Abstract
Many animals display static coloration (e.g. of feathers or fur) that can serve as a reliable sexual or social signal, but the communication function of rapidly changing colours (as in chameleons and cephalopods) is poorly understood. We used recently developed photographic and mathematical modelling tools to examine how rapid colour changes of veiled chameleons Chamaeleo calyptratus predict aggressive behaviour during male–male competitions. Males that achieved brighter stripe coloration were more likely to approach their opponent, and those that attained brighter head coloration were more likely to win fights; speed of head colour change was also an important predictor of contest outcome. This correlative study represents the first quantification of rapid colour change using organism-specific visual models and provides evidence that the rate of colour change, in addition to maximum display coloration, can be an important component of communication. Interestingly, the body and head locations of the relevant colour signals map onto the behavioural displays given during specific contest stages, with lateral displays from a distance followed by directed, head-on approaches prior to combat, suggesting that different colour change signals may evolve to communicate different information (motivation and fighting ability, respectively).
Keywords: agonistic signalling, combat, communication, Chamaeleo calyptratus, colour signals, physiological colour change
1. Introduction
The colour of most animals is relatively fixed (e.g. in dead tissues like exoskeleton, scales, feathers and hair), but some animals are capable of undergoing rapid, physiological colour change which allows them to display different colours and patterns in response to changing environmental contexts (e.g. predators [1], temperature [2] and humidity [3]). A few taxa (predominantly cephalopods, fish and reptiles) also are capable of physiological colour change during intraspecific interactions. Chameleons (Squamata: Chameleonidae) represent an intriguing subject for research on dynamic coloration because, unlike organisms that undergo localized colour change [4] or rely on achromatic pattern alterations [5], they exhibit complex colour changes during social interactions [6]. In fact, selection for conspicuous signals is likely to have driven the evolution of display colours for some chameleons [7]. The complexity of chameleon colour change may permit dynamic signalling opportunities for chameleons, whereby they use diverse chromatic elements on the body to reveal distinct information, either over the course of social interactions or across different behavioural contexts.
To date, most research on physiological colour-change signals has focused on simplified on/off signals [4,5] or mechanistic (e.g. cellular and endocrine) controls [8], with less emphasis on adaptive significance and information content [9]. The relative paucity of investigations undertaken on the signalling role of physiological colour change may be due, in part, to the technological and methodological challenges associated with quantifying such a dynamic trait. However, recent advances in the photographic quantification of colour [10,11] and the visual systems of animals [12] now enable rigorous, non-invasive analyses of colour change, as it occurs and is perceived by conspecifics during social interactions. Here, we present the first study using these photographic [10,11] and analytical methods [11,13], as well as organism-specific visual models [12,14], to examine how dynamic colour changes of veiled chameleons Chamaeleo calyptratus predict behaviours in male–male contests. Though animals engaged in contests have conflicting aims, there is a shared benefit in avoiding unnecessary escalation of such contests. Dynamic signals, for example those provided by multi-component colour changes, may allow contestants to flexibly communicate motivation or ability during contests and could therefore contribute to evolutionarily stable strategies [15].
Male veiled chameleons are well known for intense intrasexual aggression (see electronic supplementary material, S1), yet agonistic encounters are frequently settled prior to physical contact, putatively through visual signals including rapid, body-wide colour change [6]. Here, we attempt to uncover the components of colour change linked to escalation behaviour (likelihood of approaching an opponent) and contest outcome (likelihood of winning a fight) by evaluating 28 different colour patches (figure 1) from displaying veiled chameleons during staged agonistic encounters. Specifically, we focused on the rate and degree of colour change (calculated using known sensitivities of chameleon photoreceptors [14]), as well as the maximum brightness of each colour patch, during aggressive interactions.
Figure 1.
Colour patches measured during male–male competition between veiled chameleons. (a,b) Interindividual variation in location of colour patches can be seen by comparing the location of colour patches in (a) to those in (b). Because exact locations of colour patches varied among individuals, we focused on similarly located colour patches of equivalent pigmentary and structural makeup. Descriptions of colour patches and relevant principal component loadings are located in the electronic supplementary material, table S4.
