Abstract
Morphine exhibits important pharmacological effects for which it has been used in medical practice for quite a long time. However, it has a high addictive potential and can be abused. Long-term use of this drug can be connected with some pathological consequences including neurotoxicity and neuronal dysfunction, hepatotoxicity, kidney dysfunction, oxidative stress and apoptosis. Therefore, most studies examining the impact of morphine have been aimed at determining the effects induced by chronic morphine exposure in the brain, liver, cardiovascular system and macrophages. It appears that different tissues may respond to morphine diversely and are distinctly susceptible to oxidative stress and subsequent oxidative damage of biomolecules. Importantly, production of reactive oxygen/nitrogen species induced by morphine, which have been observed under different experimental conditions, can contribute to some pathological processes, degenerative diseases and organ dysfunctions occurring in morphine abusers or morphine-treated patients. This review attempts to provide insights into the possible relationship between morphine actions and oxidative stress.
Keywords: Morphine, oxidative stress, reactive oxygen and nitrogen species.
1. INTRODUCTION
Morphine is a natural alkaloid occurring in opium poppy [1]. This opioid drug is frequently used for treatment of severe pain because of its powerful analgesic and sedative effects [2, 3]. Morphine suppresses the affective reaction to pain by inhibiting transmission of pain impulses especially in the spinal cord and through modulation of central pain processing. However, it may cause a host of adverse effects when not prescribed properly. Besides others, administration of high doses of morphine can lead to different respiratory, cardiovascular, gastrointestinal or psychiatric problems [3-7].
Morphine exerts its function through opioid receptors that belong to the superfamily of plasma membrane-bound G protein-coupled receptors [8, 9]. However, there is a growing body of evidence that besides activating the relevant receptors and their classical signaling pathways morphine can also elicit oxidative stress under certain conditions. Importantly, oxidative stress seems to play a significant role in the development of different pathological processes [10-13]. On this basis, it is reasonable to assume that formation of reactive oxygen (ROS) or reactive nitrogen (RNS) species may underlie also some adverse physiological effects of morphine.
2. MORPHINE IS AN AGONIST OF OPIOID RECEPTORS
The chemical structure of morphine is derived from phenanthrene molecule consisting of five condensed rings (Fig. 1). The partially hydrogenated phenanthrene core incorporates benzene ring (A-ring) and two partially unsaturated cyclohexane rings (B- and C-rings). It consists of two hydroxyl function groups at positions 3 and 6 (C3 phenolic and C6 alcoholic hydroxyl groups) and amino group at position 17. Its full systematic name is 7,8-didehydro-4,5-epoxy-17-methyl(5α,6α)-morphinan-3,6-diol [14-16].
Fig. (1).
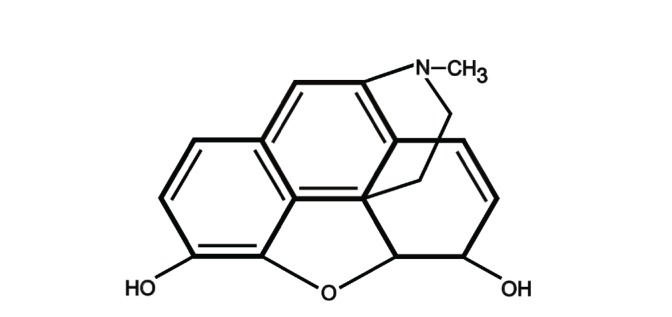
Chemical structure of morphine, an alkaloid of the phenanthrene type.
Morphine acts at the same receptors as endogenous opioids, i.e. the opioid receptors (ORs). According to their pharmacological properties, ORs have been divided into three subtypes: μ, δ, and κ [17]. Morphine is an agonist ligand with high affinity for μ-ORs but it is capable of binding to κ- and δ-ORs with lower affinities, too (Table 1). Nevertheless, it is often used as a selective agonist for μ-ORs in pharmacological studies [16]. The models of interaction between morphine and μ-ORs suggest that the amino and phenolic hydroxyl groups as well as A-, C- and D-rings of morphine interact with transmembrane domains III, V, VI and VII of the receptor [18].
Table 1.
Affinity of Opioid Receptors to Morphine
| OR | µ1/ µ2 | δ | κ |
| Ki (nM) | 0.26 ± 0.03/8.6 ± 1.2 | 358 ± 47 | 52 ± 12 |
These data were obtained from competition binding studies performed by Chen et al. [81].
After activation by agonists, ORs transfer information to a subset of trimeric G proteins (pertussis toxin sensitive Gi/o family), that in turn regulate enzyme activity of adenylyl cyclase (AC) and specific ion channels [19, 20]. More specifically, acute treatment with morphine leads to inhibition of the enzyme activity of AC and reduction of cAMP production. In parallel, the βγ subunits of Gi/o proteins through direct interaction with K+ channels may cause a partial hyperpolarization of the plasma membrane and thus change the sensitivity of excitable cells to stimulation. These effects are schematically illustrated in (Fig. 2). It is well known that treatment of cells with agonists usually results in desensitization of the relevant signal transduction pathways. The process of desensitization is characterized by un-coupling of the receptors from their cognate G proteins, which occurs within seconds to minutes. Subsequently, the receptors become internalized. The sequestered receptors can be recycled back to the plasma membrane or degraded by either the lysosomal or the ubiquitin-proteasome pathway [21-23]. Receptor down-regulation is typically a much slower process that accounts for massive desensitization after prolonged exposure to an agonist [24].
