Abstract
Macrophages ingest and kill microbes by phagocytosis and delivery to lysosomes. In this issue, Prashar et al. (2013, J. Cell Biol. http://dx.doi.org/10.1083/jcb.201304095) demonstrate that the elongated morphology of filamentous bacteria does not prevent ingestion by macrophages or the fusion of lysosomes, but creates a chimeric, unclosed phagolysosomal compartment whose leakiness blunts the toxicity of lysosomal enzymes, thereby increasing bacterial survival.
The tremendous variety of morphologies among microorganisms may allow them to thwart ingestion by the amoeboid cells that patrol pond water and the tissues of animals. Indeed, filament formation by bacteria can limit uptake by phagocytes (Justice et al., 2008). In this issue, Prashar et al. provide mechanistic support for the concept that a filamentous morphology allows bacteria to resist death by phagocytosis.
In the normal sequence of events in phagocytosis, outlined in the late nineteenth century and refined by 130 years of microscopy and cell biology, phagocytic cells such as macrophages engage particles by receptor-mediated binding to ligands on microbe surfaces. Receptor signaling leads to the formation of actin-rich, cup-shaped extensions of the cell surface, which enclose particles into membrane-bound intracellular organelles (Swanson, 2008). These phagosomes then mature by progressive fusion with other membranous compartments in the cell, including secretory granules, endosomes, and lysosomes (Flannagan et al., 2009). The mixing of phagosomal and lysosomal contents kills ingested microbes by delivering them into an acidic, hydrolase-rich environment. Ingestion, maturation, and killing have long been considered sequential, nonoverlapping activities.
But to be a useful hunting tool or a robust arm of host defense, phagocytosis must overcome microbes in all their various microscopic forms (Fig. 1). To ask how the framework for killing by phagocytosis is altered when a macrophage engages an elongate object, Prashar et al. (2013) examined phagocytosis of filamentous bacteria, which begins after macrophage exploratory movements locate a free filament end (Möller et al., 2012). Once engaged, the macrophage constructed an elongated tubular phagocytic cup comprised of a sleeve of plasma membrane with an actin-rich cuff, which pulled the filament into the cell as if sucking in a long spaghetti noodle. The cup membrane remained contiguous with the plasma membrane until it reached the distal end of the filament, at which point the cup closed into a discrete phagosome inside the cell. Earlier fluorescence microscopy of phagocytosis by macrophages and by Dictyostelium discoideum amebae had shown that membrane phospholipid and protein profiles change even before a phagocytic cup closes into the cell (Botelho et al., 2000; Dormann et al., 2004; Mercanti et al., 2006; Golebiewska et al., 2011). Prashar et al. (2013) identified similar lateral heterogeneity in membrane organization. Remarkably, fusion with early endosomes, late endosomes, and lysosomes began before filaments were fully internalized into the cell, such that the various stages of phagosome maturation were arranged along the length of the tubular phagocytic cups. This extends the earlier studies by showing that the mechanisms which maintain distinct organelle identities do not require organelles to be physically separate from each other inside the cell.
Figure 1.
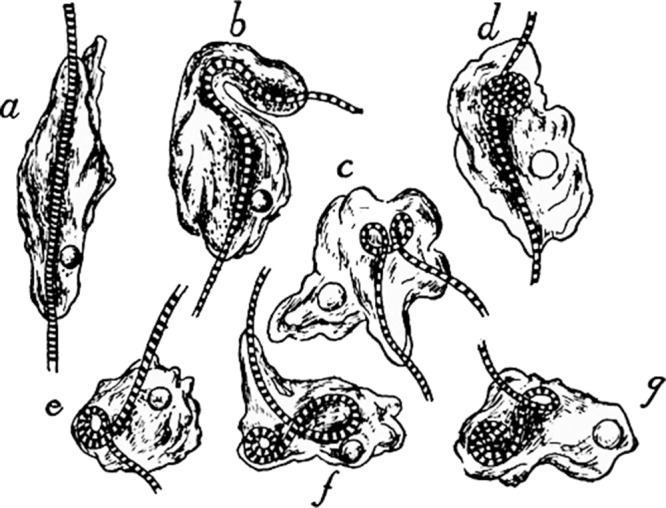
Extreme phagocytosis. H.S. Jennings’ account (Jennings, 1976) of observations by Rhumbler in 1898: “Ameba verrucosa coiling up and ingesting a filament of Oscillaria. The animal settles upon the middle of an Oscillaria filament, envelopes it, and lengthens out along it (a). Then one end bends over (b), so that a loop is formed in the filament (c). The amoeba then stretches out on the filament again, bends it over anew, and the process is repeated until the filament forms a close coil within the amoeba (c to g).”
