Abstract
BACKGROUND:
Surgical treatments for deep-seated intracranial lesions have been limited by morbidities associated with resection. Real-time magnetic resonance imaging–guided focused laser interstitial thermal therapy (LITT) offers a minimally invasive surgical treatment option for such lesions.
OBJECTIVE:
To review treatments and results of patients treated with LITT for intracranial lesions at Washington University School of Medicine.
METHODS:
In a review of 17 prospectively recruited LITT patients (34-78 years of age; mean, 59 years), we report demographics, treatment details, postoperative imaging characteristics, and peri- and postoperative clinical courses.
RESULTS:
Targets included 11 gliomas, 5 brain metastases, and 1 epilepsy focus. Lesions were lobar (n = 8), thalamic/basal ganglia (n = 5), insular (n = 3), and corpus callosum (n = 1). Mean target volume was 11.6 cm3, and LITT produced 93% target ablation. Patients with superficial lesions had shorter intensive care unit stays. Ten patients experienced no perioperative morbidities. Morbidities included transient aphasia, hemiparesis, hyponatremia, deep venous thrombosis, and fatal meningitis. Postoperative magnetic resonance imaging showed blood products within the lesion surrounded by new thin uniform rim of contrast enhancement and diffusion restriction. In conjunction with other therapies, LITT targets often showed stable or reduced local disease. Epilepsy focus LITT produced seizure freedom at 8 months. Preliminary overall median progression-free survival and survival from LITT in tumor patients were 7.6 and 10.9 months, respectively. However, this small cohort has not been followed for a sufficient length of time, necessitating future outcomes studies.
CONCLUSION:
Early peri- and postoperative clinical data demonstrate that LITT is a safe and viable ablative treatment option for intracranial lesions, and may be considered for select patients.
ABBREVIATION:
LITT, laser interstitial thermal therapy
KEY WORDS: Laser; Laser interstitial thermal therapy; LITT, Minimally invasive surgery; Stereotactic surgery
Intracranial lesions such as brain tumors and epilepsy foci are targets for medical therapy, surgical resection, and radiotherapy. Traditional surgical treatments typically include craniotomy for resection. Unfortunately, many therapeutic targets are not surgically accessible via traditional means due to the morbidity or mortality associated with surgical excision. Treatment options for patients with surgically inaccessible lesions have generally been limited to medical therapy and radiotherapy.1,2
Minimally invasive surgical treatments for deep brain tumors that avoid the morbidities associated with surgical resection are highly desirable. Thermoablation has been used in general surgery to treat multiple organ systems.3-6 Recent advances in technology have improved the ability of neurosurgeons to use lasers to treat brain tumors via minimally invasive methods. Laser interstitial thermal therapy (LITT) is a thermoablative procedure that uses a laser to produce a precise and minimally invasive heat injury to target tissue. Magnetic resonance imaging (MRI)–guided real-time LITT offers a promising new technique that can be used to treat brain tumors,7-19 radiation necrosis,20 and epileptic foci.21
OBJECTIVES
We previously reported our initial experience using LITT in the treatment of brain metastasis.9 We now report an analysis of a prospectively recruited cohort including outcome data for patients treated with MRI-guided focused LITT for intracranial lesions at Washington University School of Medicine.
PATIENTS AND METHODS
Patient Selection
Patients were selected for the Neuroblate System (Monteris Medical Corporation, Plymouth, Minnesota) by the senior author (E.C.L.). Application of LITT fell into 4 general categories: (1) deep-seated glial neoplasms, (2) recurrent gliomas, (3) recurrent brain metastases status post radiosurgery, and (4) deep-seated epilepsy focus. Tumor patients were evaluated by a multidisciplinary team of neurosurgeons, medical oncologists, and radiation oncologists. If surgical resection was indicated but traditional craniotomy carried unacceptable risks to the patient, the patient was then evaluated for LITT. General characteristics that made the lesion favorable to treatment include the following: (1) the lesion was supratentorial and accessible from a cephalad approach (ie, top one third of the head), (2) the lesion was unilateral, (3) the lesion was relatively well circumscribed, (4) the volume of lesion could be encompassed by two 3-cm cylinders (ie, 2 treatment trajectories), (5) a safe trajectory could be established relative to functional structures (ie, eloquent cortex and corticospinal tract), and (6) the patient's body habitus could fit into the bore of the MRI. Trajectories were chosen to maximize lesion ablation and minimize the number of passes. For 4 multitrajectory cases, trajectories were preplanned to maximize ablation. For the remaining 2 multitrajectory cases, additional trajectories were designed intraoperatively to maximize ablation volume. Some patients required stereotactic biopsy either before or as part of their procedure. Patient 10 had failed an awake craniotomy for resection of an insular lesion due to emesis and was selected for awake LITT. The epilepsy patient was selected for LITT after invasive monitoring confirmed that the epileptogenic focus correlated with a radiographic abnormality in the deep insula.
Study Design
After obtaining approval from the Washington University Institutional Review Board for medical research, patients undergoing LITT were prospectively enrolled in the study. Clinical records, radiological findings, operative details, and complications were reviewed. Patients were treated at Washington University between September 2010 and November 2012. Follow-up data were collected from hospital and clinic charts.