Fig. (2).
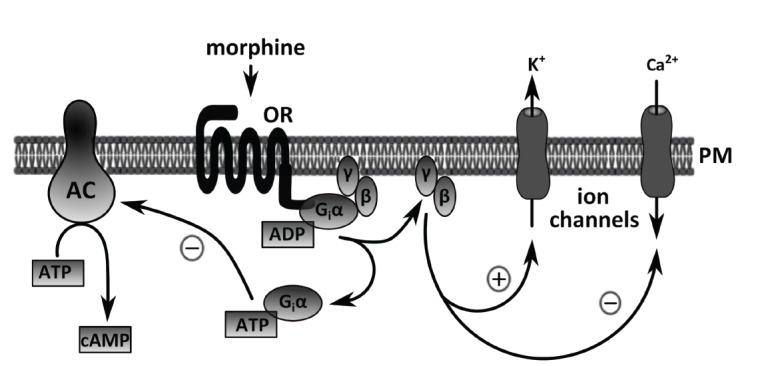
Simplified scheme of opioid receptor downstream signaling pathways. The action of morphine is initiated by the cell surface opioid receptors (ORs) and mediated through a G protein cascade. OR-activated Gi/o proteins inhibit adenylyl cyclase (AC), which results in reduced formation of the key second messenger cAMP from ATP. In parallel, G protein βg dimers may interact with and activate some voltage-sensitive K+ channels and inhibit Ca2+ channels in the plasma membrane (PM), leading to cell hyperpolarization.
Interestingly, sustained treatment with morphine may cause quite different effects than those observed after brief administration of the drug. In contrast to most other opioid agonists, morphine produces much weaker desensitization of ORs [25]. A large number of ORs remain at the plasma membrane even after prolonged or repeated treatment with morphine [26, 27]. In this case, desensitization may occur at a post-receptor level [28, 29]. Some investigators observed altered expression of some G protein subunits and/or AC [30-35]. Moreover, some experiments revealed increased phosphorylation of the β subunit of G proteins after chronic morphine exposure [36]. Besides that, these changes are typically accompanied by supersensitization and super-inhibition of AC, i.e. by a higher or a lower stimulatibility of the enzyme activity, respectively. Interestingly, different AC isoforms seem to be responsible for this sort of phenomena. Whereas AC type I, V, VI and VIII apparently play a role in super-sensitization, AC type II, IV and VII can be super-inhibited [37-41]. Altered sensitivity of the AC signaling system to stimulation observed after sustained treatment with opioid agonists can underlie the development opioid tolerance and dependence.
3. FREE RADICALS AND OXIDATIVE STRESS
Oxidative stress is characterized by excessive formation of free radicals or reactive oxygen (ROS) and reactive nitrogen (RNS) species that exceeds the capacity of antioxidant mechanisms to eliminate these radicals. ROS derived from oxygen include radical species such as superoxide (O2–) and hydroxyl radical (HO●), along with non-radical species such as hydrogen peroxide (H2O2). The most common RNS are nitric oxide (NO·) and peroxynitrite (ONOO–) [42]. The extent of ROS/RNS generation depends on the speed of cellular metabolism. The higher metabolic rate is usually connected with increased generation of free radicals. Low levels of ROS/RNS produced under normal physiological conditions may function as signals important for cell proliferation and survival [43]. However, excessive amounts of ROS/RNS can cause tissue damage and contribute to the development of different pathologies [44-46].
Cells possess powerful antioxidant defense systems that maintain redox homeostasis. Among the most important molecules participating in these regulations are enzymes such as superoxide dismutase (SOD), glutathione-peroxidase (GSHPx) and catalase (CAT). Another molecule known to play a crucial role in cell protection against oxidative damage is the tripeptide glutathione (GSH). The oxidizing agents react with the -SH group of cysteine of the glutathione instead of causing damage elsewhere [47-48]. As outlined above, oxidative stress can arise as a consequence of insufficient ability of cells to cope with and neutralize free radicals that may be produced at inordinately large amounts under certain conditions. There are some indications that, besides many other different agents, opioids can potentially initiate oxidative stress, as well.
4. OXIDATIVE STRESS AS A POSSIBLE CONSEQUENCE OF MORPHINE TREATMENT
Morphine has received far more attention than other opioids in connection with oxidative stress. In general, there are two conceivable ways how this drug could participate in the development of oxidative stress. It can either promote formation of free radicals or reduce activity of different components of antioxidant systems in target cells. Both these ways of action can be possibly combined.
