Summary
Development of the hematopoietic system proceeds in a multistep manner. Primitive erythrocytes are the first hematopoietic cells to be observed that were produced transiently in developing embryos. Multilineage lymphohematopoiesis occurs after the primitive erythropoiesis. However, the lineage relationship of cells that comprise embryonic hematopoietic system is not well characterized. To clarify this process, careful analyses of the embryonic cells that differentiate into these cell lineages are necessary. We identified the common precursors of primitive erythrocytes and multipotent hematopoietic cells in mouse embryonic stem cell cultures and mouse embryos. A subset defined as CD45−CD41+AA4.1− cells showed bipotential capability to produce primitive erythrocytes and lymphomyeloid cells at the single-cell level. The cell population was present in vivo before hematopoietic stem cells (HSCs) appeared. Our results show that primitive erythrocytes and lymphomyeloid cells are not completely separate cell lineages, and these precursors comprise the embryonic hematopoietic system before HSC emergence.
Highlights
-
•
Primitive erythrocytes and lymphomyeloid progenitors have bipotent precursors
-
•
The precursors form primitive erythroid and lymphomyeloid cells in distinct waves
-
•
The common precursors are noted in the yolk sac and embryo proper
-
•
Primitive erythrocytes and lymphocytes can be derived from a single precursor cell
Yamane and colleagues show that there is a bipotential cell stage for the primitive erythrocytes that are produced transiently in embryos and multipotent hematopoietic progenitors that supply lymphoid and myeloid cells during the early stage of mouse development. Primitive erythrocytes and lymphomyeloid cells are not completely separate cell lineages.
Introduction
Hematopoietic cells are produced in mesoderm-derived tissues during the early stages of embryonic development. The first blood cells to appear during ontogeny are primitive erythrocytes. Primitive erythropoiesis is a transient wave of hematopoiesis that specifically occurs in the yolk sac (ventral) blood islands in a lineage-restricted manner (Haar and Ackerman, 1971; Kingsley et al., 2004; Turpen et al., 1981). Primitive erythropoiesis is followed by multilineage hematopoiesis, which produces the entire repertoire of myeloid and lymphoid lineages. This type of lymohohematopoieisis, which is called definitive hematopoiesis, occurs in the para-aortic region (Cumano et al., 1996; Medvinsky and Dzierzak, 1996; Turpen et al., 1981), the vitelline and umbilical arteries (de Bruijn et al., 2000), late yolk sac (Huang and Auerbach, 1993; Yoder et al., 1997a), or placenta (Gekas et al., 2005; Ottersbach and Dzierzak, 2005). Multipotent hematopoietic progenitors produced in an initial wave of definitive hematopoiesis lack adult-repopulating capability (Cumano et al., 1996; Yamane et al., 2009). The authentic hematopoietic stem cells (HSCs) that can repopulate the body over a long period of time after they are transferred into adults appears after the first lymphomyeloid progenitors are noted (Gekas et al., 2005; Medvinsky et al., 2011). Recent studies suggested that myeloid-restricted progenitors are also present before or in parallel with the appearance of definitive lymphohematopoietic progenitors (Chen et al., 2011; Schulz et al., 2012).
The ex vivo culture of embryonic and extraembryonic tissues revealed the embryonic origin of definitive hematopoietic lineages in mice (Cumano et al., 1996; Medvinsky and Dzierzak, 1996; Yokota et al., 2006). Along with the unwavering observation that primitive erythropoiesis exclusively occurs in the extraembryonic yolk sac, these observations suggest that the primitive and definitive hematopoietic cells have a distinct tissue origin and support the view that these cells have distinct progenitor populations. The appearance of these two lineages in vivo and in vitro during different time periods also promoted this view (Nakano et al., 1996). However, classical and recent cell-tracking studies showed that definitive hematolymphoid lineages may not necessarily originate only from the embryonic portion, but also from the extraembryonic yolk sac (Fontaine-Perus et al., 1981; Samokhvalov et al., 2007; Weissman et al., 1978; Yoder et al., 1997a). Therefore, the yolk sac provides a suitable microenvironment for both primitive and definitive hematopoiesis, although the potency of the yolk sac to generate authentic transplantable HSCs is still controversial.
The studies showing the overlapping tissue source of primitive and definitive hematopoietic cells imply the existence of common progenitors for these lineages, and the existence of a bipotential precursor for primitive erythrocytes and definitive hematopoietic progenitors has been evidenced by data from experimental models. Analysis of clonal colonies derived from embryonic stem (ES) cells indicated the presence of bipotential primitive and definitive hematopoietic progenitor cells (Kennedy et al., 1997; Perlingeiro et al., 2001). Orthotopic and heterotopic transplantation of hematopoietic tissues in Xenopus embryos also implied the presence of bipotential precursors (Turpen et al., 1997). However, the developmental stages of the cells that were recognized as bipotent were unclear in these studies because uncommitted mesodermal cells may have been the source of the bipotential readout pattern, which would make the results unconvincing.
To establish precisely the relationships between cell lineages, cell identity must be defined at the branching point of these two hematopoietic lineages, and individual cells that are free of the influence of environmental signals should be analyzed. In the adult hematopoietic system, various differentiation stages of cells, from hematopoietic stem cells to unipotent progenitors, were documented (Akashi and Weissman, 2001), mostly on the basis of cell-surface-marker expression determined using monoclonal antibodies and the subsequent analysis of isolated cell subsets. Using this strategy, we previously identified and isolated the earliest definitive lymphohematopoietic cells in mouse ontogeny (Yamane et al., 2009). To attain a better understanding of hematopoiesis, in the present study we traced the origin of the previously isolated earliest definitive progenitors to the mesodermal stage of development.
Results
Fractionation of Mesodermal Cell Populations
To trace the early mesodermal cells that differentiate into different lineages, we carefully analyzed cells in intermediate stages in culture that appeared during ES cell differentiation. Mesoderm cells and their derivatives were permanently marked using ES cells harboring cre at the early mesoderm-specific Mesp1 locus (Saga et al., 1999) and the Rosa26-eYfp reporter (Soriano, 1999). Mesp1-cre is known to mark mesodermal cells ingressing through the primitive streak that later contribute to the extraembryonic mesoderm and lateral embryonic mesoderm in vivo (Saga et al., 1999). EYFP+ cells first appeared in the developing colony on day 3 of differentiation (Figure S1A available online), and the increased ratio of EYFP-labeled mesodermal cells persisted up to day 5 of differentiation (Figure S1B). Consistent with the proposed endothelial origin of hematopoietic cells (Chen et al., 2009; Eilken et al., 2009; Lancrin et al., 2009), almost all EYFP-labeled cells, first observed on day 3, expressed FLK-1, an endothelial marker, in our culture conditions (Okuyama et al., 2003).
We fractionated the mesodermal cell populations based on three markers: AA4.1 (CD93), CD41, and CD45. AA4.1 is expressed in the earliest hematolymphoid progenitors found in mice at embryonic day 9.5 (E9.5) (Yamane et al., 2009). CD41 has been used to distinguish the earliest hematopoietic cell population from nonhematopoietic endothelial cells and other cell types (Ferkowicz et al., 2003; Mikkola et al., 2003; Mitjavila-Garcia et al., 2002). CD45 expression lags behind CD41 expression but functions as a pan marker for definitive hematopoietic cell lineages (Eilken et al., 2009), although the primitive erythroid lineage never goes through CD45+ cell stages during development (Mikkola et al., 2003). EYFP+ cells, first observed on day 3, were essentially negative for AA4.1, CD41, and CD45 (Figure S1B). On day 4, detectable populations of AA4.1+CD41− (AA4.1 single-positive [AA4SP]), AA4.1−CD41+ (CD41 single-positive [CD41SP]), and a few AA4.1+CD41+ (double-positive [DP]) cells appeared within the EYFP+CD45− cell fraction (Figure S1B). Increased numbers of CD41SP and DP cells were observed on day 5 of differentiation with detectable amounts of CD45+ cells (Figures S1B and 1A).