Participants
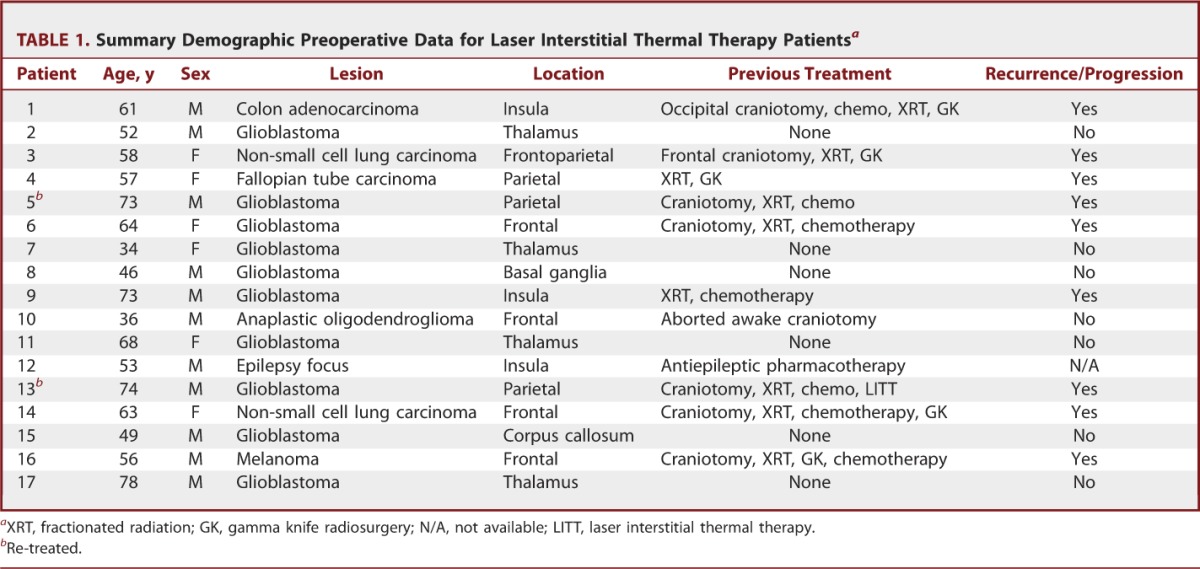
This review includes 17 patients (11 male, 6 female; M:F, 1.8:1). Ages ranged from 38 to 78 years (mean age, 59 years). One patient was undergoing repeat treatment. Lesions treated included glial neoplasms (n = 11), metastatic tumors (n = 5), and an epilepsy focus (n = 1). Locations included frontal/parietal lobe (n = 8), thalamus (n = 4), insula (n = 3), basal ganglia (n = 1), and corpus callosum (n = 1). Six patients were treated de novo, whereas the remaining patients had undergone chemo/pharmacotherapy, previous surgery and/or radiotherapy. Nine patients were treated with LITT for disease progression (Table 1). Karnofsky Performance Status Scale score before treatment was 74.1 ± 9.4.
TABLE 1.
Summary Demographic Preoperative Data for Laser Interstitial Thermal Therapy Patientsa
LITT Procedure
Neuroblate System (Monteris Medical Corporation) treatment was performed similarly as described previously.9 After initiation of general anesthesia and placement of an MRI-compatible head holder, the Medtronic STEALTH MRI-guided navigation system (Medtronic, Minneapolis, Minnesota) was registered using preoperative MRI to define the centroid of the target lesion. After inserting the probe in the lesion and positioning the patient in the IMRIS intraoperative 1.5-T MRI unit (IMRIS Inc, Minnetonka, Minnesota), the laser was activated and real-time thermal isodose MRI was monitored to achieve sufficient treatment (Figures 1A and 1B). As previously described,19 the Neuroblate software provided predictive thermal dosage lines to estimate ablation volume (Figure 1C). These thermal dosage lines were used to establish treatment duration, movement of the probe, and additional trajectories. For some patients, additional trajectories were chosen preoperatively; for other patients, additional trajectories were chosen based on thermal dosage lines of the initial trajectory. After successful ablation, the probe was removed and the wound was closed in routine fashion. The patient was admitted to the intensive care unit (ICU) postoperatively for observation.
FIGURE 1.
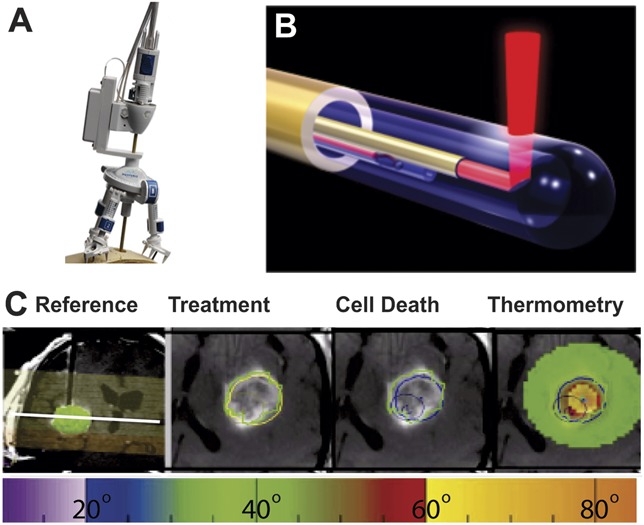
Magnetic resonance imaging (MRI)–guided Neuroblate focused laser interstitial thermal therapy. A, tripod base affixed to surface with laser probe visible. B, rendering of Neuroblate probe with laser emerging orthogonally to tip. C, trajectory strategy, treatment, and cell death areas, and thermometry measurements. The target region was defined preoperatively based on MRI (green sphere, on left and green lines). Intraoperative axial images show enhancing lesion within target region (green line), 45°C thermal dose line (yellow), and 52°C thermal dosage line (blue). Thermometry measurements (right) in degrees Celsius are plotted in space relative to the MRI within the region of thermometry monitoring (light green). A thermometry temperature color key is shown at bottom.
Data Analysis
Postoperative MRI scans were reviewed to assess temporal imaging characteristics and to evaluate for local and/or distant recurrence. Ellipsoid volumes of enhancing lesions were measured9 on preoperative and postoperative images. MRI scans were binned into 5 or 10 months postoperatively and percentages of baseline were averaged. Clinical outcomes were evaluated by a neurosurgeon postoperatively and Karnofsky performance data on last follow-up were documented when available. Follow-up time, survival, and time to radiographic progression were recorded. Kaplan-Meier curves were generated using outcome data and plotted in GraphPad Prism (GraphPad Software Inc, La Jolla, California). Statistical analysis included the Fisher exact test, with data reaching statistical significance at P < .05.
