Abstract
The prepartum surge in plasma cortisol concentrations in humans and sheep promotes fetal lung and surfactant system maturation in the support of air breathing after birth. This physiological process has been used to enhance lung maturation in the preterm fetus using maternal administration of betamethasone in the clinical setting in fetuses as young as 24 weeks gestation (term = 40 weeks). Here, we have investigated the impact of fetal intravenous cortisol infusion during the canalicular phase of lung development (from 109- to 116-days gestation, term = 150 ± 3 days) on the expression of genes regulating glucocorticoid (GC) activity, lung liquid reabsorption, and surfactant maturation in the very preterm sheep fetus and compared this to their expression near term. Cortisol infusion had no impact on mRNA expression of the corticosteroid receptors (GC receptor and mineralocorticoid receptor) or HSD11B-2, however, there was increased expression of HSD11B-1 in the fetal lung. Despite this, cortisol infusion had no effect on the expression of genes involved in lung sodium (epithelial sodium channel -α, -β, or -γ subunits and sodium–potassium ATPase-β1 subunit) or water (aquaporin 1, 3, and 5) reabsorption when compared to the level of expression during exposure to the normal prepartum cortisol surge. Furthermore, in comparison to late gestation, cortisol infusion does not increase mRNA expression of surfactant proteins (SFTP-A, -B, and -C) or the number of SFTP-B-positive cells present in the alveolar epithelium, the cells that produce pulmonary surfactant. These data suggest that there may be an age before which the lung is unable to respond biochemically to an increase in fetal plasma cortisol concentrations.
Keywords: Glucocorticoid, liquid reabsorption, lung, preterm, surfactant
Introduction
Before birth the placenta performs the role of gas exchange for the developing fetus (Longo and Reynolds 2010). Successful transition from the intrauterine to the extrauterine environment depends on the ability of the neonatal lungs to assume this vital function at birth. The fetal lung develops as a fluid-filled sac (Hooper and Harding 1995) and it is the reabsorption of the liquid which has bathed the alveolar epithelium throughout gestation that is an important determinant of the successful transition to air breathing at birth. Lung liquid reabsorption is driven by an osmotic gradient generated by transepithelial sodium movement within the lung. The process of sodium transport from the lung lumen is controlled by the amiloride-sensitive voltage-gated epithelial sodium channel (SCNN1), comprised of -α (SCNN1-A), -β (SCNN1-B) and -γ (SCNN1-G) subunits, which is present on the luminal surface of the pulmonary epithelium (Snyder 2005) and sodium–potassium active transport pumps (ATP1), comprised of catalytic α (ATP1A1) and glycolytic β (ATP1B1) subunits, which are present on the basal surface of the pulmonary epithelium (Ewart and Klip 1995). The rapid loss of water from the lung lumen in response to the net movement of sodium is regulated by differential expression of the aquaporin (AQP; subtypes 1, 3, 4 and 5) family of transmembrane channel proteins in the lung (Liu and Wintour 2005).
With the first breath, an air–liquid interface is generated within the alveoli, as the lung fluid is reduced to a thin layer, this aqueous hypophase creates high surface tension. This makes inflation difficult and as a result, a stabilizing force within the lung that prevents alveolar collapse throughout the breathing cycle is required. Pulmonary surfactant is secreted from type II alveolar epithelial cells (AEC) lining the alveoli. Surfactant is a complex mixture of lipids and surfactant proteins (SFTP), which primarily functions to reduce surface tension (Creuwels et al. 1997). The surfactant lipid component forms a stable surface film at the air-liquid interface to reduce and vary the surface tension with changing lung volume (Goerke and Clements 1985). In addition, SFTP-B and -C play important roles in regulating surface tension as they function cooperatively to promote adsorption and spreading of surfactant lipids (Hawgood et al. 1998; Johansson 1998). SFTP-A and -D play important roles in innate immunity within the lung (LeVine and Whitsett 2001; Haagsman et al. 2008). Type II AECs, which produce the components of surfactant, appear from mid-gestation during the canalicular phase of lung development (humans, ∼16–25 weeks, term = 40 weeks; sheep, ∼80–120 days, term = 150 ± 3 days) (Harding and Bocking 2001).