Several studies demonstrated that acute and chronic exposure to morphine may result in a significant decrease in GSH levels in rodent and human brain as well as in mouse liver [49-55]. It was also shown that chronic morphine treatment affects enzyme activities of SOD, CAT and GSHPx, i.e. the enzymes involved in the endogenous antioxidant defense. Although morphine-dependent rhesus macaques were found to have increased levels of SOD and GSHPx after 20 weeks of morphine treatment [56], a number of concordant results presented in several other studies indicated that activities of these enzymes are decreased after morphine exposure [50, 52, 54, 55, 57, 58]. Additionally, the impact of chronic morphine treatment on the enzyme activity of CAT seems to be ambiguous [50, 52, 56, 57]. Apparently, many different factors may influence the effect of morphine on antioxidant enzymes. However, the exact role of other factors (e.g., dosage, exposure time and species) in alterations of the enzyme activities induced by morphine treatment has not yet been clearly elucidated.
It is obvious that the activity of antioxidants and antioxidant enzymes is very closely associated with the production of ROS. In contrast to the activity of antioxidant enzymes, results of several studies exploring ROS production after morphine treatment are rather contradictory. ROS production was observed after short-term or long-term treatment even with low doses of morphine in human or mouse vascular endothelial cells [59, 60]. Morphine-induced formation of superoxide was also detected in macrophages [61]. By contrast, short-term or long-term morphine exposure did not lead to changed ROS production in HEK293 cells or rat hippocampus, respectively [58, 62]. Besides that, morphine did not affect formation of superoxide and hydrogen peroxide in the human neuroblastoma SH-SY5Y cell line. Intriguingly, it was able to inhibit ROS production and apoptotic processes induced by antitumor drug doxorubicin in these cells [63].
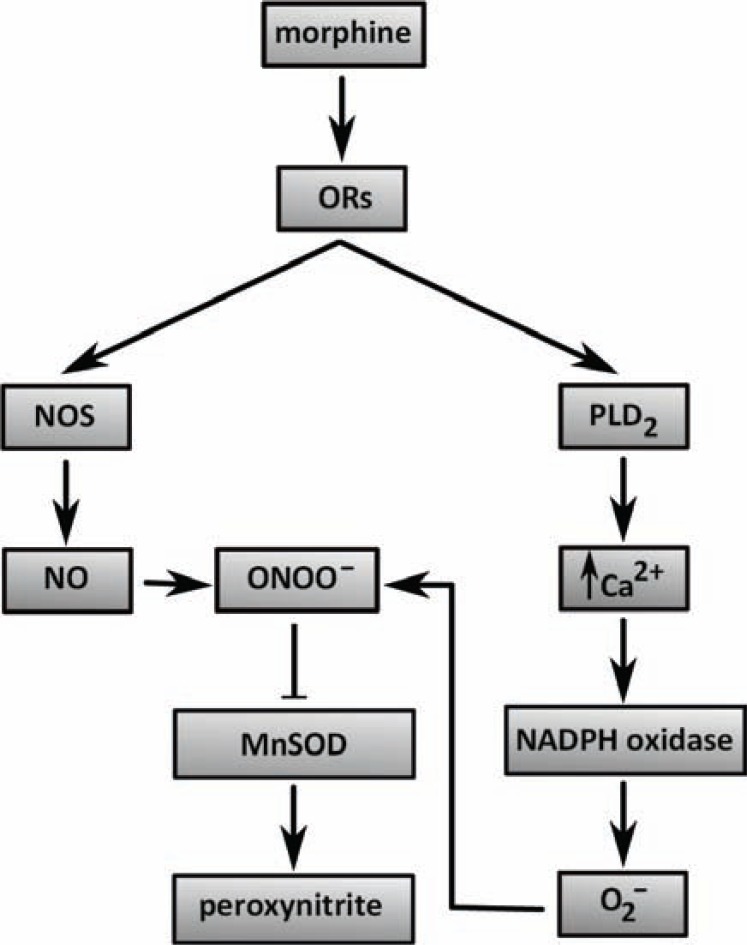
An important oxygen species participating in cell processes mediated by morphine is peroxynitrite that is formed from its precursors, superoxide and nitric oxide. It is worth mentioning here that all these molecules are involved in the development of pain, opiate-induced hyperalgesia and opiate antinociceptive tolerance [64]. It has been shown that the development of morphine antinociceptive tolerance is blocked by inhibition of the formation of peroxynitrite precursors [65]. The enzymes participating in the formation of peroxynitrite and its precursors are nitric oxide synthase (NOS), spinal manganese superoxide dismutase (MnSOD) and NADPH oxidase. Whereas activation of NOS leads to NO production, nitration and subsequent inactivation of MnSOD leads to peroxynitrite formation and activation of NADPH-oxidase results in superoxideproduction [64]. Importantly, inactivation of NOS by inhibitors or inhibition of nitration and inactivation of MnSOD prevented the development of morphine-induced antinociceptive tolerance [65, 66]. Consistently, morphine was not able to induce antinociceptive tolerance in NOS-deficient mice [67]. Morphine-elicited activation of NADPH oxidase and production of superoxide, which contribute to macrophage injury, are apparently mediated by activation of μ-ORs and subsequent activation of the phospholipase D pathway and increase in intracellular Ca2+ concentration [61]. Nevertheless, nitration and inactivation of MnSOD can be inhibited by co-administration of morphine and peroxynitrite scavengers, cationic Mn(III) N-alkylpyridylporphyrins [68, 69]. Hence, these substances may be considered as potent adjuncts to opiates. It is also worth to note that other cell processes such as neuroimmune activation including release of pro-inflammatory cytokines (tumor necrosis factor α, interleukin 1β and interleukin 6) and activation of N-methyl-D-aspartate (NMDA) receptors can contribute to peroxynitrite formation and morphine dependence and tolerance [58, 61, 64, 70, 71]. The detailed molecular mechanisms related to the initiation of oxidative stress by morphine have not yet been described. A simple diagram indicating the putative pathways affected by morphine-stimulated ORs, which can be involved in ROS/RNS production, is shown in (Fig. 3).