RESULTS
LITT: Descriptive Data
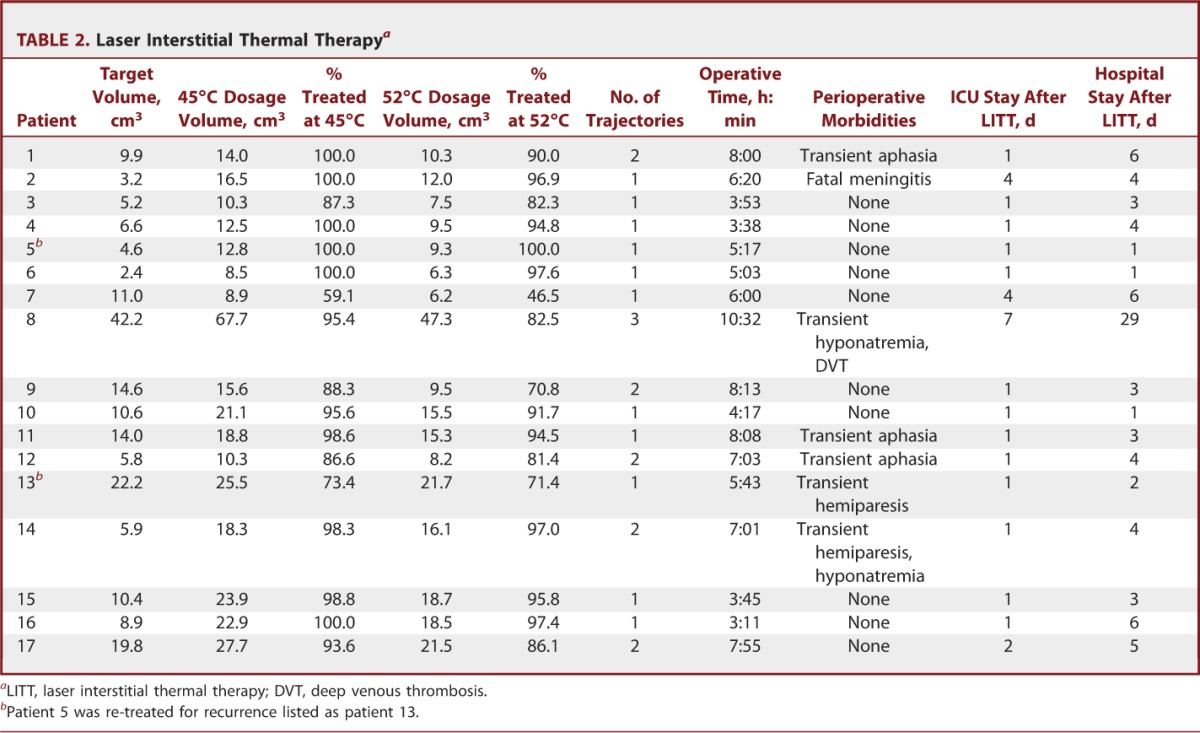
Operative details are displayed in Table 2. Mean target volume was 11.6 ± 9.6 cm3. Intraoperative thermometry measured the lesion boundary reaching 45°C and 52°C, which represents tumor temperatures necessary to induce apoptosis and necrosis, respectively.9 Mean volumes of target reaching 45°C and 52°C were 19.7 ± 13.7 cm3 and 14.9 ± 9.8 cm3, respectively. Mean target percentages treated at 45°C and 52°C were 92.6 ± 11.2% and 86.9 ± 13.8%, respectively. Eleven lesions were treated with a single pass and 6 lesions required more than 1 pass. Average target sizes for 1-pass and more than 1-pass targets were 9.0 ± 5.7 cm3 and 11.2 ± 6 cm3, respectively. Operative time was 6:07 ± 2:02 hours. Single-trajectory cases took 5:01 ± 1:28 hours, whereas multitrajectory cases required 8:07 ± 1:17 hours (P < .001).
TABLE 2.
Laser Interstitial Thermal Therapya
Perioperative Outcome Data
Ten patients experienced no morbidities. Seven patients experienced perioperative morbidities (Table 2) such as transient aphasia (n = 3), transient hemiparesis (n = 3), transient hyponatremia (n = 2), and a lower extremity deep venous thrombosis (n = 1). Patients with aphasia or hemiparesis improved over time with steroid therapy. Hyponatremia resolved spontaneously. One patient experienced fatal meningitis. In-depth analysis revealed that this meningitis was due to an operating room infrastructure-related contamination and not due to equipment contamination related to performance of procedure. Average time spent in the ICU was 1.8 ± 1.7 days, and the average total hospital stay was 5.0 ± 6.4 days (Table 2). Hospital stay was 3.2 ± 1.7 days for superficial targets and 9.4 ± 11.0 days for thalamic/basal ganglia targets. Intensive care unit stay was significantly shorter for superficial targets (1 ± 0 days) compared with thalamic/basal ganglia targets (3.6 ± 2.3 days, P < .05).
Postoperative Imaging Characteristics: Descriptive Data
Laser ablation produced consistent postoperative imaging characteristics that changed over time. Figure 2 shows a case example of a thalamic glioblastoma (Figure 2A) treated with LITT de novo (Figure 2B). MRI on postoperative day 1 shows a ring of diffusion restriction at the edge of the treatment zone (Figure 2C), new surrounding thin uniform ring of contrast enhancement (Figure 2D), peritumoral edema (Figure 2D), and blood products within the lesion on susceptibility-weighted sequences. The new rim of enhancement typically persists for several months but often decreases with time (Figures 2E-2G). Peritumoral edema initially increases in the postoperative period but often declines after a year (Figures 2E-2G).
FIGURE 2.