Figure 1.
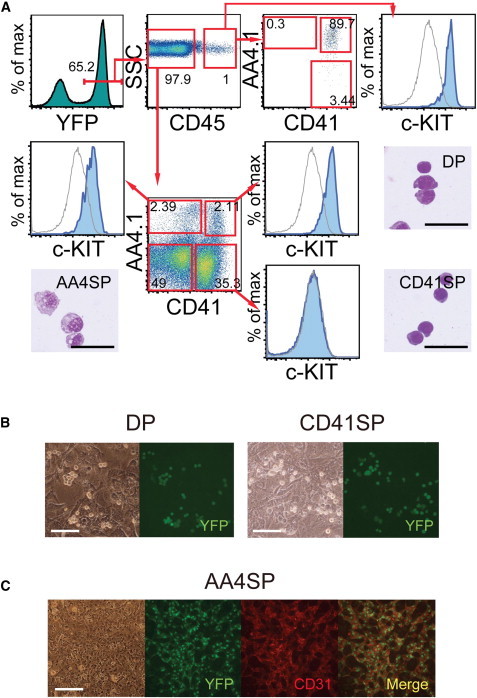
Cell Subsets within CD45− Differentiation Stage
(A) Cell subsets present on day 5 of differentiation from Mesp1-cre/Rosa26-eYfp ES cells. The c-KIT expression and May-Grunwald/Giemsa-stained cytospin images of each cell subset are shown. Gray lines in the c-KIT expression panels represent expression in DN cells, which are shown for comparison. Scale bars: 50 μm.
(B and C) Differentiation potentials of each cell subset. EYFP-labeled DP and CD41SP cells formed hematopoietic cell colonies on OP9 cells (B), whereas AA4SP cells (anti-EYFP [Alexa Fluor 488; green]) predominantly produced endothelial sheets (anti-CD31 [Cy3; red]) on OP9 cells (C); signals for the two markers were colocalized in identical cells. Scale bars, 200 μm (B) and 400 μm (C). Representative results from three separate experiments are shown.
See also Figure S1.
Identification and Isolation of Hematopoietic Cell Subsets
Next, we isolated each cell fraction by fluorescence-activated cell sorting (FACS) and then cultured them for a short period to determine which cell fraction showed hematopoietic activity. Because the expression of the markers used (AA4.1, CD41, and CD45) were mesoderm restricted, isolation of cells on basis of the Mesp1-cre/Rosa26-eYfp reporter was not necessary. However, use of the reporter reduced contamination with cells having high background fluorescence, such as fibroblasts and stromal cells, and the reporter was therefore used for the following cell-sorting experiments. Consistent with previous reports (Ferkowicz et al., 2003; Mikkola et al., 2003), CD41+ cells in the CD45− cell fraction (CD41SP and DP cells) produced hematopoietic cell clusters but rarely formed endothelial foci (Figure 1B). This indicated that most CD41+ cells belong to committed hematopoietic populations. In contrast, AA4SP cells produced endothelial cell sheets but not hematopoietic cell clusters (Figure 1C). Morphologically, AA4SP cells were distinctly different from hematopoietic CD41SP and DP cells; i.e., AA4SP cells had larger cytoplasm with vacuoles (Figure 1A). The continuous flow cytometric patterns of CD41SP and AA4SP with CD41/AA4 double-negative (DN) cells and the time course presented in Figure S1B indicate that DN cells are precursors of CD41SP and AA4SP cells. However, DN cells isolated on day 5 rarely produced hematopoietic cells, indicating that most of the uncommitted mesodermal cells are lost from DN cell populations by day 5 and that at least one additional marker is required to isolate the uncommitted mesodermal cell subset.
We compared c-KIT expression of hematopoietic CD41SP and DP cells. As shown in Figure 1A, the c-KIT expression level of DP cells was comparable to that of the first-appearing CD45+ cells, most of which have the AA4.1+CD41+ earliest hematolymphoid progenitor phenotype (Yamane et al., 2009). In contrast, CD41SP cells did not upregulate c-KIT expression relative to DN cells. This result, together with the continuous flow cytometric patterns of CD41SP and DP cells and the time course of their appearance, implies that CD41SP cells are precursors of DP cells and that DP cells are in a transitional stage to becoming the CD45+ earliest hematolymphoid progenitors. We tested this notion by cell sorting and subsequent cell culture. As shown in Figures S2A–S2C, almost all DP cell derivatives transformed into CD45+ cells after 2 days of culture, including c-KIT+AA4.1+CD41+ earliest hematolymphoid progenitors. Thus, DP cells are the immediate precursors of CD45+ definitive hematopoietic cell lineages.
Some DP and CD45+ cells were formed by day 1 in CD41SP cell cultures (Figures 2A–2C). Approximately one-third of CD45+ cells did not show AA4.1 upregulation at this point (Figure 2C), indicating that CD45 is sometimes upregulated before AA4.1 upregulation. The remaining two-thirds of CD45+ cells had the c-KIT+AA4.1+CD41+ earliest hematolymphoid progenitor phenotype. Consistent with this observation, CD41SP cells had the potential to produce B and T lymphocytes (Figures 2F and 2G) and mature myeloid cells (Figure 2H). CD41SP cells produced only 8%–10% of CD45+ cells after 2 days of culture (Figure 2D), which contradicts the result for DP cells, in which the vast majority of cells were CD45+ cells (Figure S2C). Interestingly, most CD45− cells were TER119+ erythroid cells (Figure 2D). RT-PCR analysis revealed that the cells expressed embryonic (β-H1, ε-y) and adult (β-major) globins (Figure 2E); i.e., the cells were primitive erythrocytes (Baron et al., 2012; McGrath et al., 2011; Whitelaw et al., 1990).
Figure 2.
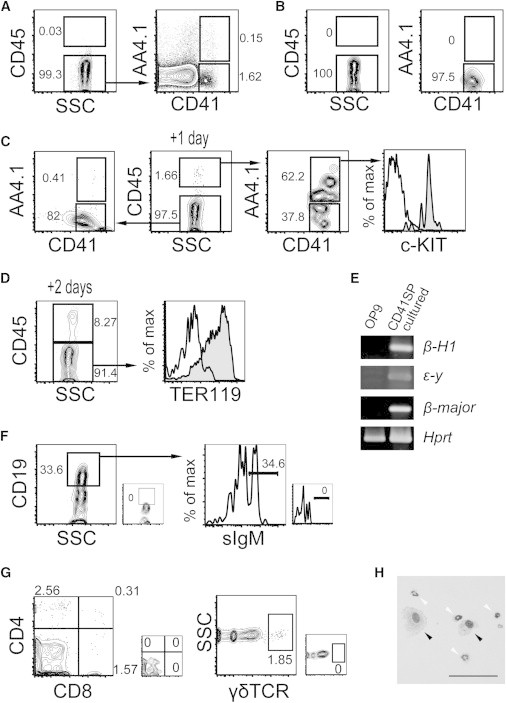
Differentiation Properties of CD41SP Cells
(A and B) Sort gates (A) and postsort analysis (B) of CD41SP cells.
