Abstract
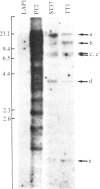
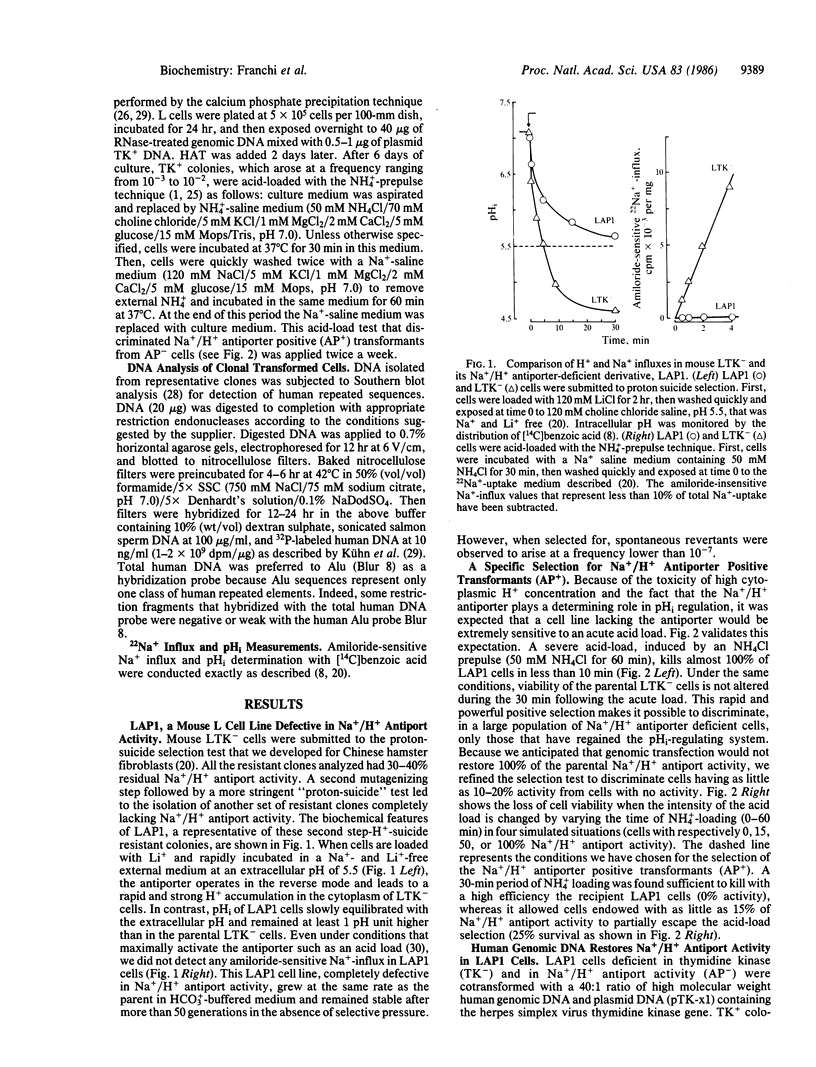
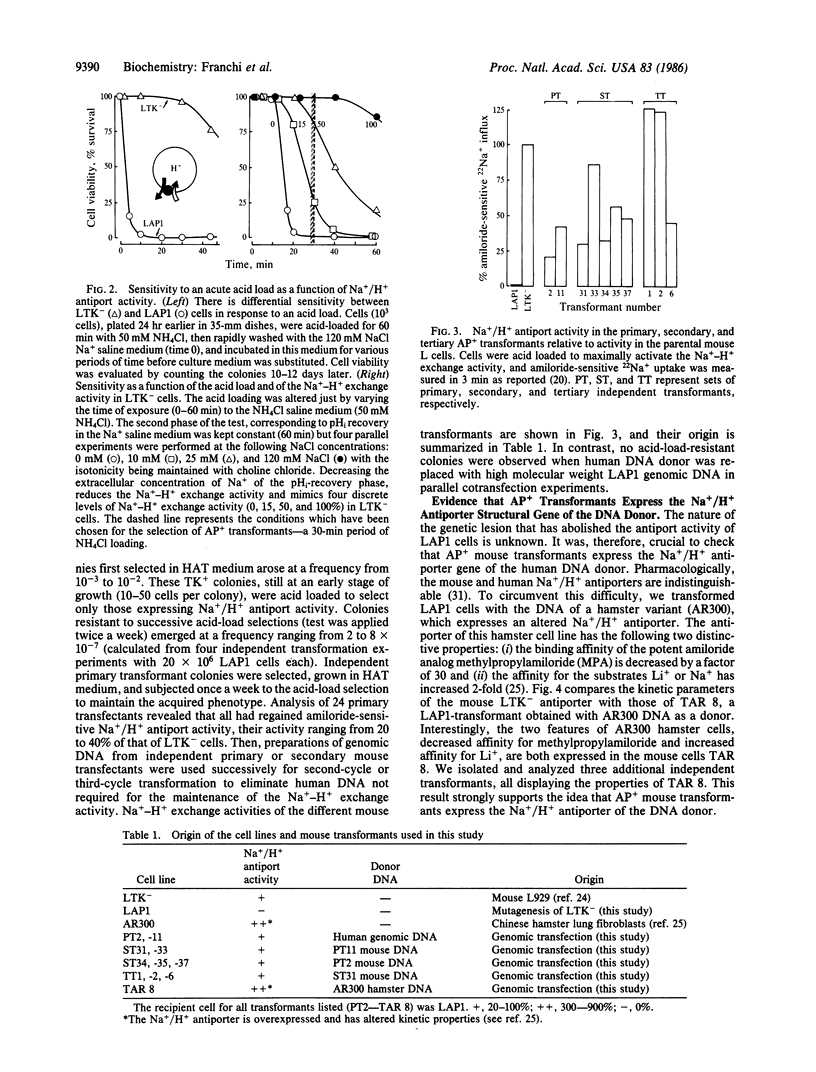
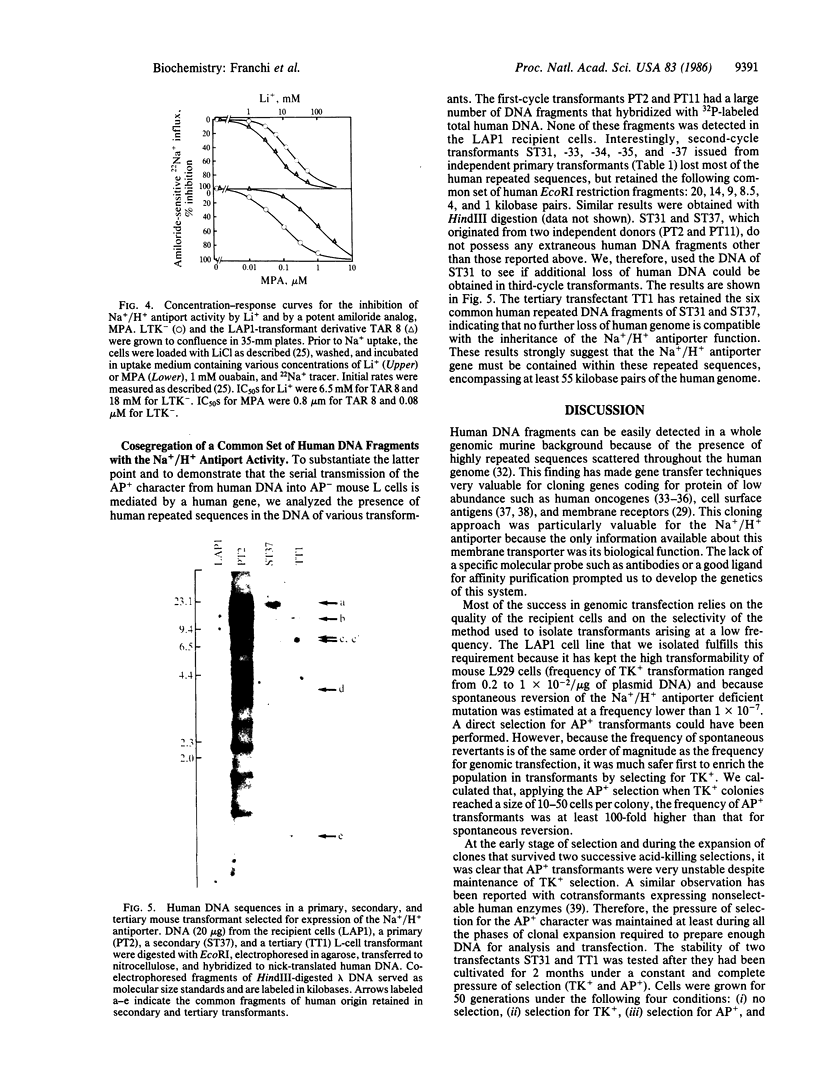
To clone the gene for the human Na+/H+ antiporter, we first constructed a stable mouse LTK- cell line (LAP1) lacking Na+/H+ antiport activity. Second, we devised a selective technique based on acid killing that specifically sorts out cells expressing low levels of Na+/H+ antiport activity from a population of antiporter-deficient cells (AP-). LAP1 cells (TK- and AP-) were cotransformed with human genomic DNA and the thymidine kinase (TK) gene. TK+ transformants, first selected, were submitted to acid loading. The rare transformants that survived (frequency, 2-8 X 10(-7) expressed Na+/H+ antiport activity (AP+). We found that: transformation with mouse LAP1 DNA did not give rise to AP+ transformants; transformation of LAP1 cells with DNA from an altered Na+/H+ antiporter hamster variant led to AP+ transformants expressing the altered Na+/H+ antiporter of the DNA donor; human repeated sequences were present in all primary, secondary, and tertiary mouse AP+ transformants; six identical EcoRI human DNA fragments (55 kilobase pairs of the human genome) cosegregated with the Na+/H+ antiport activity in secondary and tertiary transformants. These results strongly suggest that we have stably expressed the structural gene for the human Na+/H+ antiporter in mouse cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- Burns C. P., Rozengurt E. Serum, platelet-derived growth factor, vasopressin and phorbol esters increase intracellular pH in Swiss 3T3 cells. Biochem Biophys Res Commun. 