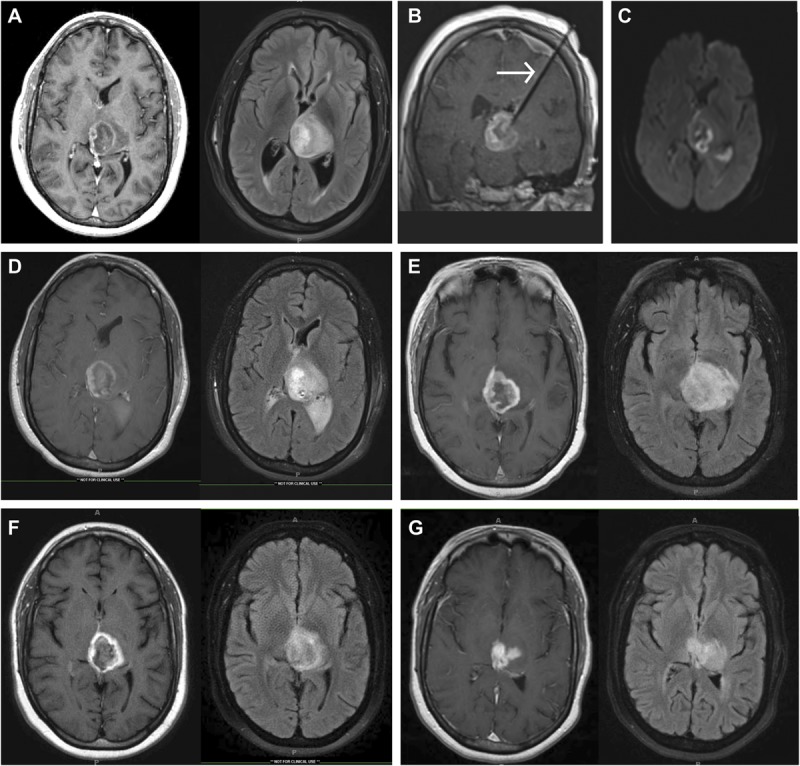
Magnetic resonance imaging (MRI) showing a thalamic glioblastoma treated with focused laser interstitial thermal therapy (LITT). A, preoperative T1-weighted MRI with gadolinium and T2-weighted MRI showing a 3.5 × 4.0-cm left thalamic lesion. B, intraoperative coronal T1-weighted MRI with contrast showing the probe targeting the tumor. Diffusion-weighted image (C) and T1-weighted MRI with gadolinium and T2-weighted MRI (D) were performed on postoperative day 1. T1-weighted MRI with gadolinium and T2-weighted MRI 3 (E), 7 (F), and 15 (G) months postoperatively. Arrow in B indicates LITT probe.
LITT was also used to treat 4 recurrent glioblastomas. Figure 3 shows an example of a recurrent left frontal glioblastoma treated with LITT cytoreduction. Surveillance MRI revealed a recurrent left frontal nodule (Figure 3A). MRI on postoperative day 1 showed successful target ablation (Figure 3B). The 5-month postoperative MRI showed no recurrence and reduced peritumoral edema (Figure 3C). Tumor recurrence was found on an MRI performed 11 months postoperatively (Figure 3D).
FIGURE 3.
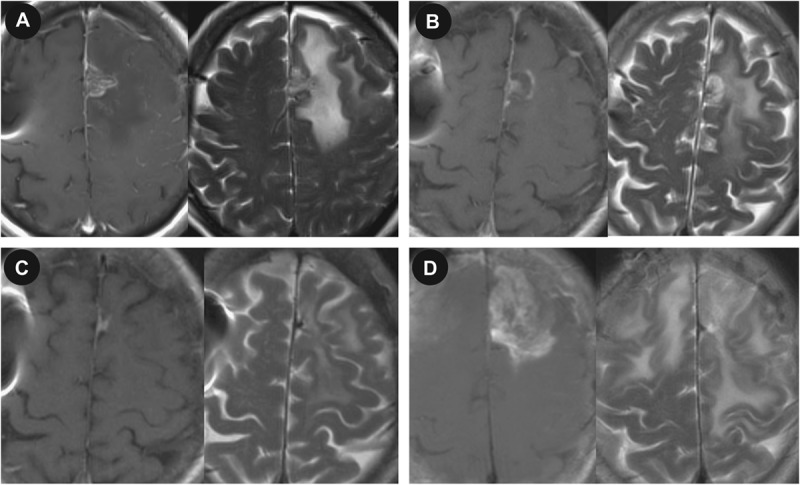
Magnetic resonance imaging (MRI) showing a recurrent glioblastoma treated with focused laser interstitial thermal therapy. A, preoperative T1-weighted MRI with gadolinium and T2-weighted MRI showing a 1.3 × 0.8-cm left frontal lesion. T1-weighted MRI with gadolinium and T2-weighted MRI on postoperative days 1 (B) and 5 (C) and 11 months (D) postoperatively.
LITT was used to treat brain metastasis in 5 patients. Figure 4 shows a right frontal melanoma (Figure 4A) treated with LITT (Figure 4B). Postoperative MRI is shown for postoperative day 1 (Figure 4C). MRI performed 1 month postoperatively shows stable disease and edema (Figure 4D). MRI performed 3 months postoperatively showed an increase in lesion size (Figure 4E).
FIGURE 4.