(C–F) Differentiation potential of CD41SP cells. CD41SP cells cultured on OP9 cells (C–F and H) or OP9DL1 cells (G) for additional 1 day (C), 2 days (D), 3 days (E), 10 days (H), 2 weeks (G), or 3 weeks in the presence of interleukin-7 (F) were analyzed by flow cytometry (C, D, F, and G), RT-PCR (E), or May-Grunwald/Giemsa staining (H). Small plots shown at bottom right (F and G) and open lines in histograms (C and D) show control staining. The black and white arrowheads in (H) indicate macrophages and neutrophils, respectively. Scale bar, 100 μm. Representative results from at least two separate experiments are shown.
See also Figures S2 and S3.
We traced the differentiation of CD41SP cells by daily flow cytometric analysis (Figures 3A–3C). CD45−TER119+ and a few CD45+TER119− cells appeared within 1 day. By the next day, CD45+TER119− cells, including c-KIT+AA4.1+CD41+ hematolymphoid progenitors, were apparent. The number of c-KIT+AA4.1+CD41+ hematolymphoid progenitors peaked on day 8 of differentiation. The number of CD45−TER119+ cells gradually declined to an almost undetectable level by day 9 of differentiation. A considerable number of CD45−TER119+ cells appeared again, together with CD45+TER119+ cells, on day 10 of differentiation. We compared the initial and late CD45−TER119+ erythroid cells. Day 6 erythroid cells showed lower TER119 expression than day 10 erythroid cells, but the day 6 cells were larger (Figure 3D). These are the properties of primitive erythrocytes (Kingsley et al., 2004; Ogawa et al., 1993). We examined β-globin expression to confirm which of primitive or definitive erythroid cell were detected by real-time RT-PCR. Day 6 erythroid cells expressed β-H1, ε-y, and β-major, although day 10 erythroid cells expressed only β-major (Figure 3E). These results indicate that day 6 and day 10 cells represent primitive and definitive erythroid cells, respectively. Therefore, CD41SP cells are common precursors of primitive erythrocytes and CD45+ definitive hematopoietic cell lineages. The separate waves of primitive and definitive hematopoiesis would be explained by the differentiation property of CD41SP cells, which directly produce primitive erythrocytes and supply definitive erythroid cells through the immature hematopoietic progenitor stage.
Figure 3.
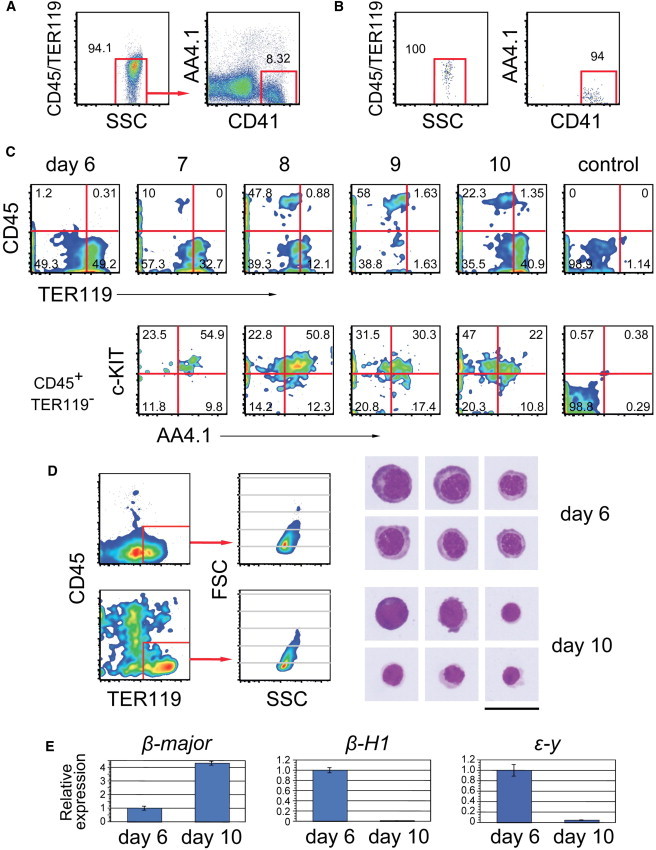
Differentiation Kinetics of CD41SP Cells
(A and B) Sort gates (A) and postsort analysis (B) of CD41SP cells used for the experiments shown below.
(C) The time course of CD41SP cell differentiation. Lower panels show the expression pattern within the CD45+TER119− cell fraction. Control staining using day 10 cells is shown on the right.
(D) Comparison of day 6 and day 10 CD45−TER119+ cells. TER119 expression level and cell size (FSC) (left) and representative May-Grunwald/Giemsa-stained cytospin images of sorted CD45−TER119+ cells (right) are shown. Scale bars, 25 μm.
(E) Real-time quantitative PCR for β-globin expression on day 6 and day 10 cells. Gene expression levels are shown after internal normalization with Hprt and shown as expression relative to day 6 cells (value = 1). Values are expressed as mean ± SD. Representative results from at least two separate experiments are shown.
See also Figure S4.
Next, Runx1−/− ES cells, which are known to lack the potential to differentiate into definitive hematopoietic cell lineages (Mikkola et al., 2003; Wang et al., 1996), were analyzed (Figure S3). Consistent with previous reports, Runx1−/− cells did not proceed to the CD45+ cell fraction (Mikkola et al., 2003), and a few residual CD45+ cells did not have the c-KIT+AA4.1+ hematolymphoid progenitor phenotype (Figure S3A) but produced primitive erythrocytes, although the number of cells produced was smaller (Figure S3B). At an earlier time point (day 5), DP cells were considerably reduced in the Runx1−/− cell population (Figures S3C and S3D). Few residual DP cells failed to upregulate c-KIT expression (Figure S3E). Therefore, the development of “authentic” DP cells was completely abolished on the Runx1−/− background. Runx1−/− cells always produced a consistent number of CD41SP cells, albeit in reduced numbers compared with wild-type and heterozygous cells. The decrease in the number of CD41SP cells would account for the decrease in the number of primitive erythrocytes. The number of AA4SP cells produced by Runx1−/− cells was similar to that produced by wild-type and heterozygous cells (Figures S3C and S3D). Therefore, the Runx1−/− ES cells that we used in the study did not have general defects in differentiation. Overall, the observations here suggest that the most important checkpoint where Runx1 needs to act is at the transition stage to DP cells.
Gene expression profiles of CD41SP cells and CD45+c-KIT+AA4.1+ earliest hematolymphoid progenitors were compared (Figure S4). Reflecting the developmental stage, gene expression of transcription factors involved in mesoderm formation was downregulated in CD45+c-KIT+AA4.1+ cells during the transition from CD41SP cells. Conversely, expression of genes involved in definitive hematopoiesis was upregulated in CD45+c-KIT+AA4.1+ cells. Along with the expression of definitive hematopoietic genes, many hematopoietic cytokine receptors were upregulated during the transition to CD45+c-KIT+AA4.1+ cells, suggesting that responsiveness to various cytokine stimulations is acquired during differentiation.