Fig. (3).
The relationship between morphine and free radical generation. A diagram schematically depicts putative pathways whereby morphine-activated opioid receptors may induce oxidative stress in target cells.
Morphine effects have also been explored in relation with viral infection because of frequent spread of human immunodeficiency virus (HIV) infection in drug-abusing population. It was found that morphine induces the replication of HIV-1 in peripheral blood mononuclear cells and Kupffer cells and acts synergistically with HIV proteins to suppress immune function. In this way, this drug may promote propagation of the disease [72-75]. Opiate-abusing HIV patients also suffer from neuroAIDS characterized by damage and inflammation in the nervous system, sensory neuropathies, myelopathies, behavioral/cognitive perturbations and dementia. NeuroAIDS also includes infiltration of macrophages into CNS and formation of microglial nodules and multinucleated giant cells [74, 75]. It seems that these changes in nervous system may ensue as a direct consequence of macrophage/microglial activation and subsequent production of ROS and release of pro-inflammatory cytokines [74, 75]. Measurement of ROS (hydrogen peroxide, superoxide and peroxynitrite) generation revealed that combined HIV-Tat and morphine action raised oxidative stress in cultured microglia [74]. Increased ROS production resulted in protein oxidation. The role of morphine in the development of oxidative stress under these conditions was corroborated by experiments with the opioid antagonist naloxone, which inhibited morphine-induced formation of ROS [74]. Yet another study showed that the levels of intracellular GSH and activity of SOD, CAT and GSHPx were decreased in morphine-dependent rhesus macaques infected by simian human immunodeficiency virus (SHIV) as compared with non-infected morphine-dependent monkeys [56]. There are some recent indications that HIV-1 strain-specific differences in gp120 are critical determinants in shaping both the timing and pattern of neurotoxic interactions with opioid drugs and the possible ROS production [76].
Current proteomic-based techniques allow determining the possible changes in expression levels of many proteins in parallel. Besides others, some authors used two-dimensional differential gel electrophoresis to assess the levels of SOD in the brain of rats or rhesus monkeys affected by morphine. While the amount of MnSOD (SOD2) was decreased in the brain of rats decapitated three days after a single dose of morphine [77, 78], the levels of [Cu-Zn]SOD (SOD1) and glutathione S-transferase P were increased in the nucleus accumbens of the brain of rhesus macaques treated for 90 days with increasing drug doses [79]. Besides that, administration of morphine to rhesus monkeys resulted in concomitant decrease in the expression of peroxiredoxin 2 [79]. Interestingly, application of the quantitative iTRAQ technique for proteomic analysis of the rat heart after prolonged morphine exposure did not reveal any significant alterations in the levels of several proteins involved in the control of redox state (SOD1, SOD2, GSHPx, GSHPx 4 isoform A precursor, peroxiredoxin 2, peroxiredoxin 3, peroxiredoxin 5, peroxiredoxin 6, CAT and others) [80]. These data support the view that morphine may exert rather different effects in different situations.
It is evident that morphine-induced ROS formation and decrease in the activity of antioxidant enzymes, which was observed under various experimental conditions, can lead to oxidative damage of different types of biomolecules, including DNA, lipids and proteins [50, 53]. In order to reverse the deleterious morphine effects on the endogenous antioxidant defense system, researchers aimed to find substances capable of moderating the drug-induced oxidative stress. Among the most effective substances with the ability to normalize morphine-induced depletion of GSH, alterations in enzyme activities of SOD, CAT and GSHPx or decrease in cell viability, are organic acid taurine (present in the mammalian brain), opioid antagonist naltrexone, exo-genous antioxidant N-acetylcysteine, triterpenoid bacoside-A from the plant Bacopa monniera and oil from Nigella sativa seeds [52, 54, 55, 57]. All these substances may serve as a potential means of attenuating oxidative stress.
CONCLUDING REMARKS
As shown in this short review, apart from analgesia, morphine can also produce some undesirable side effects. This is especially true for long-term or uncontrolled use of the drug, which can be accompanied by different health problems and pathologies. Morphine has been shown to cause oxidative stress under certain conditions by increasing formation of different kinds of free radicals. The imbalance of antioxidant levels and suppression of antioxidant enzymes can lead to oxidative damage in various tissues. Lipid peroxidation, DNA damage, protein oxidation and induction of apoptosis are typical harmful events associated with this deleterious process. The impact of morphine on cellular redox balance may differ according to concrete conditions and it depends on multiple factors, such as species, age and sex of an organism, tissue type and organ, dosage and length of usage, and simultaneous administration of other drugs. Increased generation of cellular ROS can also contribute to HIV replication and progress of neuroAIDS by immune suppression in morphine-treated experimental models. Overall, oxidative stress must be taken into account when considering the possible impacts of morphine treatment which may have serious pathological consequences. Because the molecular details of how morphine may initiate increased ROS/RNS production are still not well understood, future studies should focus on unraveling these mechanisms.