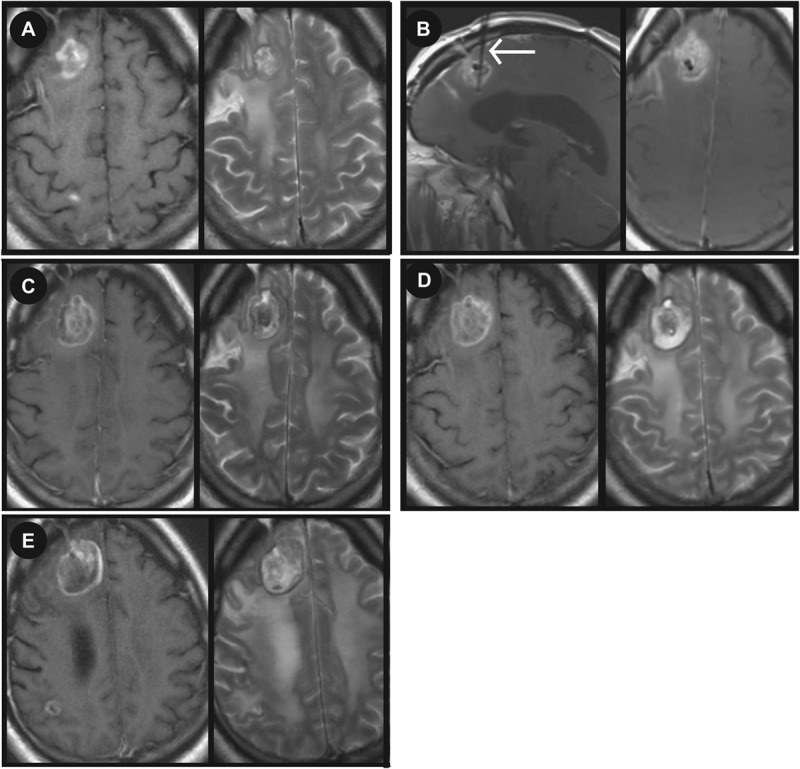
Magnetic resonance imaging (MRI) showing a melanoma metastasis treated with focused laser interstitial thermal therapy (LITT). A, preoperative T1-weighted MRI with gadolinium and T2-weighted MRI showing a 2.5 × 2.1-cm right frontal lesion. B, intraoperative sagittal and axial T1-weighted MRI with contrast showing the probe targeting the tumor. T1-weighted MRI with gadolinium and T2-weighted MRI (C) were performed on postoperative day 1. T1-weighted MRI with gadolinium and T2-weighted MRI 1 (D) and 3 (E) months postoperatively. Arrow in B indicates LITT probe.
One patient was treated with LITT for ablation of an epilepsy focus. Preoperative MRI showed a lesion in the left insula (Figure 5A). Interictal brain positron emission tomography showed hypometabolism in the left insula. Ictal single-photon emission computed tomography showed increased activity in the left insular and temporal lobes (Figure 5B). The patient underwent a craniotomy for placement of grids, strips, and an insular depth electrode. Subsequent invasive monitoring confirmed that the left insular lesion was the seizure focus, and LITT was performed to ablate the seizure focus. Figures 5C and 5D show the response to LITT 1 day and 5 months postoperatively.
FIGURE 5.
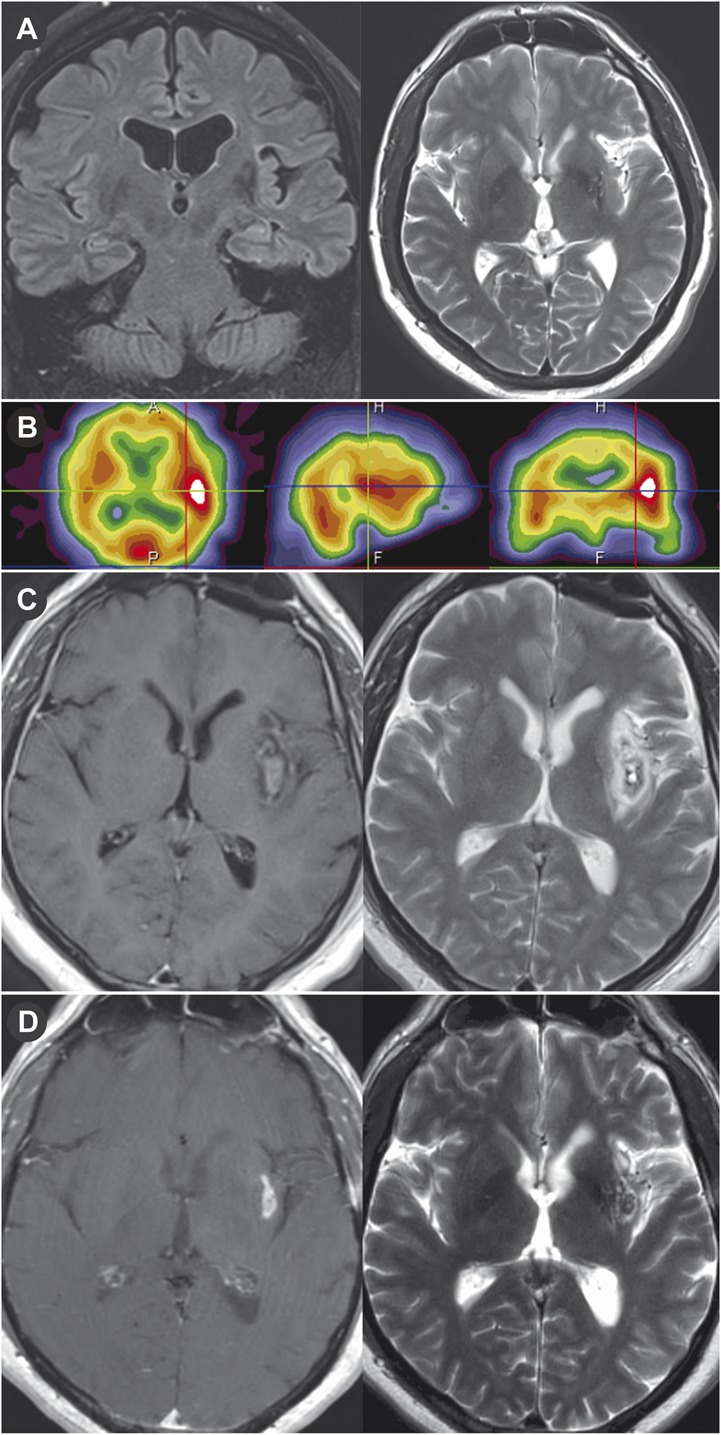
Magnetic resonance imaging (MRI) showing architectural distortion in the left subinsular region that corresponded to an abnormality on the ictal and interictal single-photon emission computed tomography (SPECT) study and was treated with focused laser interstitial thermal therapy. A, preoperative coronal fluid-attenuated inversion recovery MRI and T2-weighted MRI showing a 2.5 × 2.1-cm left subinsular lesion. B, preoperative ictal SPECT study showing increased activity in left insula and temporal lobe. T1-weighted MRI with gadolinium and T2-weighted MRI on postoperative day 1 (C) and 5 months postoperatively (D).