In the bulk culture of CD41SP cells, we did not observe cardiomyocytes, an FLK-1+ cell lineage (Kattman et al., 2006). However, we did observe a few endothelial cells, although such a cell population is rare compared with the dominant hematopoietic cell population. CD41 expression is associated with the hematopoietic commitment event within the hemogenic endothelium (or hemangioblasts) (Ferkowicz et al., 2003; Mikkola et al., 2003). However, the fraction of CD41+ cells that has the residual potential to proliferate as endothelial cells (or to produce endothelial cells) remains unknown. To examine the remaining endothelial differentiation potential, we performed a frequency analysis for the endothelial potential of CD41SP cells on OP9 stromal cells. As shown in Figure 4, only approximately 1 of 150 CD41SP cells had the potential to produce endothelial cells, whereas approximately one of three AA4SP cells formed endothelial cell sheets under the same culture conditions. This observation indicated that most of the CD41SP cells that we isolated lacked the potential to produce endothelial cells.
Figure 4.
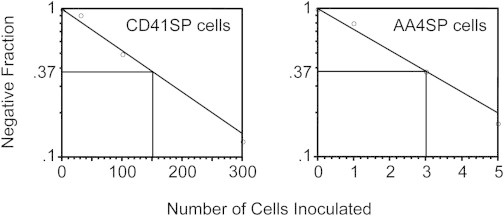
Limiting Dilution Analysis to Estimate the Residual Endothelial Potency of CD41SP Cells
CD41SP cells were cultured on OP9 cells for 6 days at varying cell concentrations. Endothelial cells were detected by staining with anti-CD31 and anti-EYFP antibodies. AA4SP cells were analyzed for comparison. The fraction of negative wells was plotted at each cell concentration. Representative results from two separate experiments are shown.
We next examined the in vivo counterparts of CD41SP and DP cells (Figure 5). In these experiments, homozygous transgenic male mice that expressed GFP under the control of the ubiquitous promoter GFP-Tg were crossed with wild-type female mice and GFP+ embryonic cells were analyzed to avoid possible contamination of the analysis with a few maternal blood cells. Both CD41SP and DP cells were abundant in the yolk sac and sparse in the embryo proper of E8.5 mice. At E9.5, the numbers of these cells decreased in the yolk sac but increased in the embryo proper (Figure 5A). CD41SP and DP cells isolated from E9.5 embryos formed cobblestone-like areas on stromal cells (Figure 5B), indicating that these cell fractions are hematopoietic, as noted in the culture of ES cells. In addition, we confirmed that CD41SP cells had the potential to produce mature myeloid cells (Figure 5C), B lymphocytes (Figure 5D), and primitive erythrocytes (Figure 5E), indicating that CD41SP cells are the common precursors of primitive erythrocytes and definitive hematopoietic lineages in vivo. Interestingly, the CD41SP cells present in the embryo proper, where primitive erythrocytes are not normally produced, also produced primitive erythrocytes if the CD41SP cells were separated from the cells surrounding them (Figure 5E). This result implied that environments in the embryo proper hamper the differentiation of CD41SP cells into primitive erythrocytes.
Figure 5.
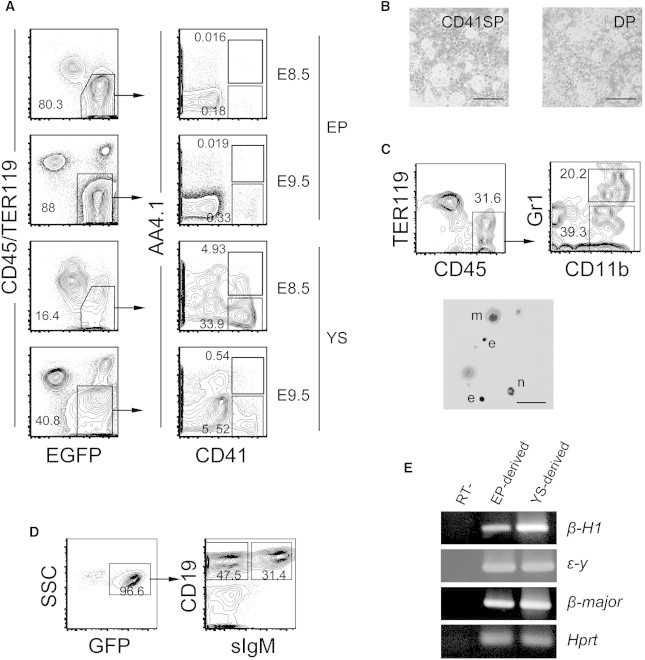
In Vivo Counterparts of DP and CD41SP Cells
(A) Presence of CD41SP and DP cells in the embryo proper (EP) and yolk sac (YS) of E8.5 and E9.5 embryos. GFP-Tg males were crossed with non-Tg females to avoid possible contamination of maternal cells in the analysis. Note that GFPlow cell populations in the left shoulders of the gated CD45−TER119−EGFP+ cells were primitive erythrocytes.
(B) Myeloid lineage colonies developed from E9.5 CD41SP and DP cells on OP9 cells after culturing for 2 weeks. Scale bars, 200 μm.
(C and D) CD41SP cells isolated from E9.5 YS were cultured on OP9 cells for 5 days (C) or 3 weeks in the presence of interleukin-7 (D). Then, they were analyzed for the presence of myeloid cells (C) or B lymphocytes (D) by flow cytometry. A representative May-Grunwald/Giemsa-stained cytospin image is also shown in (C). m, macrophage; n, neutrophil; e, erythroblast. Scale bar, 50 μm.
(E) E9.5 CD41SP cells isolated from EP or YS cells were cultured for 1 day on OP9 and then analyzed for β-globin expression by RT-PCR to determine whether primitive erythrocytes were present. The expression of Hprt was evaluated as an internal control. Representative results from at least two separate experiments are shown.
During mouse ontogeny, the first adult-repopulating HSCs appear at E10.5–11 (Gekas et al., 2005; Medvinsky et al., 2011). It is known that hematopoietic progenitors derived from ES cells and embryos before the time point do not engraft in adult environments unless genetically modified (Kyba et al., 2002). We evaluated the transplantability of CD41SP cells derived from E9.5 embryos and ES cells. In these experiments, CD41SP cells were injected intravenously (104 cells per recipient) into sublethally irradiated adult immunodeficient Rag2−/−/Il2rγc−/− mice that lacked T, B, and NK cells. However, donor cell engraftment was not observed when mice were analyzed at 3 and 6 weeks after transplantation (ES cell derived, n = 3; E9.5 YS derived, n = 6). It was reported that injection of E9 cells into neonate mice allowed donor cells to repopulate (Yoder et al., 1997a, 1997b). Therefore, E9.5 CD41SP cells isolated from the embryo proper and yolk sac were injected together (3,000 cells per recipient) through the facial vein into sublethally irradiated newborn Rag2/Il2rγc-deficient mice <48 hr after birth. GFP+-donor-derived cells were searched for rigorously 3–5 weeks after transplantation. However, this attempt did not alter the transplantability of CD41SP cells again (n = 6), although adult bone marrow cells transferred in this manner repopulated recipient mice steadily, suggesting the validity of this method. These results suggested that isolation and transfer of the most immature hematopoietic compartment were insufficient to override transplantation barriers in neonatal or adult recipients. Injection of E9.5 CD45+c-KIT+AA4.1+ cells, which are the earliest hematolymphoid progenitors isolated from the yolk sac and caudal halves of the embryo proper (three embryo equivalents per recipient), did not lead to repopulation in neonatal recipients (n = 4).