ACKNOWLEDGEMENTS
Experimental work conducted in the authors’ laboratory was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (IAA501110901), the Charles University Grant Agency (429511), and the Ministry of Education, Youth and Sport of the Czech Republic (MSM0021620858).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Vallejo R, Barkin RL, Wang VC. Pharmacology of opioids in the treatment of chronic pain syndromes. Pain Phys. 2011;14(4):E343–E360. [PubMed] [Google Scholar]
- 2.Ueda H, Ueda M. Mechanisms underlying morphine analgesic tolerance and dependence. Front. Biosci. 2009;14:5260–5272. doi: 10.2741/3596. [DOI] [PubMed] [Google Scholar]
- 3.Flemming K. The use of morphine to treat cancer related pain a synthesis of quantitative and qualitative research. J. Pain Sympt. Man. 2010;39(1):139–154. doi: 10.1016/j.jpainsymman.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Asgary S, Sarrafzadegan N, Naderi GA, Rozbehani R. Effect of opium addiction on new and traditional cardi-ovascular risk factors do duration of addiction and route of administration matter. Lipids Health Dis. 2008;7:42. doi: 10.1186/1476-511X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultan P, Gutierrez MC, Carvalho B. Neuraxial morphine and respiratory depression: finding the right balance. Drugs. 2011;71(14):1807–1819. doi: 10.2165/11596250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Miaskowski C. A review of the incidence causes consequences and management of gastrointestinal effects associated with postoperative opioid administration. J. Perianesth. Nurs. 2009;24(4):222–228. doi: 10.1016/j.jopan.2009.05.095. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anes-thesiology. 2011;115(6):1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1(8):761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- 9.Insel PA, Snead A, Murray F, Zhang L, Yokouchi H, Katakia T, Kwon O, Dimucci D, Wilderman A. GPCR expression in tissues and cells are the optimal receptors being used as drug targets. Br. J. Pharmacol. 2012;165(6):1613–1616. doi: 10.1111/j.1476-5381.2011.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Patel VP, Chu CT. Nuclear transport oxidative stress and neurodegeneration. Int. J. Clin. Exp. Pathol. 2011;4(3):215–229. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease updated experimental and clinical evidence. J. Dig. Dis. 2012;13(3):133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez-Del-Rio M, Velez-Pardo C. The bad the good and the ugly about oxidative stress. Oxid. Med. Cell Longev. 2012;2012:163913. doi: 10.1155/2012/163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braenden OJ, Eddy NB, Halbach H. Synthetic substances with morphine-like effect relationship between chemical structure and analgesic action. Bull. World Health Organ. 1955;13(6):937–998. [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen G, Christrup L, Sjogren P. Relationships among morphine metabolism, pain and side effects during long-term treatment an update. J. Pain Symptom Manag. 2003;25(1):74–91. doi: 10.1016/s0885-3924(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 16.Janecka A, Fichna J, Janecki T. Opioid receptors and their ligands. Curr. Top. Med. Chem. 2004;4(1):1–17. doi: 10.2174/1568026043451618. [DOI] [PubMed] [Google Scholar]
- 17.Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr. Drug Targets. 2012;13(2):230–246. doi: 10.2174/138945012799201612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eguchi M. Recent advances in selective opioid receptor agonists and antagonists. Med. Res. Rev. 2004;24(2):182–212. doi: 10.1002/med.10059. [DOI] [PubMed] [Google Scholar]
- 19.Piros ET, Hales TG, Evans CJ. Functional analysis of cloned opioid receptors in transfected cell lines. Neurochem. Res. 1996;21(11):1277–1285. doi: 10.1007/BF02532368. [DOI] [PubMed] [Google Scholar]
- 20.Standifer KM, Pasternak GW. G proteins and opioid receptor-mediated signalling. Cell Signal. 1997;9(3-4):237–248. doi: 10.1016/s0898-6568(96)00174-x. [DOI] [PubMed] [Google Scholar]
- 21.Wojcikiewicz RJ. Regulated ubiquitination of proteins in GPCR-initiated signaling pathways. Trends Pharmacol. Sci. 2004;25(1):35–41. doi: 10.1016/j.tips.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan ML, Vasudevan NT, Gupta MK, Martelli EE, NagaPrasad SV. G-protein coupled receptor resensitization-appreciating the balancing act of receptor function. Curr. Mol. Pharmacol. 2012;0 [PMC free article] [PubMed] [Google Scholar]
- 24.vonZastrow M. Role of endocytosis in signalling and regulation of G-protein-coupled receptors. Biochem. Soc. Trans. 2001;29 (Pt 4):500–504. doi: 10.1042/bst0290500. [DOI] [PubMed] [Google Scholar]