The delayed closure of elongated phagocytic cups compromised macrophage antimicrobial activities. The actin cuff around the filament formed a tight ring that could limit egress of large dextrans delivered into the phagocytic cup from lysosomes. Nonetheless, while the phagocytic cup membranes remained contiguous with the plasma membrane, protons and lysosomal enzymes leaked out. Complete cup closure was required for phagosome acidification and the full degradative capacity of macrophage defenses. This suggests that adopting a filamentous morphology allows bacteria to lessen the toxicity of microbicidal compounds delivered into the phagosome. Consistent with this idea, Prashar et al. (2013) determined that the survival and growth of Legionella pneumophila in macrophages correlated with filament length.
Studies of phagocytosis are undoubtedly important for understanding host defense against infections, but they also provide a vantage point for asking how cells organize cytoplasm for the complexities of microscopic life. Fc receptor–mediated phagocytosis of particles coated with IgG proceeds by a zipper-like mechanism, in which the patterns of IgG ligands on a particle surface guide the distribution of phagocyte signaling that shapes a phagosome (Swanson and Baer, 1995). This localized signaling is modulated by feedback regulation related to the physical properties of the particle. For example, macrophages presented with long, rod-shaped particles coated with IgG respond differently depending on the orientation of their initial contact with those particles. Particles contacted end-on are readily ingested, but particles contacted along their long face are not (Champion and Mitragotri, 2006). This regulation of Fc receptor signaling by surface topology could explain why macrophages ingest filaments only after locating a filament end. But it is a puzzling observation, not least because a macrophage will engage a planar surface coated with IgG as if to engulf it—a response termed “frustrated phagocytosis” (Wright and Silverstein, 1984). Why should phagocytosis proceed on an impossibly large planar surface but not against the side of a rod-shaped particle? Although still unresolved, these fascinating questions about shape-sensing in phagocytosis have been addressed by recent theoretical and experimental studies (Clarke et al., 2010; Dieckmann et al., 2010; Tollis et al., 2010).
Another puzzling relationship between Fc receptor signaling and the physical dimensions of the prey concerns the role of phosphatidylinositol 3-kinase (PI3K) in phagocytosis. PI3K is required for phagocytosis of microspheres larger than 3 µm in diameter, but not for phagocytosis of smaller microspheres (Araki et al., 1996; Cox et al., 1999). This suggests that phagocytosis is regulated by a PI3K-dependent feedback related to particle size. How does that work? It could be that PI3K relieves a feedback inhibition of Fc receptor signaling that begins only after some delay, such that it becomes rate-limiting only for the phagocytosis of larger particles that take more time to ingest. Prashar et al. (2013) excluded that possibility by examining the effects of PI3K inhibition on the phagocytosis of filaments. In the presence of the PI3K inhibitor LY294002, phagocytosis of IgG-opsonized sheep erythrocytes was inhibited but the phagocytosis of filaments was not. This suggests that formation of the narrow aperture for sucking in a noodle occurs below the PI3K-dependent size threshold. Moreover, once a phagocytic response was initiated, it continued for as long as necessary to ingest the filament, showing that PI3K-dependent regulation of phagocytosis is spatial rather than temporal. The 3′ phosphoinositide products of PI3K may be part of a system for gauging the three-dimensional distribution of phagocytic receptor signaling, a mechanism of spatial integration that regulates cellular commitment to phagocytosis (Zhang et al., 2010). In the battles with the exotic geometries of microbial life, it may be necessary for a phagocyte to decide whether a particle is small enough to eat or should instead be held outside and engaged as extracellular prey. Accordingly, the narrow end of a filament presents a stimulus that is below the size threshold for PI3K-dependent regulation, so the macrophage begins slurping it in, with a considerable amount of lysosomal spittle dribbling out.