Bipotential Activity of Single CD41SP Cells
To test whether single CD41SP cells produce both primitive erythrocytes and definitive hematopoietic cells, we performed a clonal analysis of ES cell-derived CD41SP cells. In this assay, we sorted and placed CD41SP cells (Figures 6A and 6B) in semisolid media with cytokines to produce fewer than five colonies/cm2 in the culture dish. Under these culture conditions, primitive erythrocytes survived over the period that was observable on OP9 cells without adding cytokines (Figure 3) (Nakano et al., 1996). After culture, individual colonies were isolated and half of the cells from a single colony were subjected to flow cytometry to examine CD45 expression. Approximately two-thirds (28/43) of the colonies contained CD45+ cells (definitive hematopoietic cells) and CD45− cells (primitive and definitive erythroid cells) (Figure 6C). The remaining one-third of colonies consisted of either CD45+ cells or CD45− cells. The former colonies were further subjected to RT-PCR analysis to examine β-globin expression, half of the cells being used to determine the presence of primitive erythrocytes. Because a transient definitive cell population may express embryonic β-H1 globin, ε-y globin expression is considered the most reliable marker for the detection of primitive erythrocytes (Baron et al., 2012; McGrath et al., 2011). In total, 19 of the 24 examined colonies were positive for ε-y expression (Figure 6D). Among these clones, 15 and 4 clones were positive and negative for β-H1, respectively. The latter clones may contain relatively mature primitive erythtocytes (Baron et al., 2012; McGrath et al., 2011). These experiments indicated that at least 40% of the CD41SP cells in ES cell culture were common precursors of primitive erythrocytes and definitive hematopoietic cells. It was also found that at least 4 of these 19 clones that contained primitive erythrocytes showed simultaneous presence of CD45+TER119+ erythroblasts that were in a transitional stage of definitive erythroid differentiation before the loss of CD45 expression. This indicated that at least some CD41SP cells may give rise to both primitive and definitive erythrocytes at the single-cell level (Figure S5).
Figure 6.
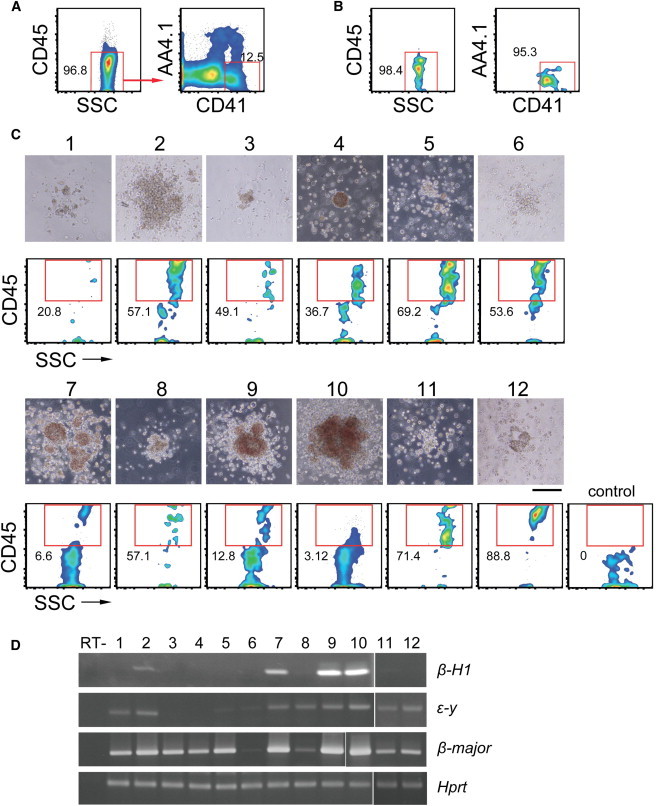
Bipotential Primitive and Definitive Hematopoietic Activity of Single CD41SP Cells
(A and B) Sort gates (A) and postsort analysis (B) of ES cell-derived CD41SP cells used for the experiments shown below.
(C and D) Clonal analysis of CD41SP cells for potency to differentiate into primitive erythrocytes and definitive hematopoietic cells. Each of the colonies developed from CD41SP cells at a clonal density were isolated and analyzed for CD45 expression by using half of the cells. Colonies consisting of both CD45+ and CD45− cells (C, numbered 1–12) were further analyzed for β-globin expression by RT-PCR (D) using the remaining half of the cells. Nested RT-PCR was performed for Hprt as an internal control. Upper and lower panels in (C) show images of the analyzed colonies (nos. 1–12) and the flow cytometry pattern for CD45 expression, respectively. Control staining is shown on the right. Scale bar, 200 μm. Representative results from two separate experiments are shown.
See also Figure S5.
We also tested CD41SP cells isolated from in vivo sources (Figure S6). In this experiment, single cells were individually deposited into methylcellulose medium with cytokines. The frequency of colony formation in three independent experiments was 17.4%. We performed the same assay as that used for ES cell-derived cells to examine whether the cells were bipotential primitive-definitive hematopoietic precursors. Approximately 70% (34/48) of the colonies contained CD45+ and CD45− cells (Figure S6A). These clones were further analyzed by RT-PCR. Seven of the 34 colonies analyzed were positive for ε-y expression (Figure S6B). Among these clones, three and four were positive and negative for β-H1, respectively. Therefore, at least 1 out of 7 CD41SP cells were bipotential precursors in vivo also.
It was recently reported that myeloid-restricted progenitors may be present during mouse development independent of lymphomyeloid stem/progenitor cells (Schulz et al., 2012). Although CD41SP cells had lymphoid potential in bulk culture (Figures 2F, 2G, and 5D), the question remained whether the detected bipotential capability of single CD41SP cells was for myeloid-restricted progenitors or multipotent lymphomyeloid progenitors. To address this question, single CD41SP cells isolated from E9.5 yolk sac were individually deposited into methylcellulose medium with cytokines. In these experiments, the lymphopoietic cytokine IL-7 was added to the cytokine cocktail. After culture, individual colonies were isolated, and then half of the cells from a single colony were subjected to RT-PCR analysis to examine β-globin expression as described above. Using the other half of the cells, genomic DNA was isolated, and then the DH to JH rearrangement status was examined by seminested PCR for the detection of B lymphocytes. Of the 77 clones analyzed, 22 showed DHJH rearrangement and 15 expressed ε-y. Four clones were observed to overlap between these two categories. Therefore, at least some of the CD41SP cells in vivo were bipotential precursors of primitive erythrocytes and B cell progenitors (Table 1).
Table 1.
ε-y Expression and DH-JH Rearrangement in CD41SP Cell Clones
| Classification | No. of Clones |
|---|---|
| ε-y−DH-JH recombination− | 44 |
| ε-y+DH-JH recombination− | 11 |
| ε-y−DH-JH recombination+ | 18 |
| DH-JH1+ | 2 |
| DH-JH2+ | 10 |
| DH-JH3+ | 3 |
| DH-JH1+ & DH-JH2+ | 1 |
| DH-JH1+ & DH-JH3+ | 1 |
| DH-JH2+ & DH-JH+ | 1 |
| ε-y+DH-JH recombination+ | 4 |
| DH-JH2+ | 2 |
| DH-JH3+ | 2 |
The data shown are summarized from two separate experiments. See also Figure S6.