- 25.vonZastrow M. A cell biologist's perspective on physiological adaptation to opiate drugs. Neuropharmacology. 2004;47 (Suppl 1 ):286–292. doi: 10.1016/j.neuropharm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, vonZastrow M. Morphine activates opioid receptors without causing their rapid internalization. J. Biol. Chem. 1996;271(32):19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 27.Dang VC, Williams JT. Morphine-Induced mu-opioid receptor desensitization. Mol. Pharmacol. 2005;68(4):1127–1132. doi: 10.1124/mol.105.013185. [DOI] [PubMed] [Google Scholar]
- 28.Svoboda P, Novotny J. Hormone-induced subcellular redistribution of trimeric G proteins. Cell Mol. Life Sci. 2002;59(3):501–512. doi: 10.1007/s00018-002-8441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gintzler AR, Chakrabarti S. Post-opioid receptor adaptations to chronic morphine altered functionality and associations of signaling molecules. Life Sci. 2006;79(8):717–722. doi: 10.1016/j.lfs.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Chakrabarti S, Rivera M, Yan SZ, Tang WJ, Gintzler AR. Chronic morphine augments G(beta)(gamma)/Gs(alpha) stimulation of adenylyl cyclase relevance to opioid tolerance. Mol. Pharmacol. 1998;54(4):655–662. [PubMed] [Google Scholar]
- 31.Kaewsuk S, Hutamekalin P, Ketterman AJ, Khotchabhakdi N, Govitrapong P, Casalotti SO. Morphine induces short-lived changes in G-protein gene expression in rat prefrontal cortex. Eur. J. Pharmacol. 2001;411(1-2):11–16. doi: 10.1016/s0014-2999(00)00768-8. [DOI] [PubMed] [Google Scholar]
- 32.Fabian G, Bozo B, Szikszay M, Horvath G, Coscia CJ, Szucs M. Chronic morphine-induced changes in mu-opioid receptors and G proteins of different subcellular loci in rat brain. J. Pharmacol. Exp. Ther. 2002;302(2):774–780. doi: 10.1124/jpet.102.036152. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Wang X, Zimmerman D, Boja ES, Wang J, Bilsky EJ, Rothman RB. Chronic morphine up-regulates G alpha12 and cytoskeletal proteins in Chinese hamster ovary cells expressing the cloned mu opioid receptor. J. Pharmacol. Exp. Ther. 2005;315(1):248–255. doi: 10.1124/jpet.105.089367. [DOI] [PubMed] [Google Scholar]
- 34.Nalepa I, Zelek-Molik A, Bielawski A, Roman A, Vetulani J. Does the presence of morphine counteract adaptive changes in expression of G-protein alpha subunits mRNA induced by chronic morphine treatment? Pharmacol. Rep. 2007;59(1):34–45. [PubMed] [Google Scholar]
- 35.Skrabalova J, Neckar J, Hejnova L, Bartonova I, Kolar F, Novotny J. Antiarrhythmic effect of prolonged morphine exposure is accompanied by altered myocardial adenylyl cyclase signaling in rats. Pharmacol. Rep. 2012;64(2):351–359. doi: 10.1016/s1734-1140(12)70775-2. [DOI] [PubMed] [Google Scholar]
- 36.Chakrabarti S, Regec A, Gintzler AR. Chronic morphine acts via a protein kinase Cgamma-G(beta)-adenylyl cyclase complex to augment phosphorylation of G(beta) and G(betagamma) stimulatory adenylyl cyclase signaling. Brain Res. Mol. Brain Res. 2005;138(1):94–103. doi: 10.1016/j.molbrainres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Avidor-Reiss T, Nevo I, Saya D, Bayewitch M, Vogel Z. Opiate-induced adenylyl cyclase superactivation is isozyme-specific. J. Biol. Chem. 1997;272(8):5040–5047. doi: 10.1074/jbc.272.8.5040. [DOI] [PubMed] [Google Scholar]
- 38.Nevo I, Avidor-Reiss T, Levy R, Bayewitch M, Heldman E, Vogel Z. Regulation of adenylyl cyclase iso-zymes on acute and chronic activation of inhibitory receptors. Mol. Pharmacol. 1998;54(2):419–426. doi: 10.1124/mol.54.2.419. [DOI] [PubMed] [Google Scholar]
- 39.Ammer H, Christ TE. Identity of adenylyl cyclase isoform determines the G protein mediating chronic opioid-induced adenylyl cyclase supersensitivity. J. Neurochem. 2002;83(4):818–827. doi: 10.1046/j.1471-4159.2002.01188.x. [DOI] [PubMed] [Google Scholar]
- 40.Watts VJ, Neve KA. Sensitization of adenylate cyclase by Galpha i/o-coupled receptors. Pharmacol. Ther. 2005;106(3):405–421. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Schallmach E, Steiner D, Vogel Z. Inhibition of AC-II activity following chronic agonist exposure is modulated by phosphorylation. J. Mol. Neurosci. 2006;29(2):115–122. doi: 10.1385/JMN:29:2:115. [DOI] [PubMed] [Google Scholar]
- 42.Bergendi L, Benes L, Durackova Z, Ferencik M. Chemistry physiology and pathology of free radicals. Life Sci. 1999;65(18-19):1865–1874. doi: 10.1016/s0024-3205(99)00439-7. [DOI] [PubMed] [Google Scholar]
- 43.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid. Redox. Signal. 2008;10(8):1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc. Hematol. Disord. Drug Targets. 2006;6(1):1–19. doi: 10.2174/187152906776092659. [DOI] [PubMed] [Google Scholar]
