants. Tex Rep Biol Med. 1980;40:143–156. [PubMed] [Google Scholar]
- Basset P., Beuzard Y., Garel M. C., Rosa J. Isoelectric focusing of human hemoglobin: its application to screening, to the characterization of 70 variants, and to the study of modified fractions of normal hemoglobins. Blood. 1978 May;51(5):971–982. [PubMed] [Google Scholar]
- Benesch R. E., Kwong S., Edalji R., Benesch R. alpha Chain mutations with opposite effects on the gelation of hemoglobin S. J Biol Chem. 1979 Sep 10;254(17):8169–8172. [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L., Ranney H. M. Structure and properties of hemoglobin C-Harlem, a human hemoglobin variant with amino acid substitutions in 2 residues of the beta-polypeptide chain. J Biol Chem. 1967 Jan 25;242(2):248–255. [PubMed] [Google Scholar]
- Brennan S. O., Williamson D., Whisson M. E., Carrell R. W. Hemoglobin Palmerston North beta 23 (B5) Val replaced by Phe. A new variant identified in a patient with polycythemia. Hemoglobin. 1982;6(6):569–575. doi: 10.3109/03630268209046450. [DOI] [PubMed] [Google Scholar]
- Brittenham G. M., Schechter A. N., Noguchi C. T. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985 Jan;65(1):183–189. [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal M., North M. L., Thillet J., Albrecht-Ellmer K., Garel M. C., Blouquit Y., Rosa J., Valentin C. Haemoglobin Strasbourg alpha2beta2 23 (B5) Val replaced by Asp: revised structure anf functional properties. FEBS Lett. 1978 Jun 15;90(2):286–288. doi: 10.1016/0014-5793(78)80387-1. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- Drapeau G. R. Cleavage at glutamic acid with staphylococcal protease. Methods Enzymol. 1977;47:189–191. doi: 10.1016/0076-6879(77)47023-x. [DOI] [PubMed] [Google Scholar]
- Edelstein S. J., Poyart C., Blouquit Y., Kister J. Self-association of haemoglobin Olympia (alpha 2 beta 2 20 (B2) Val----Met). A human haemoglobin bearing a substitution at the surface of the molecule. J Mol Biol. 1986 Jan 20;187(2):277–289. doi: 10.1016/0022-2836(86)90234-2. [DOI] [PubMed] [Google Scholar]
- Galacteros F., Kleman K., Caburi-Martin J., Beuzard Y., Rosa J., Lubin B. Cord blood screening for hemoglobin abnormalities by thin layer isoelectric focusing. Blood. 1980 Dec;56(6):1068–1071. [PubMed] [Google Scholar]
- Garel M. C., Domenget C., Galacteros F., Martin-Caburi J., Beuzard Y. Inhibition of erythrocyte sickling by thiol reagents. Mol Pharmacol. 1984 Nov;26(3):559–565. [PubMed] [Google Scholar]
- Goossens M., Dumez Y., Kaplan L., Lupker M., Chabret C., Henrion R., Rosa J. Prenatal diagnosis of sickle-cell anemia in the first trimester of pregnancy. N Engl J Med. 1983 Oct 6;309(14):831–833. doi: 10.1056/NEJM198310063091405. [DOI] [PubMed] [Google Scholar]
- Goossens M., Garel M. C., Auvinet J., Basset O., Ferreira Gomes P., Rosa J., Arous N. Hemoglobin C Ziguinchor alphaA2 beta62 (A3) Glu leads to Val beta58 (E2) Pro leads to Arg: the second sickling variant with amino acid substitutions in 2 residues of the beta polypeptide chain. FEBS Lett. 1975 Oct 15;58(1):149–154. doi: 10.1016/0014-5793(75)80246-8. [DOI] [PubMed] [Google Scholar]
- Lacombe C., Craescu C. T., Blouquit Y., Kister J., Poyart C., Delanoe-Garin J., Arous N., Bardakdjian J., Riou J., Rosa J. Structural and functional studies of hemoglobin Poissy alpha 2 beta 2(56) (D7) Gly----Arg and 86 (F2) Ala----Pro. Eur J Biochem. 1985 Dec 16;153(3):655–662. doi: 10.1111/j.1432-1033.1985.tb09350.x. [DOI] [PubMed] [Google Scholar]
- Moo-Penn W. F., Schmidt R. M., Jue D. L., Bechtel K. C., Wright J. M., Horne M. K., 3rd, Haycraft G. L., Roth E. F., Jr, Nagel R. L. Hemoglobin S Travis: a sickling hemoglobin with two amino acid substitutions [beta6(A3)glutamic acid leads to valine and beta142 (h20) alanine leads to valine). Eur J Biochem. 1977 Aug 1;77(3):561–566. doi: 10.1111/j.1432-1033.1977.tb11699.x. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T., Miwa S., Ohba Y., Hattori Y., Miyaji T., Miyata H., Shinohara T., Hori T., Takayama J. Hemoglobin Miyashiro (beta 23[B5] val substituting for gly) an electrophoretically silent variant discovered by the isopropanol test. Hemoglobin. 1981;5(7-8):653–666. doi: 10.3109/03630268108991833. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Thermodynamics of gelation of sickle cell deoxyhemoglobin. J Mol Biol. 1977 Sep 15;115(2):111–134. doi: 10.1016/0022-2836(77)90093-6. [DOI] [PubMed] [Google Scholar]
- Salvo G., Caprari P., Samoggia P., Mariani G., Salvati A. M. Human erythrocyte separation according to age on a discontinuous "Percoll" density gradient. Clin Chim Acta. 1982 Jul 1;122(2):293–300. doi: 10.1016/0009-8981(82)90290-x. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. B., Shelton J. R., Powars D. Hemoglobin Sunshine Seth - alpha 2 (94 (G1) Asp replaced by His) beta 2. Hemoglobin. 1979;3(2-3):145–159. doi: 10.3109/03630267908998910. [DOI] [PubMed] [Google Scholar]