Discussion
Although the presence of bipotential precursors was suggested on the basis of results obtained with different experimental models, it was always difficult to distinguish the cell population from uncommitted mesoderm or hemangioblasts. In this study, we identified the bipotential cell stage at which cells are committed to a hematopoietic fate. The bipotent cells were defined as CD45−AA4.1−CD41+ cells. Cells of the CD41SP subset produced primitive erythrocytes and definitive hematopoietic lineages at the single-cell level. The upregulation of AA4.1 expression in the CD41SP cell population (transition to the DP cell stage) was associated with commitment to a definitive hematopoietic lineage. In developing animals, primitive erythropoiesis precedes definitive lymphohematopoiesis in the blood islands. This phenomenon was recapitulated in cultured CD41SP cells. We demonstrated that two distinct waves occurred because primitive erythrocytes were produced directly from CD41SP cells, whereas mature definitive hematopoietic cell lineages were produced through the CD45+c-KIT+AA4.1+CD41+ earliest hematolymphoid progenitors. Further studies are required to understand the mode of branching of the primitive and definitive hematopoietic pathways.
There is no doubt about the close relation between hematopoietic cells and endothelial cells. Expression of endothelial cell markers in cells with hematopoietic activity and in vivo observations of embryonic vasculatures with budding hematopoietic foci increase the probability of the existence of the hemogenic endothelium. However, it is not known whether these hematopoietic cells originate from genuine endothelial cells within the endothelial lining or the cells with hematopoietic activity have just entered the endothelial lining and whether a stage comprising truly bipotent hematopoietic-endothelial cell progenitors is present. Because CD41SP cells, the most immature hematopoietic cells described here, gave rise only to hematopoietic cell lineages, the identification of their immediate uncommitted precursors and characterization of their differentiation potential and in vivo localization will answer these questions.
The most immature hematopoietic cell subset CD41SP cells were present in the yolk sac and embryo proper, although CD41SP cells appeared slightly earlier in the yolk sac than in the embryo proper. A vast majority of CD41SP cells at E8.5 were present in the yolk sac, even with limited blood flow (McGrath et al., 2003); therefore, it is possible that CD41SP cells found in the yolk sac may have developed independent of the embryo proper. However, CD41SP cells in the embryo proper at E8.5 were rare and only certain numbers of CD41SP cells were detected from E9.5, when the embryonic circulation was established. Therefore, it is conceivable that CD41SP cells found in the embryo proper may have migrated from the yolk sac. Alternately, these cells may have developed in situ. Although the precise localization of CD41SP cells and their tissue origin has not been determined in the present study, these factors should be carefully examined before any conclusions are drawn because there is considerable cell movement during development, which is independent of embryonic circulation. Interestingly, CD41SP cells isolated from the embryo proper produced primitive erythrocytes in vitro. However, the site of overt production of primitive erythrocytes is restricted to the yolk sac in vivo; therefore, it is conceivable that there could be a conflict between the in vitro potential and physiological fate in vivo. If this is the case, the embryonic microenvironment may suppress the differentiation of CD41SP cells into primitive erythrocytes. Alternatively, the microenvironment of the yolk sac may specifically promote the differentiation of primitive erythrocytes. Either way, our data suggest that cell isolation on the basis of cell-surface markers allows the prediction of cell-intrinsic differentiation potentials of embryonic hematopoietic cells.
It is a generally accepted that adult-repopulating HSCs appear at E10.5–11 (Gekas et al., 2005; Medvinsky et al., 2011). At this stage, HSCs are considered to be rare and estimated to be less than one per single entire embryo (Gekas et al., 2005; Taoudi et al., 2008). A dramatic increase in the number of transplantable stem cells occurs thereafter. In accordance with these observations, CD41SP cells that are isolatable from earlier embryos (E8.5–E9.5) and ES cells lacked the capability to engraft postnatal mice. The CD45+c-KIT+AA4.1+ multipotent progenitors that are committed to definitive hematolymphoid lineages isolated from E9.5 lacked the capability of repopulation. In contrast to these observations, it was reported that E9 yolk sac cells contained neonate-repopulating HSCs (Yoder et al., 1997a, 1997b). Although there is a possibility that a distinct cell population other than committed hematopoietic cells may settle specifically in neonatal hematopoietic microenvironments, the existence of authentic HSCs would be unequivocally demonstrated if experimental data fulfilled the following criteria: (1) exclusion of the possible maternal contamination of circulating HSCs; (2) clear identification of the cell fraction containing stem cell activity; and (3) proof of self-renewal examined by secondary transplantation or cell-tracking technology to monitor the persistence of the stem cell fraction and supply of mature progenies. Because lineage-committed progenies themselves derived from E9.5 or from earlier embryos can be sustained in the body for long term and independent from HSCs (Ito et al., 2013; Schulz et al., 2012), this would be critical for judgment of stem cell activity. To the best of our knowledge, reports that fulfilled these criteria and demonstrated the presence of postnatal mice-repopulating HSCs at and before E9.5 embryos have not been published yet.
Most studies that locate budding hematopoietic cell foci that lead to transplantable HSCs in the major arterial regions were undertaken at or after E10.5. However, the cellular characteristics of the precursors of HSCs before E10.5 are currently unknown. HSCs could develop de novo directly from uncommitted mesodermal cells or hemogenic endothelial cells. Alternately, HSCs may be generated from pre-existing multipotent hematopoietic cells such as CD41SP cells and CD45+c-KIT+AA4.1+ cells. Attempts of in vivo labeling or orthotopic transplantation of the candidate precursor cell populations for HSCs in early embryos may resolve this question.
In summary, we identified and isolated the common precursor of primitive erythrocytes and multipotent hematolymphoid progenitors. CD41SP cells were present before the appearance of transplantable HSCs. These bipotential precursors could constitute the embryonic hematopoietic system during the early stage of mouse development before authentic HSCs become the exclusive source of multiple hematolymphoid cell lineages. The discovery of an equivalent system for the generation of primitive erythrocytes and hematolymphoid cells in other vertebrates would be of interest. The use of cell-isolation techniques and single-cell tracking would help in the discovery of this system in animals.
Experimental Procedures
Mice
C57BL/6JJcl mice were purchased from Crea Japan. EGFP-transgenic mice [C57BL/6-Tg (CAG-EGFP)] and BALB/cA-Rag2−/−/Il2rγc−/− mice, originally purchased from the Central Institute for Experimental Animals, were maintained at the Institute of Laboratory Animals at Mie University. All animal experiments were performed according to the Mie University guidelines for laboratory animals.
Antibodies
The following antibodies were used in this study: AA4.1 (eBioscience), CD4 (GK1.5, BioLegend), CD8 (53-6.7, BioLegend), CD11b (M1/70, BioLegend), CD19 (6D5, BioLegend), CD31 (390, eBioscience), CD41 (MWReg30, BD Pharmingen), CD45 (30-F11, eBioscience), c-KIT (2B8, BD Pharmingen), FLK-1 (Avas12α1, eBioscience), γδTCR (UC7-13D5, BioLegend), Gr-1 (RB6-8C5, BioLegend), immunoglobulin (Ig) M (II/41, eBioscience), TER119 (eBioscience), and YFP (rabbit anti-GFP, Invitrogen).
Cell Sorting and Analysis
All cell-sorting experiments were performed on FACSAria (BD). Cells were analyzed on FACSAria or FACSCanto II (BD). Subsequent analysis was performed using the FlowJo software (Tree Star).