- 45.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases role in joint diseases. Joint Bone Spine. 2007;74(4):324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011;301(6):H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Qiao M, Mieyal JJ, Asmis LM, Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized low-density lipoprotein-induced cell injury in human macrophages role of glutathione reductase and glutaredoxin. Free Radic. Biol. Med. 2006;41(5):775–785. doi: 10.1016/j.freeradbiomed.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Chen JH, Xu T, Zhou AS, Yang HK. Rice protein improves oxidative stress by regulating glutathione metabolism and attenuating oxidative damage to lipids and proteins in rats. Life Sci. 2012;91(11-12):389–394. doi: 10.1016/j.lfs.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Goudas LC, Langlade A, Serrie A, Matson W, Milbury P, Thurel C, Sandouk P, Carr DB. Acute decreases in cerebrospinal fluid glutathione levels after intracerebroventricular morphine for cancer pain. Anesth. An-alg. 1999;89(5):1209–1215. [PubMed] [Google Scholar]
- 50.Zhang YT, Zheng QS, Pan J, Zheng RL. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin. Pharmacol. Toxicol. 2004;95(2):53–58. doi: 10.1111/j.1742-7843.2004.950202.x. [DOI] [PubMed] [Google Scholar]
- 51.Guzman DC, Vazquez IE, Brizuela NO, Alvarez RG, Mejia GB, Garcia EH, Santamaria D, deApreza M, Olguin HJ. Assessment of oxidative damage induced by acute doses of morphine sulfate in postnatal and adult rat brain. Neurochem. Res. 2006;31(4):549–554. doi: 10.1007/s11064-006-9053-7. [DOI] [PubMed] [Google Scholar]
- 52.Payabvash S, Beheshtian A, Salmasi AH, Kiumehr S, Ghahremani MH, Tavangar SM, Sabzevari O, Dehpour AR. Chronic morphine treatment induces oxidant and apoptotic damage in the mice liver. Life Sci. 2006;79(10):972–980. doi: 10.1016/j.lfs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Ozmen I, Naziroglu M, Alici HA, Sahin F, Cengiz M, Eren I. Spinal morphine administration reduces the fatty acid contents in spinal cord and brain by increasing oxidative stress. Neurochem. Res. 2007;32(1):19–25. doi: 10.1007/s11064-006-9217-5. [DOI] [PubMed] [Google Scholar]
- 54.Abdel-Zaher AO, Abdel-Rahman MS, FM EL. Blockade of nitric oxide overproduction and oxidative stress by Nigella sativa oil attenuates morphine-induced tolerance and dependence in mice. Neurochem. Res. 2010;35(10):1557–1565. doi: 10.1007/s11064-010-0215-2. [DOI] [PubMed] [Google Scholar]
- 55.Sumathi T, Nathiya VC, Sakthikumar M. Protective Effect of Bacoside-A against Morphine-Induced Oxidative Stress in Rats. Ind. J. Pharm. Sci. 2011;73(4):409–415. doi: 10.4103/0250-474X.95624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez-Casanova A, Husain K, Noel RJJr, Rivera-Amill V, Kumar A. Interaction of SIV/SHIV infection and morphine on plasma oxidant/antioxidant balance in macaque. Mol. Cell Biochem. 2008;308(1-2):169–175. doi: 10.1007/s11010-007-9625-0. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, Li Y, Yan G, Bu Q, Lv L, Yang Y, Zhao J, Shao X, Deng Y, Zhu R, Zhao Y, Cen X. Protective role of taurine against morphine induced neurotoxicity in C6 cells via inhibition of oxidative stress. Neurotox. Res. 2011;20(4):334–342. doi: 10.1007/s12640-011-9247-x. [DOI] [PubMed] [Google Scholar]
- 58.Rozisky JR, Laste G, deMacedo IC, Santos VS, Krolow R, Noschang C, Vanzella C, Bertoldi K, Lovatel GA, deSouza IC, Siqueira IR, Dalmaz C, Caumo W, Torres IL. Neonatal morphine administration leads to changes in hippocampal BDNF levels and antioxidant enzyme activity in the adult life of rats. Neurochem. Res. 2013;38(3):494–503. doi: 10.1007/s11064-012-0941-8. [DOI] [PubMed] [Google Scholar]
- 59.Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009;256(1-2):83–91. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Lam CF, Liu YC, Tseng FL, Sung YH, Huang CC, Jiang MJ, Tsai YC. High-dose morphine impairs vascular endothelial function by increased production of superoxide anions. Anesthesiology. 2007;106(3):532–537. doi: 10.1097/00000542-200703000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Bhat RS, Bhaskaran M, Mongia A, Hitosugi N, Singhal PC. Morphine-induced macrophage apoptosis oxidative stress and strategies for modulation. J. Leukoc. Biol. 2004;75(6):1131–1138. doi: 10.1189/jlb.1203639. [DOI] [PubMed] [Google Scholar]
- 62.Koch T, Seifert A, Wu DF, Rankovic M, Kraus J, Borner C, Brandenburg LO, Schroder H, Hollt V. mu-opioid receptor-stimulated synthesis of reactive oxygen species is mediated via phospholipase D2. J. Neurochem. 2009;110(4):1288–1296. doi: 10.1111/j.1471-4159.2009.06217.x. [DOI] [PubMed] [Google Scholar]