Maturation of the surfactant system occurs in the saccular phase of lung development in late gestation (humans, from ∼25 weeks to term; sheep, from ∼120 days to term), and is largely regulated by the prepartum rise in the concentration of plasma cortisol, the endogenous glucocorticoid (GC) (Silver and Fowden 1988; Tan et al. 1999; Bolt et al. 2001). In addition, GCs regulate the expression of genes controlling lung liquid reabsorption both in vivo and in vitro (Champigny et al. 1994; Dagenais et al. 2001; Mustafa et al. 2004; Jesse et al. 2009). Intracellular GC concentrations are regulated by two isoforms of the 11β-hydroxysteroid dehydrogenase (HSD11B) enzyme, with HSD11B-1 generating cortisol from inactive cortisone, while HSD11B-2 functions to interconvert active cortisol to cortisone (Tomlinson and Stewart 2001). GCs mediate their effects through the intracellular glucocorticoid receptor (NR3C1). Once bound, the GC-NR3C1 complex binds to glucocorticoid response elements (GRE) present on target genes to induce their expression. The SFTP promoters do not contain a GRE, however, their expression is regulated indirectly by GC through transcription factors that have a GRE and bind to the SFTP promoter to increase gene expression (Reichardt et al. 1998; Mendelson 2000). It has been suggested that this mechanism involves transcription factors such as thyroid transcription factor-1 (NKX2-1) and cofactors such as GATA-binding protein-6 (GATA6) (Stahlman et al. 1996; Mendelson 2000). In addition to the SFTPs, there is evidence to suggest that indirect GC activity is also a mechanism by which GCs regulate expression of genes involved in lung liquid reabsorption (Bremner et al. 2002). GC action within the fetal lung is also mediated through the action of the mineralocorticoid receptor (NR3C2), which is known to play a role in normal lung development and lung liquid reabsorption (Keller-Wood et al. 2009). It is not known if these genes respond when plasma cortisol concentrations reach a threshold level or if their expression is dependent on the structural development of the lung, including the number of type II AECs.
In this study, we have evaluated the effectiveness of fetal intravenous cortisol infusion to influence expression of genes regulating lung liquid reabsorption and SFTPs in the very preterm sheep fetus during the late canalicular phase of lung development, equivalent to ∼23–24 weeks gestation in humans, compared to age-matched controls and fetuses during the prepartum cortisol surge in late gestation.
Methods
All procedures were approved by the University of Adelaide Animal Ethics Committee. Tissues used in this study were available from a series of fetal sheep studies and data on neuroendocrine function from some of these animals have been published previously (Warnes et al. 1998; Ross et al. 2000).
Animals and surgery
Twenty-nine pregnant Border-Leicester × Merino and Merino ewes were used in this study. The ewes were housed in individual pens in animal holding rooms, with a 12:12 h light/dark cycle, and fed once daily with water provided ad libitum. At 103- or 119-days gestation, general anesthesia was induced in the ewe with an intravenous injection of sodium thiopentone (1.25 g, Pentothal; Rhone Merieux, Pinkenba, Qld, Australia) and maintained with 2.5–4% halothane inhalation anesthetic (Fluothane; ICI, Melbourne, Vic, Australia) in oxygen. Vascular catheters were implanted in the jugular vein of the ewe, a carotid artery, and jugular vein of the fetus, and in the amniotic cavity as previously described (Ross et al. 2000; Muhlhausler et al. 2005). Ewes received an intramuscular injection of antibiotics (3.5 mL of Norocillin [Norbrook Laboratories Ltd., Gisborne, Australia] and 2 mL of 125 mg/mL Dihydrostreptomycin in sterile saline [Sigma, St Louis, MO]) for 3 days following surgery. Antibiotics (500 mg; sodium ampicillin; Commonwealth Serum Laboratories, Melbourne, Vic, Australia) were administered intra-amniotically to all fetal sheep daily for 4 days postoperatively. Ewes were allowed at least 4 days to recover from surgery prior to the experimental protocol.