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde in PBS, blocked with 1% BSA in PBS, and then incubated with primary antibodies against CD31 (rat IgG) and YFP (rabbit IgG, Alexa Fluor 488 conjugated). Cells were then incubated with Cy3-conjugated donkey anti-rat IgG antibodies (Jackson ImmunoResearch Laboratories). Images were captured using an inverted microscope (Olympus IX71) equipped with an Olympus DP71 camera and DP controller software (Olympus).
Cell Culture
Mesp1-cre/Rosa26-eYfp ES cells were established as follows. Mesp1cre/+ male mice (Saga et al., 1999) were crossed with Rosa26eYfp/eYfp female mice (Soriano, 1999) that had been superovulated with pregnant mare’s serum and human chorionic gonadotropin. Blastocysts obtained from these mice were layered on the surface of mouse embryonic fibroblasts, and clones showing the fine morphology of ES cells were propagated and checked for genotype. In this study, one Mesp1cre/+Rosa26eYfp/+ clone with a normal karyotype was used for experiments. ES cells were maintained as described (Yamane et al., 1997). To induce hematopoietic differentiation of ES cells in vitro, cells were layered on the surface of OP9 stromal cells (Yoshida et al., 1990) in MEM Alpha Medium (Gibco) supplemented with 20% FBS (HyClone) (Nakano et al., 1994). For B cell induction, cells were cultured on OP9 in RPMI-1640 containing 5% FBS and 0.05 mM 2ME in the presence of 10 ng/ml rmIL-7. For T cell induction, cells were cultured on OP9DL1 in MEM Alpha Medium supplemented with 20% FBS. For colony formation in methylcellulose, sorted cells were incubated in Iscove’s modified Dulbecco’s medium (Gibco) containing 0.9% methylcellulose (Fluka), 30% FBS, 1% BSA (Sigma), 0.1 mM 2ME, and cytokines (50 pM rhbFGF, 10 ng/ml rmVEGF, 10 ng/ml rmSCF, 10 ng/ml rmFlt3 ligand, 10 ng/ml rhIL-11, 10 ng/ml rmIL-3, 10 ng/ml rmGM-CSF, 10 ng/ml rhTpo, and 10 ng/ml rhEpo) for 7 days in 35 mm suspension culture plates at 100–300 cells/plate or in 48-well plates (for single-cell deposition experiments). Where indicated, 10 ng/ml rmIL-7 was added to the cytokine cocktail. For FACS analysis of ES cell differentiation from day 3 to day 5, cells were collected by dissociating colonies with Hanks’-based Cell Dissociation Buffer (Gibco), and thereafter by pipetting.
Cell Preparation from Embryos
The embryo proper and yolk sac obtained from C57BL/6J females time-mated with EGFP-transgenic males were incubated with 1 mg/ml collagenase (Wako) in 2% FBS/HBSS for 30 min at 37°C. A single-cell suspension prepared by pipetting after incubation was used for the experiments.
RT-PCR and Microarray Analysis
Total RNA was isolated using Trizol reagent (Invitrogen). Reverse transcription was performed using ReverTra Ace (Toyobo) and oligo(dT) primers (Toyobo). First-strand synthesized cDNA was used for PCR with rTaq polymerase (Toyobo) and the following primers: β-major forward 5′-cacaaccccagaaacagaca-3′ and reverse 5′-gtggtacttgtgagccaagg-3′; β-H1 forward 5′-ctcaaggagacctttgctca-3′ and reverse 5′-gcctaattcagtccccatgg-3′; ε-y forward 5′-atggcaagaaggtgctgact-3′ and reverse 5′-gccgaagtgactagccaaaa-3′; and Hprt forward 5′-gtaatgatcagtcaacgggggac-3′ and reverse 5′-ccagcaagcttgcaaccttaacca-3′. After an initial denaturation step (94°C for 3 min), amplification was performed by 36 cycles of 45 s at 94°C, 45 s at 56°C, and 60 s at 72°C, followed by a final extension step of 3 min at 72°C. Where indicated, nested PCR was performed for Hprt by using the above-noted primers after the first-step PCR with the following primers: forward 5′-agttctttgctgacctgctg-3′ and reverse 5′-gctttgtatttggcttttcc-3′. For real-time quantitative PCR, first-strand synthesized cDNA was analyzed using the Fast SYBR and StepOnePlus system (Applied Biosystems) with the following primers: β-major forward 5′-atcacttggacagcctcaag-3′ and reverse 5′-cccagcacaatcacgatcat-3′; β-H1 forward 5′-ctcaaggagacctttgctca-3′ and reverse 5′-atcaccaacatgttgcccag-3′; ε-y forward 5′-atggcaagaaggtgctgact-3′ and reverse 5′-gccgaagtgactagccaaaa-3′; and Hprt forward 5′-gtaatgatcagtcaacgggggac-3′ and reverse 5′-ccagcaagcttgcaaccttaacca-3′. After initial AmpliTaq activation (20 s at 95°C), amplification was performed by two-step PCR (40 cycles of 3 s at 95°C and 30 s at 60°C) followed by melting curve analysis. Microarray analysis was performed using a 3D-Gene Mouse Oligo chip 24K (Toray). Gene expression levels were analyzed after a global normalization procedure.
PCR Detection of Immunoglobulin Gene Rearrangement
Genomic DNA isolated by a standard proteinase K-Phenol/Chloroform method was used to detect DH-JH recombination by seminested PCR. DH primer degenerated at two positions, which recognizes nine of ten DH genes (Schlissel et al., 1991), was used as forward primer in the first- and second-round PCR. Together with DH primer, DH to JH1 or JH2 rearrangement was detected by JH1–2 (first round) and JH1–2nest (second round) primers as reverse primers, and DH to JH3 rearrangement was detected by JH3 (first round) and JH3nest (second round) primers as reverse primers. After an initial denaturation step (94°C for 3 min), amplification was performed by 36 cycles of 45 s at 94°C, 60 s at 62°C (first and second rounds for JH1/JH2) or 65°C (first and second rounds for JH3), and 90 s at 72°C, followed by a final extension step of 3 min at 72°C. The oligonucleotide sequences were as follows: DH 5′-ggaattcgmtttttgtsaagggatctactactgtg-3′ (m = a or c; s = c or g); JH1-2 5′-aagaaagcatagaagagagggacctagcgg-3′; JH1–2nest 5′-gaccctttctgactcccaaggtgtccctagt-3′; JH3 5′-gtgacctatattccagcctcacctcagaga-3′; and JH3nest 5′-gtctagattctcacaagagtccgatagaccctgg-3′.
Acknowledgments
We thank K. Isono and C. Ito for technical assistance; M. Yamada for laboratory management; N.A. Speck for providing the Runx1 knockout ES cells; M. Tsuneto and S.I. Hayashi for providing materials; and T. Shigeoka for the critical reading of the manuscript. This work was supported by the Japan Society for the Promotion of Science (KAKENHI no. 23791078), the Ministry of Education, Culture, Sports, Science and Technology (KAKENHI nos. 21790912 and 23118512), the Uehara Memorial Foundation, the Mitsubishi Pharma Research Foundation, and the Takeda Science Foundation (to T.Y.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Accession Numbers
The Gene Expression Omnibus accession number for the data reported in this paper is GSE48189.