Serial MRI scans were reviewed to assess for local or tumor progression. MRI shows local and disease control for most patients for over 6 to 7 months. Two patients showed rapid distant disease progression. One patient showed local progression of disease at 6 months. Four patients showed distant progression at 8 to 9 months. Volumetric analysis 5 and 10 months postoperatively showed that target local lesions measured 91.4 ± 17.1% and 67.4 ± 8.4% of control, respectively (Figure 6).
FIGURE 6.
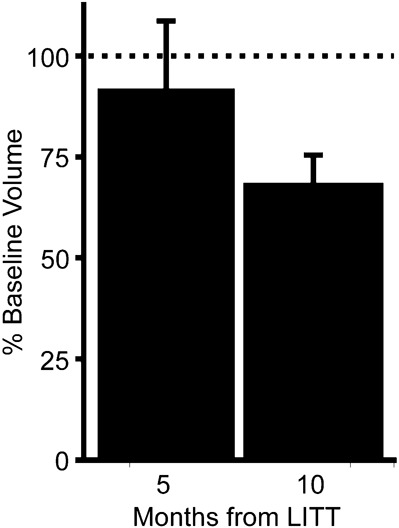
Local control of brain tumors after focused laser interstitial thermal therapy (LITT). Histogram shows volumetric analysis of postoperative imaging binned into 5 and 10 months after treatment relative to preoperative baseline volume.
Postoperative Clinical Outcome Data
Nine patients were treated postoperatively with chemotherapy, 6 were treated with radiotherapy, 1 required a craniotomy at a different location, and 1 required repeat LITT (Table 3). Eight patients experienced disease progression. Five patients died (3 patients died of central nervous system disease). Average Karnofsky score at the last follow-up of surviving tumor patients was 63.8 ± 16.0. Patient 12 has remained seizure free for 8 months postoperatively.
TABLE 3.
Postoperative Clinical Coursea
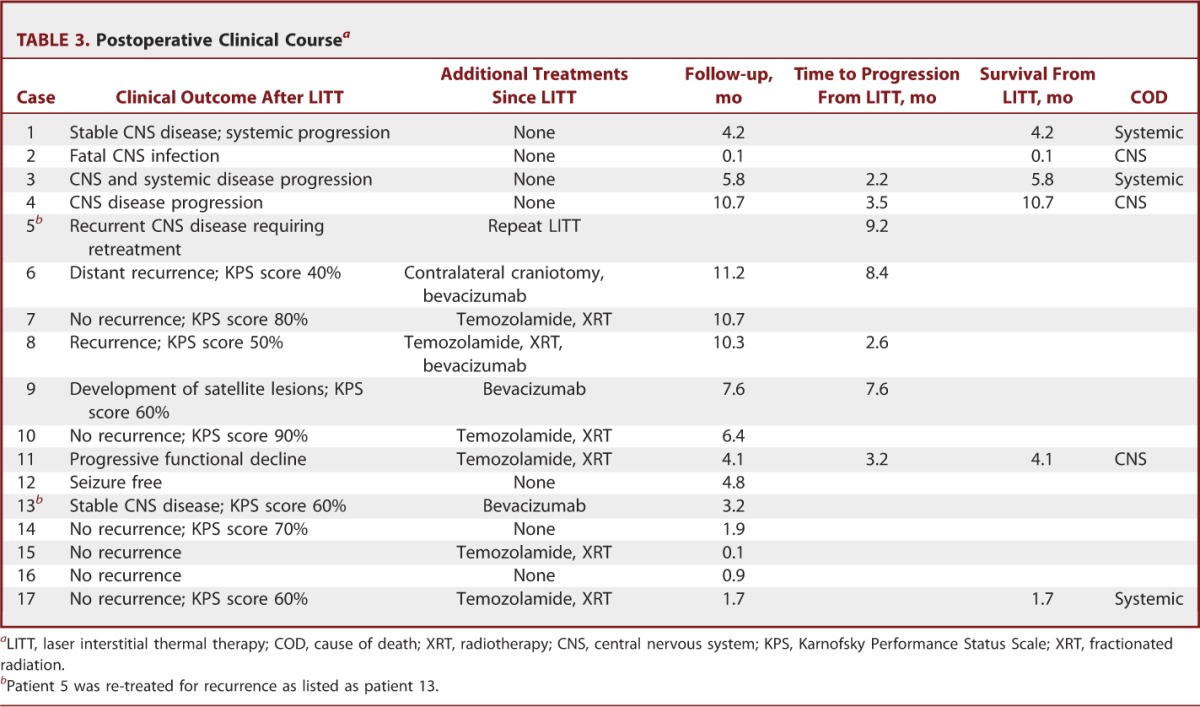
Preliminary outcomes in tumor patients were studied, and Kaplan-Meier curves were generated. Overall median recurrence-free survival for all brain tumor patients treated with LITT was 7.6 months from LITT. Median recurrence-free survival for glioma and metastasis patients was 8 and 5.8 months after LITT, respectively (Figure 7A). Median overall survival is 10.7 months after LITT (Figure 7B). Median survival for metastasis patients is 5.8 months after LITT, whereas median survival is not yet reached for glioma patients (Figure 7B). Median survival from initial intracranial diagnosis was 33.5 months overall, 33.5 months for metastasis, and has yet to be reached for gliomas (Figure 7C). Recurrent vs de novo deep-seated glioma subgroup analysis was limited due to the small cohort size and limited follow-up. Median survival has yet to be reached. Median recurrence-free survival for recurrent and de novo deep-seated gliomas was 8.4 and 2.9 months, respectively.
FIGURE 7.