References
- Araki N., Johnson M.T., Swanson J.A. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249–1260 10.1083/jcb.135.5.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho R.J., Teruel M., Dierckman R., Anderson R., Wells A., York J.D., Meyer T., Grinstein S. 2000. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151:1353–1368 10.1083/jcb.151.7.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion J.A., Mitragotri S. 2006. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA. 103:4930–4934 10.1073/pnas.0600997103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Engel U., Giorgione J., Müller-Taubenberger A., Prassler J., Veltman D., Gerisch G. 2010. Curvature recognition and force generation in phagocytosis. BMC Biol. 8:154 10.1186/1741-7007-8-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D., Tseng C.-C., Bjekic G., Greenberg S. 1999. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274:1240–1247 10.1074/jbc.274.3.1240 [DOI] [PubMed] [Google Scholar]
- Dieckmann R., von Heyden Y., Kistler C., Gopaldass N., Hausherr S., Crawley S.W., Schwarz E.C., Diensthuber R.P., Côté G.P., Tsiavaliaris G., Soldati T. 2010. A myosin IK-Abp1-PakB circuit acts as a switch to regulate phagocytosis efficiency. Mol. Biol. Cell. 21:1505–1518 10.1091/mbc.E09-06-0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D., Weijer G., Dowler S., Weijer C.J. 2004. In vivo analysis of 3-phosphoinositide dynamics during Dictyostelium phagocytosis and chemotaxis. J. Cell Sci. 117:6497–6509 10.1242/jcs.01579 [DOI] [PubMed] [Google Scholar]
- Flannagan R.S., Cosío G., Grinstein S. 2009. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat. Rev. Microbiol. 7:355–366 10.1038/nrmicro2128 [DOI] [PubMed] [Google Scholar]
- Golebiewska U., Kay J.G., Masters T., Grinstein S., Im W., Pastor R.W., Scarlata S., McLaughlin S. 2011. Evidence for a fence that impedes the diffusion of phosphatidylinositol 4,5-bisphosphate out of the forming phagosomes of macrophages. Mol. Biol. Cell. 22:3498–3507 10.1091/mbc.E11-02-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H.S. 1976. Behavior of the Lower Organisms. Indiana University Press, Bloomington, IN: 366 pp [Google Scholar]
- Justice S.S., Hunstad D.A., Cegelski L., Hultgren S.J. 2008. Morphological plasticity as a bacterial survival strategy. Nat. Rev. Microbiol. 6:162–168 10.1038/nrmicro1820 [DOI] [PubMed] [Google Scholar]
- Mercanti V., Charette S.J., Bennett N., Ryckewaert J.J., Letourneur F., Cosson P. 2006. Selective membrane exclusion in phagocytic and macropinocytic cups. J. Cell Sci. 119:4079–4087 10.1242/jcs.03190 [DOI] [PubMed] [Google Scholar]
- Möller J., Luehmann T., Hall H., Vogel V. 2012. The race to the pole: how high-aspect ratio shape and heterogeneous environments limit phagocytosis of filamentous Escherichia coli bacteria by macrophages. Nano Lett. 12:2901–2905 10.1021/nl3004896 [DOI] [PubMed] [Google Scholar]
- Prashar A., Bhatia S., Gigliozzi D., Martin T., Duncan C., Guyard C., Terebiznik M.R. 2013. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J. Cell Biol. 1081–1097 10.1083/jcb.201304095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.A. 2008. Shaping cups into phagosomes and macropinosomes. Nat. Rev. Mol. Cell Biol. 9:639–649 10.1038/nrm2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.A., Baer S.C. 1995. Phagocytosis by zippers and triggers. Trends Cell Biol. 5:89–93 10.1016/S0962-8924(00)88956-4 [DOI] [PubMed] [Google Scholar]
- Tollis S., Dart A.E., Tzircotis G., Endres R.G. 2010. The zipper mechanism in phagocytosis: energetic requirements and variability in phagocytic cup shape. BMC Syst. Biol. 4:149 10.1186/1752-0509-4-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.D., Silverstein S.C. 1984. Phagocytosing macrophages exclude proteins from the zones of contact with opsonized targets. Nature. 309:359–361 10.1038/309359a0 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hoppe A.D., Swanson J.A. 2010. Coordination of Fc receptor signaling regulates cellular commitment to phagocytosis. Proc. Natl. Acad. Sci. USA. 107:19332–19337 10.1073/pnas.1008248107 [DOI] [PMC free article] [PubMed] [Google Scholar]