Supplemental Information
References
- Akashi K., Weissman I.L. Stem cells and hematolymphoid development. In: Zon L.I., editor. Hematopoiesis A Developmental Approach. Oxford University Press; Oxford: 2001. pp. 15–34. [Google Scholar]
- Baron M.H., Isern J., Fraser S.T. The embryonic origins of erythropoiesis in mammals. Blood. 2012;119:4828–4837. doi: 10.1182/blood-2012-01-153486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.J., Yokomizo T., Zeigler B.M., Dzierzak E., Speck N.A. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.J., Li Y., De Obaldia M.E., Yang Q., Yzaguirre A.D., Yamada-Inagawa T., Vink C.S., Bhandoola A., Dzierzak E., Speck N.A. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A., Dieterlen-Lievre F., Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- de Bruijn M.F., Speck N.A., Peeters M.C., Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken H.M., Nishikawa S., Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- Ferkowicz M.J., Starr M., Xie X., Li W., Johnson S.A., Shelley W.C., Morrison P.R., Yoder M.C. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- Fontaine-Perus J.C., Calman F.M., Kaplan C., Le Douarin N.M. Seeding of the 10-day mouse embryo thymic rudiment by lymphocyte precursors in vitro. J. Immunol. 1981;126:2310–2316. [PubMed] [Google Scholar]
- Gekas C., Dieterlen-Lièvre F., Orkin S.H., Mikkola H.K. The placenta is a niche for hematopoietic stem cells. Dev. Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Haar J.L., Ackerman G.A. A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat. Rec. 1971;170:199–223. doi: 10.1002/ar.1091700206. [DOI] [PubMed] [Google Scholar]
- Huang H., Auerbach R. Identification and characterization of hematopoietic stem cells from the yolk sac of the early mouse embryo. Proc. Natl. Acad. Sci. USA. 1993;90:10110–10114. doi: 10.1073/pnas.90.21.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito C., Yamazaki H., Yamane T. Earliest hematopoietic progenitors at embryonic day 9 preferentially generate B-1 B cells rather than follicular B or marginal zone B cells. Biochem. Biophys. Res. Commun. 2013;437:307–313. doi: 10.1016/j.bbrc.2013.06.073. [DOI] [PubMed] [Google Scholar]
- Kattman S.J., Huber T.L., Keller G.M. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kennedy M., Firpo M., Choi K., Wall C., Robertson S., Kabrun N., Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- Kingsley P.D., Malik J., Fantauzzo K.A., Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- Kyba M., Perlingeiro R.C., Daley G.Q. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lancrin C., Sroczynska P., Stephenson C., Allen T., Kouskoff V., Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K.E., Frame J.M., Fromm G.J., Koniski A.D., Kingsley P.D., Little J., Bulger M., Palis J. A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo. Blood. 2011;117:4600–4608. doi: 10.1182/blood-2010-12-325357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K.E., Koniski A.D., Malik J., Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101:1669–1676. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- Medvinsky A., Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Medvinsky A., Rybtsov S., Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- Mikkola H.K., Fujiwara Y., Schlaeger T.M., Traver D., Orkin S.H. Expression of CD41 marks the initiation of definitive hematopoiesis in the mouse embryo. Blood. 2003;101:508–516. doi: 10.1182/blood-2002-06-1699. [DOI] [PubMed] [Google Scholar]
- Mitjavila-Garcia M.T., Cailleret M., Godin I., Nogueira M.M., Cohen-Solal K., Schiavon V., Lecluse Y., Le Pesteur F., Lagrue A.H., Vainchenker W. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development. 2002;129:2003–2013. doi: 10.1242/dev.129.8.2003. [DOI] [PubMed] [Google Scholar]
- Nakano T., Kodama H., Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- Nakano T., Kodama H., Honjo T. In vitro development of primitive and definitive erythrocytes from different precursors. Science. 1996;272:722–724. doi: 10.1126/science.272.5262.722. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Nishikawa S., Yoshinaga K., Hayashi S., Kunisada T., Nakao J., Kina T., Sudo T., Kodama H., Nishikawa S. Expression and function of c-Kit in fetal hemopoietic progenitor cells: transition from the early c-Kit-independent to the late c-Kit-dependent wave of hemopoiesis in the murine embryo. Development. 1993;117:1089–1098. doi: 10.1242/dev.117.3.1089. [DOI] [PubMed] [Google Scholar]
- Okuyama H., Tsuneto M., Yamane T., Yamazaki H., Hayashi S. Discrete types of osteoclast precursors can be generated from embryonic stem cells. Stem Cells. 2003;21:670–680. doi: 10.1634/stemcells.21-6-670. [DOI] [PubMed] [Google Scholar]
- Ottersbach K., Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev. Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Perlingeiro R.C., Kyba M., Daley G.Q. Clonal analysis of differentiating embryonic stem cells reveals a hematopoietic progenitor with primitive erythroid and adult lymphoid-myeloid potential. Development. 2001;128:4597–4604. doi: 10.1242/dev.128.22.4597. [DOI] [PubMed] [Google Scholar]
- Saga Y., Miyagawa-Tomita S., Takagi A., Kitajima S., Miyazaki Ji., Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Samokhvalov I.M., Samokhvalova N.I., Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- Schlissel M.S., Corcoran L.M., Baltimore D. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J. Exp. Med. 1991;173:711–720. doi: 10.1084/jem.173.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S.E., Pollard J.W. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Taoudi S., Gonneau C., Moore K., Sheridan J.M., Blackburn C.C., Taylor E., Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3:99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Turpen J.B., Knudson C.M., Hoefen P.S. The early ontogeny of hematopoietic cells studied by grafting cytogenetically labeled tissue anlagen: localization of a prospective stem cell compartment. Dev. Biol. 1981;85:99–112. doi: 10.1016/0012-1606(81)90239-6. [DOI] [PubMed] [Google Scholar]
- Turpen J.B., Kelley C.M., Mead P.E., Zon L.I. Bipotential primitive-definitive hematopoietic progenitors in the vertebrate embryo. Immunity. 1997;7:325–334. doi: 10.1016/s1074-7613(00)80354-4. [DOI] [PubMed] [Google Scholar]
- Wang Q., Stacy T., Binder M., Marin-Padilla M., Sharpe A.H., Speck N.A. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I.L., Papaioannou V.E., Gardner R.L. Fetal hematopoietic origins of the adult hematolymphoid system. In: Clarkson B., Marks P.A., Till J.E., editors. Differentiation of Normal and Neoplasmic Hematopoietic Cells. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1978. pp. 33–47. [Google Scholar]
- Whitelaw E., Tsai S.F., Hogben P., Orkin S.H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol. Cell. Biol. 1990;10:6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Kunisada T., Yamazaki H., Era T., Nakano T., Hayashi S.I. Development of osteoclasts from embryonic stem cells through a pathway that is c-fms but not c-kit dependent. Blood. 1997;90:3516–3523. [PubMed] [Google Scholar]
- Yamane T., Hosen N., Yamazaki H., Weissman I.L. Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proc. Natl. Acad. Sci. USA. 2009;106:8953–8958. doi: 10.1073/pnas.0904090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder M.C., Hiatt K., Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc. Natl. Acad. Sci. USA. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder M.C., Hiatt K., Dutt P., Mukherjee P., Bodine D.M., Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- Yokota T., Huang J., Tavian M., Nagai Y., Hirose J., Zúñiga-Pflücker J.C., Péault B., Kincade P.W. Tracing the first waves of lymphopoiesis in mice. Development. 2006;133:2041–2051. doi: 10.1242/dev.02349. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L.D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