1983 Nov 15;116(3):931–938. doi: 10.1016/s0006-291x(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Chambard J. C., Pouyssegur J. Intracellular pH controls growth factor-induced ribosomal protein S6 phosphorylation and protein synthesis in the G0----G1 transition of fibroblasts. Exp Cell Res. 1986 Jun;164(2):282–294. doi: 10.1016/0014-4827(86)90029-7. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Vande Woude G. F., Wagner M., Smiley J. R., Summers W. C. Construction and characterization of a recombinant plasmid encoding the gene for the thymidine kinase of Herpes simplex type 1 virus. Gene. 1979 Nov;7(3-4):335–342. doi: 10.1016/0378-1119(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Goetz J. D., Rothstein A., Gelfand E. W. Characterization of the activation of Na+/H+ exchange in lymphocytes by phorbol esters: change in cytoplasmic pH dependence of the antiport. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1429–1433. doi: 10.1073/pnas.82.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C., Barbosa J. A., Kamarck M. E., Ruddle F. H. An approach to the cloning of cell surface protein genes. Selection by cell sorting of mouse L-cells that express HLA or 4F2 antigens after transformation with total human DNA. Mol Biol Med. 1983 Oct;1(3):335–352. [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Pellicer A., Mehler M., Hirschhorn R. Detection, frequency, and stability of cotransformants expressing nonselectable human enzymes. Somat Cell Mol Genet. 1986 Jan;12(1):1–12. doi: 10.1007/BF01560722. [DOI] [PubMed] [Google Scholar]