Infusion regime
Cortisol (hydrocortisone succinate, Solucortef, 2–3 mg in 4.4 mL saline/24 h) was infused intravenously into the fetus from 109- to 116-days gestation (n = 9). Age-matched controls received either saline infusion from 109- to 116-days gestation (n = 4) or no infusion (n = 4). This infusion protocol has previously been shown to increase fetal plasma cortisol concentrations (saline-infused, 1.6 ± 0.1 nmol/L; cortisol-infused, 39.3 ± 2.8 nmol/L [Ross et al. 2000; Warnes et al. 1998]). Late gestation fetuses received saline infusion from 130- to 140-days gestation (n = 12) and plasma cortisol concentration at this gestational age is 5–10 nmol/L (Phillips et al. 1996; Morrison et al. 2007; Orgeig et al. 2010).
Fetal arterial blood gas measurements
Fetal arterial blood samples (1 mL) were collected daily to measure PaO2, PaCO2, pH, oxygen saturation (SaO2) and hemoglobin (Hb) using an ABL 550 analyzer (Radiometer Pacific Pty Ltd, Australia) and temperature corrected to 39°C.
Tissue collection
At 116 ± 1 day (n = 17) or 140 ± 1 day (n = 12) gestation, ewes were humanely killed with an intravenous overdose of sodium pentobarbitone (Virbac Pty Ltd, Peakhurst, NSW, Australia). Fetal sheep were delivered by hysterectomy, weighed, and humanely killed. The fetal lungs were removed, weighed, snap frozen in liquid nitrogen, and stored at −80°C for subsequent gene expression analysis. In addition, a section of lung tissue was fixed in 4% paraformaldehyde for subsequent immunohistochemical analysis.
Quantification of mRNA transcripts within the fetal lung
Total RNA extraction
Total RNA was extracted from 22 lung samples (∼50 mg) using Invitrogen Trizol Reagent Solution (Invitrogen Life Technologies, Carlsbad, CA) as per the manufacturer's guidelines and Qiagen RNeasy purification columns (Qiagen Pty Ltd., Doncaster, Vic, Australia) (Gentili et al. 2009; Soo et al. 2012). Total RNA integrity from all extracted tissue samples was assessed by running all samples (116-day saline-infused = 6; 116-day cortisol-infused = 6; 140-day saline-infused = 10) on an agarose gel stained with ethidium bromide. Total RNA was quantified by spectrophotometric measurements at 260 and 280 nm and checked for protein and DNA contamination. cDNA was synthesized using Superscript III First Strand Synthesis System (Invitrogen) using 2 μg of total RNA, random hexamers, dNTP, DTT and Superscript III in a final volume of 20 μL as per the manufacturer's guidelines. Controls containing either no RNA transcript (no template control – NTC) or no Superscript III (no amplification control, NAC) were used to test for reagent contamination and genomic DNA contamination, respectively.
Quantitative real-time RT-PCR
Initially, the geNorm component of qbaseplus 2.0 software (Biogazelle, Zwijnaarde, Belgium) was used to determine the most stable reference genes from a panel of candidate genes (Vandesompele et al. 2002) and the minimum number of reference genes required to calculate a stable normalization factor as previously described (Soo et al. 2012). The gene expression of GC regulatory genes (HSD11B-1, HSD11B-2, NR3C1, NR3C2, NKX2-1, and GATA6), genes regulating lung liquid reabsorption (SCNN1-A, SCNN1-B, SCNN1-G, ATP1A1 (Keller-Wood et al. 2009), ATP1B1, AQP1, AQP3, AQP4, and AQP5), surfactant proteins (SFTP-A, -B, -C [Orgeig et al. 2010], and -D), and reference genes (β-actin (ACTB), peptidylprolyl isomerase A (PPIA) (Passmore et al. 2009), and tyrosine 3-monooxygenase (YWHAZ)) were measured by qRT-PCR. Previously published (Keller-Wood et al. 2005; Passmore et al. 2009; Orgeig et al. 2010) or specifically designed (Table 1) primer sets were validated and optimized as previously described (Orgeig et al. 2010). mRNA transcripts in all fetal lung samples were measured by qRT-PCR using Fast SYBR® Green Master Mix (Applied Biosystems, Foster City, CA) in a final volume of 6 μL on a ViiA7 Fast Real-time PCR system (Applied Biosystems). Each qRT-PCR well contained 3 μL Fast SYBR Green Master Mix (2×), 2 μL of forward and reverse primer mixed with H2O to obtain final primer concentrations (Table 1) and 1 μL of diluted relevant cDNA. Primers were validated to generate a single transcript as confirmed by the presence of an individual double stranded DNA product of the correct size and sequence. Controls for each primer set containing no cDNA were included on each plate to test for reagent contamination (NTC). Melt curve/dissociation curves were also run to check for nonspecific product formation. Amplification efficiency reactions were performed on five triplicate serial dilutions of cDNA template for each primer set. Amplification efficiencies were determined from the slope of a plot of Ct (defined as the threshold cycle with the lowest significant increase in fluorescence) against the log of the cDNA template concentration (ranging from 1 to 100 ng). Ct values were in the linear amplification range for all genes. Each sample was run in triplicate for target genes and reference genes. The reactions were quantitated by setting the threshold within the exponential growth phase of the amplification curve and obtaining corresponding Ct values. The abundance of each transcript relative to the abundance of stable housekeeping genes (Hellemans et al. 2007) was calculated using DataAssist 3.0 analysis software (Applied Biosystems) and expressed as mean normalized expression (Soo et al. 2012).