2. Material and methods
In spring 2011, we staged a series of aggressive, dyadic encounters between 10 adult male veiled chameleons in a round-robin tournament format that matched each chameleon against every other chameleon in our study population (see electronic supplementary material, S2). Trials were recorded with two high-definition video cameras that also enabled us to take still photographs of each chameleon. Though chameleons can exhibit a complex suite of behaviours during agonistic encounters (e.g. hissing, tail-curling and rocking), the most salient signals relate to the probability of escalating and winning aggressive encounters [15]. Thus, we monitored both escalation likelihood (whether or not a chameleon moved in a directed fashion toward its opponent) and win/loss outcome (losing chameleons retreated from their opponent at some point during the trial). We then used matrices of approach behaviour and contest outcomes to determine ‘ability’ scores using Bradley–Terry models (see electronic supplementary material, S6). Both chameleons displayed aggressive behaviour and rapid colour change in 17 of our 45 contests.
We collected data on brightness and colour change from 28 different colour patches on each chameleon (encompassing a variety of chameleon body regions and colour types; figure 1) that were photographed every 4 s during display, approach and combat phases of the trials. We first equalized and linearized photographs [11], then used specialized mapping functions to convert RGB (red, green and blue) values from these photographs to relative stimulation values of the different chameleon photoreceptor types (see electronic supplementary material, S3). Photographically derived estimates of chameleon photoreceptor stimulation values obtained from an independent dataset did not differ from spectrophotometrically determined values (see electronic supplementary material, table S2 and figure S2).
Because chameleon display coloration and change has never been quantitatively analysed with respect to intraspecific variation in behaviour, we measured the amount and speed of colour change, as well as maximum display brightness (previously suggested to be an informative component of squamate colour signals [16]). We measured colour changes as: (i) the perceptual distance travelled (sum of distances between colour measurements taken at different times) and (ii) rate of colour change (perceptual distance/time), both measured using units of just noticeable differences, which capture perceivable changes in colour taking into account species-specific photoreceptor sensitivities [13,17] (see electronic supplementary material, S4). Brightness was quantified by calculating how each colour patch would stimulate chameleon double cones, though only maximum brightness was evaluated in model-averaging and regression analyses.
We used principal components analyses to reduce the number of variables within three body regions (head, stripes and main body; electronic supplementary material, S5 and table S4) and an information theoretic model-averaging approach to evaluate the relative importance (RI; the sum of Akaike weights for all models in which that variable appeared) of colour intensity and change (averaged for each chameleon across trials) in predicting the likelihood of approaching an opponent or winning aggressive interactions (see electronic supplementary material, S6). Lastly, we included predictor variables from the top models in regression analyses to determine the degree to which colour traits explained variation in approach and winning abilities.
3. Results
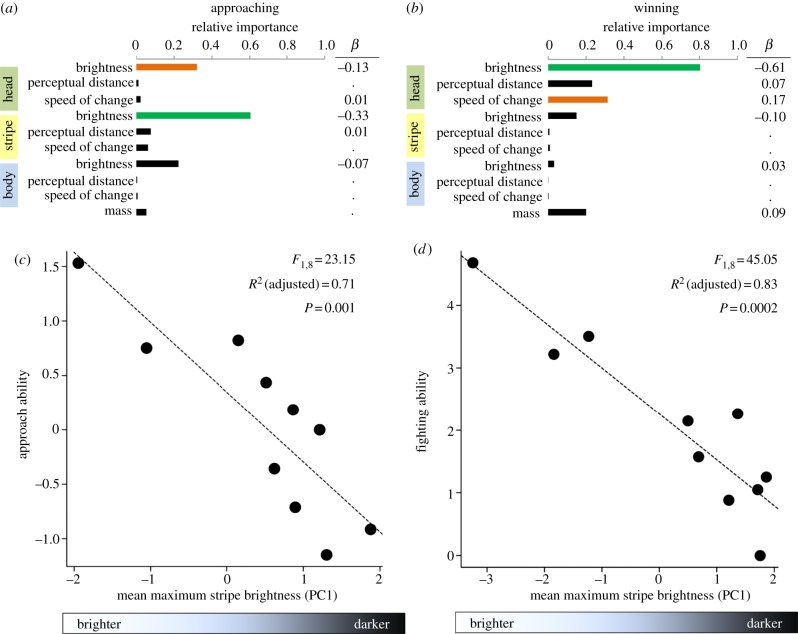
Approach and fighting abilities were highly correlated (r = 0.84, p = 0.003), though the colour metrics that best predicted these outcomes were different. Specifically, maximum head (RI = 0.32) and stripe brightness (RI = 0.60) achieved were the best predictors of approach ability during agonistic encounters (figure 2a; electronic supplementary material, table S5); in regression analysis, maximum stripe brightness explained 71% of the variation in approach likelihood (figure 2c). Chameleons who displayed brighter heads (RI = 0.80) that changed colour faster (RI = 0.31) were more likely to win physical encounters (figure 2b; electronic supplementary material, table S6). A regression containing only maximum head brightness explained 83% of the variation in fighting ability (figure 2d).