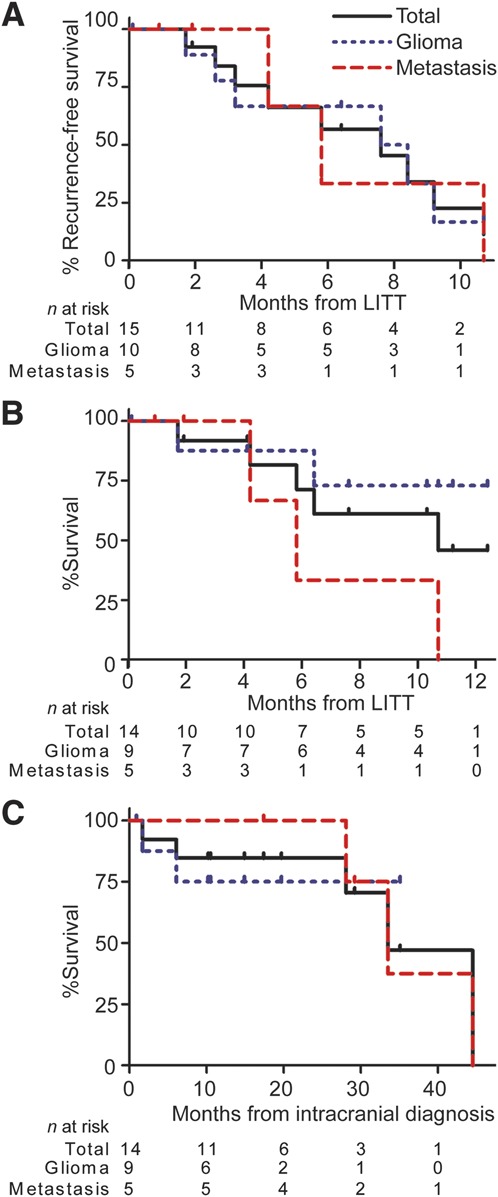
Kaplan-Meier curves for focused laser interstitial thermal therapy (LITT) tumor patients including total cohort, gliomas, and metastases. Recurrence-free survival (A), survival after LITT (B), and survival after intracranial diagnosis (C) are displayed. Patient 2 was excluded from this analysis. The number of patients (n) at risk are below the curves at listed monthly intervals.
DISCUSSION
Key Results and Interpretation
Advances in technology have pushed the field of neurosurgery and benefited patient care. Minimally invasive treatments such as LITT provide surgeons with new cytoreductive techniques to treat lesions that previously could not be addressed via open surgical approaches. Although lasers are not new, experience with real-time MRI-guided laser to treat intracranial lesions is limited.8-22 In this report, we present our institutional series and outcomes of patients treated with LITT for 11 glial neoplasms, 5 brain metastases, and a seizure focus. Five targets were located in deep gray matter (thalamus/basal ganglia), and the remaining were located in superficial structures. Nine patients were treated for progression of disease, and 6 targets were treated de novo. The cases represent 3 treatment candidates: (1) recurrent superficial tumors, (2) deep-seated brain tumors not amenable to traditional resection, and (3) deep seizure foci. Patients were selected for LITT if they had a favorable target and were not amenable to traditional resection.
Real-time intraoperative data demonstrated that LITT treated 93% of our target boundaries. As expected, the number of trajectories significantly increased the operative time. Intensive care unit and hospital stays for LITT patients were low, consistent with previous studies.18 In this report, we show that patients with superficial lesions only stay in the ICU for 1 day, significantly shorter than for deep gray matter lesions. Three of patients 5 (60%) with deep-seated lesions experienced a complication. In contrast, only 1 of 3 patients (33%) with superficial lesions experienced a complication. Post hoc chart analysis also revealed that ablation of deep-seated lesions in patients 7, 8, and 17 led to a transient period of slowed cognition, prompting extended intensive care neurological monitoring. Most significant complications were transient in nature, but 1 patient experienced fatal meningitis, which was not related to the LITT procedure.
As others have shown,21 we also find that thermal ablation of an epileptogenic focus can treat seizures. This therapy option opens new possibilities for select epilepsy patients in whom imaging or depth-electrode data support LITT for ablation. We anticipate that additional series and trials will follow this report detailing the efficacy of LITT for ablation of seizure foci.
Postoperative imaging characteristics were evaluated immediately after surgery and on postoperative clinic visits. LITT produced predictable treatment effects including (1) hemorrhagic necrosis within the lesion, (2) a new ring of diffusion restriction within the treatment zone and the treatment zone edge, (3) a new ring of enhancement at the treatment zone edge, and (4) peritumoral edema. Although somewhat variable, delayed imaging typically showed a loss of diffusion restriction, a stable or reduced ring of enhancement, and eventual decline in edema. Clinical outcomes of tumor patients varied, with distant or recurrent disease developing in some patients. Of the surviving patients, the average Karnofsky performance score was 64.
Limitations
Preliminary quantitative analysis of outcomes offers median progression-free survival and survival. Deep-seated basal ganglia and thalamic glioblastomas are thought to portend a poorer prognosis than glioblastomas elsewhere.23,24 Treatment for such tumors is limited to radiation and chemotherapy. In our small cohort (n = 4), patients with de novo deep-seated gliomas had a 2- to 10 month recurrence-free survival. Patients with recurrent glioblastomas treated with bevacizumab have a 4.2 to 6 month median recurrence-free survival.25,26 In our cohort, recurrence-free survival after LITT for recurrent high-grade gliomas was 8.4 months. However, due to the small cohort and limited follow-up, the value of quantitative outcome analysis and interpretation is limited. This glimpse into outcomes begs for future multi-institutional studies with larger cohorts and longer follow-up to allow a meaningful analysis. Previous reports suggest that focal therapies such as gamma knife may produce less than desirable outcomes for glioblastoma patients.27 LITT, although focal, produces a cytoreductive/ablative measure that can coagulate more than 93% of the tumor. Future series and trials will be necessary to further evaluate how LITT affects long-term prognosis in glioma patients, especially those with deep-seated tumors not amenable to resection.
CONCLUSION
Advances in neurosurgery depend on a marriage of surgical and technological innovation. We reviewed our initial experience and outcomes with the NeuroBlate System LITT for the treatment of intracranial lesions. LITT appears to be a safe and viable surgical option for the treatment of intracranial lesions and may be considered for select patients. However, additional studies are necessary to completely evaluate LITT for clinical efficacy and outcomes.