- 63.Lin X, Li Q, Wang YJ, Ju YW, Chi ZQ, Wang MW, Liu JG. Morphine inhibits doxorubicin-induced reactive oxygen species generation and nuclear factor kappaB transcriptional activation in neuroblastoma SH-SY5Y cells. Biochem. J. 2007;406(2):215–221. doi: 10.1042/BJ20070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salvemini D, Neumann WL. Peroxynitrite a strategic linchpin of opioid analgesic tolerance. Trends Pharmacol. Sci. 2009;30(4):194–202. doi: 10.1016/j.tips.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J. Clin. Invest. 2007;117(11):3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolesnikov YA, Pick CG, Ciszewska G, Pasternak GW. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc. Natl. Acad. Sci. USA. 1993;90(11):5162–5166. doi: 10.1073/pnas.90.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinzen EL, Pollack GM. The development of morphine antinociceptive tolerance in nitric oxide synthase-deficient mice. Biochem. Pharmacol. 2004;67(4):735–741. doi: 10.1016/j.bcp.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 68.Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic. Biol. Med. 2009;46(2):212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D. Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience. 2009;164(2):702–710. doi: 10.1016/j.neuroscience.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murray F, Harrison NJ, Grimwood S, Bristow LJ, Hutson PH. Nucleus accumbens NMDA receptor subunit expression and function is enhanced in morphine dependent rats. Eur. J. Pharmacol. 2007;562(3):191–197. doi: 10.1016/j.ejphar.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 71.Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, Petrusca DN, Mollace V, Mazzon E, Li D, Petrache I, Matuschak GM, Salvemini D. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. J. Pharmacol. Exp. Ther. 2009;329(1):64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson PK, Gekker G, Hu S, Anderson WR, Kravitz F, Portoghese PS, Balfour HHJr, Chao CC. Morphine amplifies HIV-1 expression in chronically infected promonocytes cocultured with human brain cells. J. Neuroimmunol. 1994;50(2):167–175. doi: 10.1016/0165-5728(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 73.Schweitzer C, Keller F, Schmitt MP, Jaeck D, Adloff M, Schmitt C, Royer C, Kirn A, Aubertin AM. Morphine stimulates HIV replication in primary cultures of human Kupffer cells. Res. Virol. 1991;142(2-3):189–195. doi: 10.1016/0923-2516(91)90056-9. [DOI] [PubMed] [Google Scholar]
- 74.Turchan-Cholewo J, Dimayuga FO, Gupta S, Keller JN, Knapp PE, Hauser KF, Bruce-Keller AJ. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress possible role in cytokine regulation. J. Neurochem. 2009;108(1):202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dave RS, Khalili K. Morphine treatment of human monocyte-derived macrophages induces differential miRNA and protein expression impact on inflammation and oxidative stress in the central nervous system. J. Cell Biochem. 2010;110(4):834–845. doi: 10.1002/jcb.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Podhaizer EM, Zou S, Fitting S, Samano KL, El-Hage N, Knapp PE, Hauser KF. Morphine and gp120 toxic interactions in striatal neurons are dependent on HIV-1 strain. J. Neuroimmune Pharmacol. 2012;7(4):877–891. doi: 10.1007/s11481-011-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bierczynska-Krzysik A, Bonar E, Drabik A, Noga M, Suder P, Dylag T, Dubin A, Kotlinska J, Silberring J. Rat brain proteome in morphine dependence. Neurochem. Int. 2006;49(4):401–406. doi: 10.1016/j.neuint.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 78.Bierczynska-Krzysik A, PradeepJohn JP, Silberring J, Kotlinska J, Dylag T, Cabatic M, Lubec G. Proteomic analysis of rat cerebral cortex hippocampus and striatum after exposure to morphine. Int. J. Mol. Med. 2006;18(4):775–784. [PubMed] [Google Scholar]
- 79.Bu Q, Yang Y, Yan G, Hu Z, Hu C, Duan J, Lv L, Zhou J, Zhao J, Shao X, Deng Y, Li Y, Li H, Zhu R, Zhao Y, Cen X. Proteomic analysis of the nucleus accumbens in rhesus monkeys of morphine dependence and withdrawal intervention. J. Proteomics. 2012;75(4):1330–1342. doi: 10.1016/j.jprot.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 80.Drastichova Z, Skrabalova J, Jedelsky P, Neckar J, Kolar F, Novotny J. Global changes in the rat heart pro-teome induced by prolonged morphine treatment and withdrawal. PLoS One. 2012;7(10):e47167. doi: 10.1371/journal.pone.0047167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine morphine fentanyl butorphanol and nalbuphine. Life Sci. 1993;52(4):389–396. doi: 10.1016/0024-3205(93)90152-s. [DOI] [PubMed] [Google Scholar]