Figure 2.
Explanatory variables associated with escalating and winning contests. Multi-model averaging indicates that average maximum stripe brightness (principal component, PC) best explains the likelihood of (a,c) a chameleon approaching his opponent and (b,d) that average maximum head brightness (PC) best explains the likelihood of a chameleon winning a fight. (a,b) Bar length is proportional to RI values of variables predicting (a) likelihood of approaching an opponent or (b) likelihood of winning an aggressive interaction, with green bars indicating RI values greater than 0.60 and orange bars indicating RI values greater than 0.30. Average parameter estimates of regression coefficients (β) were obtained using multi-model-averaging approaches and dots indicate parameter estimates with absolute values less than 0.005. (c) Regression of chameleon stripe brightness and approach ability calculated across trials. Average stripe brightness values represent PC scores, all of which had negative loadings of maximum brightness values (chameleons with brighter bodies had negative PC scores). (d) Regression of head brightness on fighting ability. Colour-metric predictor variables in (a–d) represent principal component scores from multiple colour patches within a region (see electronic supplementary material, table S4). Abilities plotted in (c,d) represent Bradley–Terry ‘ability’ scores.
4. Discussion
We found that different aspects of chameleon competition (approach likelihood and contest outcome) were best predicted by separate components of display coloration—maximum stripe brightness and maximum head brightness, respectively. We also found that rate of colour change was an informative component of aggressive displays; chameleons whose head coloration changed faster were more likely to win agonistic encounters. Taken together, these correlational findings represent the first demonstration that multiple components of rapid colour change can be used to signal different aspects of competitive behaviour (e.g. motivation and fighting ability).
Selection may favour separate signal components for motivation and fighting ability [15] because these two aspects of competition can differentially affect contest outcomes; for example, regardless of true ability, animals who fight harder to defend mates or territories can often overcome less-motivated competitors. Separate signals may also be favoured when rival assessment is prolonged and animals progress through a series of escalation and threat behaviours, as occurs for slow-moving chameleons that have the opportunity to sequentially evaluate competitor intent and quality. Interestingly, the physical locations of the key colour-signalling elements of veiled chameleons align closely with the behaviours they exhibit during agonistic encounters. Aggressive chameleons display laterally to one another from a distance before approaching, providing their opponents the opportunity to assess body stripe coloration (which best predicted escalation likelihood in our study). Next, as they approach and prepare to engage in head-to-head combat (see electronic supplementary material, S1 and video S1), they have close visual access to head coloration (which best predicted win/loss outcome). Separate plumage patches in lark buntings Calamospiza melanocorys also convey different information about approach intensity and physical aggression [18], but our study is the first to document the use of multiple, behaviourally accentuated colour-change signals to communicate different information.
Costly intraspecific conflict over resources is common throughout the animal kingdom, yet rapid colour change as an agonistic signal is incredibly rare and warrants special examination. One possible explanation for the link between rate of colour change and fighting ability is that expression of bright, rapidly changing colours is causally related to the physiological processes (e.g. hormone status and energetic reserves) associated with fighting ability [4]. An alternative, though not exclusive, explanation suggests that it is more costly for strong individuals facing strong opponents to signal weakness before signalling strength (analogous to brightening slowly) than it is to initially signal strength [15], providing the evolutionary pressure to rapidly signal fighting ability when strong. Regardless of the explanation, documenting the behavioural contexts in which colour change signals occur is an important first step in understanding the function and evolution of this relatively rare signal type and should markedly inform our views on competitive signalling theory. Moreover, future detailed studies of the physiological mechanisms underlying display coloration will be key for revealing the information communicated by rapidly changing colours.