- Molski T. F., Naccache P. H., Volpi M., Wolpert L. M., Sha'afi R. I. Specific modulation of the intracellular pH of rabbit neutrophils by chemotactic factors. Biochem Biophys Res Commun. 1980 May 30;94(2):508–514. doi: 10.1016/0006-291x(80)91260-7. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Shilo B. Z., Shih C., Cowing D., Hsu H. W., Weinberg R. A. Three different human tumor cell lines contain different oncogenes. Cell. 1981 Aug;25(2):355–361. doi: 10.1016/0092-8674(81)90054-4. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+. J Biol Chem. 1984 Sep 10;259(17):10989–10994. [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., L'Allemain G., Paris S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985 Oct 7;190(1):115–119. doi: 10.1016/0014-5793(85)80439-7. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Robbins K. C., Barbacid M. Oncogenes in human tumor cell lines: molecular cloning of a transforming gene from human bladder carcinoma cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2845–2849. doi: 10.1073/pnas.79.9.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Stein L. F., Cantley L. C. Phorbol esters induce differentiation in a pre-B-lymphocyte cell line by enhancing Na+/H+ exchange. J Biol Chem. 1984 Jun 10;259(11):7056–7060. [PubMed] [Google Scholar]
- Rothenberg P., Glaser L., Schlesinger P., Cassel D. Activation of Na+/H+ exchange by epidermal growth factor elevates intracellular pH in A431 cells. J Biol Chem. 1983 Oct 25;258(20):12644–12653. [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Lazdunski M. The Na+/H+ antiport is activated by serum and phorbol esters in proliferating myoblasts but not in differentiated myotubes. Properties of the activation process. J Biol Chem. 1985 Jul 5;260(13):8008–8013. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]