Disclosures
Dr Leuthardt has a consulting relationship with Monteris Medical. The other authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article.
Footnotes
Correspondence: Eric C. Leuthardt, MD, Washington University School of Medicine, Department of Neurological Surgery, 660 S. Euclid Ave., Campus Box 8057, St. Louis, MO 63110. E-mail: LeuthardtE@wudosis.wustl.edu
Figure.
No available caption
REFERENCES
- 1.Ewend MG, Elbabaa S, Carey LA. Current treatment paradigms for the management of patients with brain metastases. Neurosurgery. 2005;57(suppl 5):S66-S77; discussion S61-S64 [DOI] [PubMed] [Google Scholar]
- 2.Ewend MG, Morris DE, Carey LA, Ladha AM, Brem S. Guidelines for the initial management of metastatic brain tumors: role of surgery, radiosurgery, and radiation therapy. J Natl Compr Canc Netw. 2008;6(5):505-513; quiz 514 [DOI] [PubMed] [Google Scholar]
- 3.Flanders VL, Gervais DA. Ablation of liver metastases: current status. J Vasc Interv Radiol. 2010;21(suppl 8):S214-S222 [DOI] [PubMed] [Google Scholar]
- 4.Karam JA, Ahrar K, Matin SF. Ablation of kidney tumors. Surg Oncol Clin N Am. 2011;20(2):341-353 [DOI] [PubMed] [Google Scholar]
- 5.Lian H, Guo H, Gan W, et al. Cryosurgery as primary treatment for localized prostate cancer. Int Urol Nephrol. 2011;43(4):1089-1094 [DOI] [PubMed] [Google Scholar]
- 6.Sonntag PD, Hinshaw JL, Lubner MG, Brace CL, Lee FT., Jr Thermal ablation of lung tumors. Surg Oncol Clin N Am. 2011;20(2):369-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maroon JC, Onik G, Quigley MR, Bailes JE, Wilberger JE, Kennerdell JS. Cryosurgery re-visited for the removal and destruction of brain, spinal and orbital tumours. Neurol Res. 1992;14(4):294-302 [DOI] [PubMed] [Google Scholar]
- 8.Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943-950 [DOI] [PubMed] [Google Scholar]
- 9.Hawasli AH, Ray WZ, Murphy RK, Dacey RG, Jr, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: technical case report. Neurosurgery. 2012;70(2 suppl operative):332-337; discussion 338 [DOI] [PubMed] [Google Scholar]
- 10.Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser irradiation of recurrent glioblastomas. J Magn Reson Imaging. 2005;22(6):799-803 [DOI] [PubMed] [Google Scholar]
- 11.Schwarzmaier HJ, Eickmeyer F, von Tempelhoff W, et al. MR-guided laser-induced interstitial thermotherapy of recurrent glioblastoma multiforme: preliminary results in 16 patients. Eur J Radiol. 2006;59(2):208-215 [DOI] [PubMed] [Google Scholar]
- 12.Carpentier A, Chauvet D, Reina V, et al. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med. 2012;44(5):361-368 [DOI] [PubMed] [Google Scholar]
- 13.Kahn T, Bettag M, Ulrich F, et al. MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr. 1994;18(4):519-532 [DOI] [PubMed] [Google Scholar]
- 14.Anzai Y, Lufkin R, DeSalles A, Hamilton DR, Farahani K, Black KL. Preliminary experience with MR-guided thermal ablation of brain tumors. AJNR Am J Neuroradiol. 1995;16(1):39-48; discussion 49-52 [PMC free article] [PubMed] [Google Scholar]
- 15.Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1 suppl 1):ONS21-ONS28; discussion ONS28-ONS29 [DOI] [PubMed] [Google Scholar]
- 16.Leonardi MA, Lumenta CB. Stereotactic guided laser-induced interstitial thermotherapy (SLITT) in gliomas with intraoperative morphologic monitoring in an open MR: clinical expierence. Minim Invasive Neurosurg. 2002;45(4):201-207 [DOI] [PubMed] [Google Scholar]
- 17.Bettag M, Ulrich F, Schober R, et al. Stereotactic laser therapy in cerebral gliomas. Acta Neurochir Suppl (Wien). 1991;52(1):81-83 [DOI] [PubMed] [Google Scholar]
- 18.Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery. 2012;71(1 suppl operative):133-144; 144-145 [DOI] [PubMed] [Google Scholar]
- 19.Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma. J Neurosurg. 2013;118(6):1202-1219 [DOI] [PubMed] [Google Scholar]
- 20.Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90(3):192-200 [DOI] [PubMed] [Google Scholar]
- 21.Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. 2012;24(4):408-414 [DOI] [PubMed] [Google Scholar]
- 22.Kahn T, Harth T, Kiwit JC, Schwarzmaier HJ, Wald C, Modder U. In vivo MRI thermometry using a phase-sensitive sequence: preliminary experience during MRI-guided laser-induced interstitial thermotherapy of brain tumors. J Magn Reson Imaging. 1998;8(1):160-164 [DOI] [PubMed] [Google Scholar]
- 23.Grigsby PW, Thomas PR, Schwartz HG, Fineberg BB. Multivariate analysis of prognostic factors in pediatric and adult thalamic and brainstem tumors. Int J Radiat Oncol Biol Phys. 1989;16(3):649-655 [DOI] [PubMed] [Google Scholar]
- 24.Pathy S, Jayalakshmi S, Chander S, Singh R, Julka PK, Rath GK. Prognostic factors influencing the outcome of thalamic glioma. Neurol India. 2002;50(1):37-40 [PubMed] [Google Scholar]
- 25.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733-4740 [DOI] [PubMed] [Google Scholar]
- 26.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722-4729 [DOI] [PubMed] [Google Scholar]
- 27.Crowley RW, Pouratian N, Sheehan JP. Gamma knife surgery for glioblastoma multiforme. Neurosurg Focus. 2006;20(4):E17. [DOI] [PubMed] [Google Scholar]