Acknowledgements
We thank Ellis Loew, Jim Bowmaker, Matthew Toomey, Martin Stevens, Thomas Pike, Kristen McCartney, Sarah Bruemmer, Megan Best and Brianna Bero-Buell for invaluable assistance, Dale DeNardo, the McGraw lab and two anonymous reviewers for helpful manuscript suggestions, and David, Sandy and Veronica Ligon for support. See full acknowledgements in the electronic supplementary material, S8.
Funding statement
Additional financial support was provided by an ASU GPSA grant.
References
- 1.Allen JJ, Mäthger LM, Barbosa A, Buresch KC, Sogin E, Schwartz J, Chubb C, Hanlon RT. 2010. Cuttlefish dynamic camouflage: responses to substrate choice and integration of multiple visual cues. Proc. R. Soc. B 277, 1031–1039 (doi:10.1098/rspb.2009.1694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veron JEN. 1974. The role of physiological colour change in the thermoregulation of Austrolestes annulosus (Selys) (Odonata). Aust. J. Zool. 22, 457–469 (doi:10.1071/ZO9740457) [Google Scholar]
- 3.Hinton HE, Jarman GM. 1973. Physiological colour change in the elytra of the hercules beetle, Dynastes hercules. J. Insect Physiol. 19, 533–549 (doi:10.1016/0022-1910(73)90064-4) [Google Scholar]
- 4.Summers CH, Greenberg N. 1994. Somatic correlates of adrenergic activity during aggression in the lizard, Anolis carolinensis. Horm. Behav. 28, 29–40 (doi:10.1006/hbeh.1994.1003) [DOI] [PubMed] [Google Scholar]
- 5.Adamo SA, Hanlon RT. 1996. Do cuttlefish (Cephalopoda) signal their intentions to conspecifics during agonistic encounters? Anim. Behav. 52, 73–81 (doi:10.1006/anbe.1996.0153) [Google Scholar]
- 6.Nečas P. 1999. Chameleons: nature's hidden jewels. Frankfurt, Germany: Chimaira [Google Scholar]
- 7.Stuart-Fox D, Moussalli A. 2008. Selection for social signalling drives the evolution of chameleon colour change. PLoS Biol. 6, e25 (doi:10.1371/journal.pbio.0060025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nery LEM, Castrucci AML. 1997. Pigment cell signalling for physiological color change. Comp. Biochem. Physiol. A 29, 1135–1144 (doi:10.1016/S0300-9629(97)00045-5) [DOI] [PubMed] [Google Scholar]
- 9.Stuart-Fox D, Moussalli A. 2009. Camouflage, communication and thermoregulation: lessons from colour changing organisms. Phil. Trans. R. Soc. B 364, 463–470 (doi:10.1098/rstb.2008.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens M, Párraga CA, Cuthill IC, Partridge JC, Troscianko TS. 2007. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 90, 211–237 (doi:10.1111/j.1095-8312.2007.00725.x) [Google Scholar]
- 11.Pike TW. 2011. Using digital cameras to investigate animal colouration: estimating sensor sensitivity functions. Behav. Ecol. Sociobiol. 65, 849–858 (doi:10.1007/s00265-010-1097-7) [Google Scholar]
- 12.Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633 (doi:10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 13.Pike TW. 2012. Preserving perceptual distances in chromaticity diagrams. Behav. Ecol. 23, 723–728 (doi:10.1093/beheco/ars018) [Google Scholar]
- 14.Bowmaker JK, Loew ER, Ott M. 2005. The cone photoreceptors and visual pigments of chameleons. J. Comp. Physiol. A 191, 925–932 (doi:10.1007/s00359-005-0014-4) [DOI] [PubMed] [Google Scholar]
- 15.Enquist M. 1985. Communication during aggressive interactions with particular reference to variation in choice of behaviour. Anim. Behav. 33, 1152–1161 (doi:10.1016/S0003-3472(85)80175-5) [Google Scholar]
- 16.Molnár O, Bajer K, Török J, Herczeg G. 2012. Individual quality and nuptial throat colour in male European green lizards. J. Zool. 287, 233–239 (doi:10.1111/j.1469-7998.2012.00916.x) [Google Scholar]
- 17.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B 265, 351–358 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaine AS, Lyon BE. 2008. Intrasexual selection on multiple plumage ornaments in the lark bunting. Anim. Behav. 76, 657–667 (doi:10.1016/j.anbehav.2008.03.014) [Google Scholar]