Table 1.
qRT-PCR primer sequences and final primer concentrations for target and housekeeping genes
| Primer name | Sequence 5′→3′ | Primer conc (μmol/L) | Accession no. |
|---|---|---|---|
| HSD11B-1 | NM_001009395.1 | ||
| Forward | GCGCCAGATCCCTGTCTGAT | 0.90 | |
| Reverse | AGCGGGATACCACCTTCTTT | 0.90 | |
| HSD11B-2 | NM_001009460.1 | ||
| Forward | GAGACATGCCGTTTCCATGC | 0.45 | |
| Reverse | TGATGCTGACCTTGACACCC | 0.45 | |
| NR3C1 | NM_001114186.1 | ||
| Forward | ACTGCCCCAAGTGAAAACAGA | 0.90 | |
| Reverse | ATGAACAGAAATGGCAGACATTTTATT | 0.90 | |
| NR3C2 | AF349768.1 | ||
| Forward | ATGACAGCTCCAAACCAAACACGG | 0.90 | |
| Reverse | AAATCCTGGAAGTACCTTCGCCCA | 0.90 | |
| NKX2-1 | FJ177515 | ||
| Forward | ACACAAAGACCAAACTGCTGGACG | 0.90 | |
| Reverse | GCGTGGGAAACCCATTTGAATCAC | 0.90 | |
| GATA6 | DQ126151 | ||
| Forward | TCTACAGCAAGATGAACGGCCTCA | 0.90 | |
| Reverse | TAGAGTCCACAGGCATTGCACACA | 0.90 | |
| SCNN1-A | AF232715.1 | ||
| Forward | ACGACAAGAACAGCTCCAACCTCT | 0.90 | |
| Reverse | GCCGCAGATTAAAGCCAGCATCAT | 0.90 | |
| SCNN1-B | AF065146.1 | ||
| Forward | AGTGGTTCTGGACCTGTTTGAGGA | 0.45 | |
| Reverse | CATGTGGTTCCATTGTGGCTGCAT | 0.45 | |
| SCNN1-G | AF250862.1 | ||
| Forward | TCGTGCTTCCAGGCAAAGATGGTA | 0.45 | |
| Reverse | TTAAAGCTGCAGGCTTCCTTGCAC | 0.45 | |
| ATP1B1 | NM_001009796 | ||
| Forward | TGCCTTTCGTCCTAACGATCCCAA | 0.45 | |
| Reverse | CTGGGCACATTGCCACAATCTTCA | 0.45 | |
| AQP1 | NM_001009194.1 | ||
| Forward | AAAGTGTCACTGGCCTTTGGGTTG | 0.45 | |
| Reverse | ATGTACATGATGGCCCGGAGGATA | 0.45 | |
| AQP3 | AF123316.1 | ||
| Forward | TCACTTGAACCCTGCTGTGACCTT | 0.05 | |
| Reverse | ACCCGAAGATAATTCCAGCACCCA | 0.05 | |
| AQP4 | NM_001009279 | ||
| Forward | TGGGAAATTGGGAGAACCACTGGA | 0.45 | |
| Reverse | GGCAGCTTTGCTGAAGGCTTCTTT | 0.45 | |
| AQP5 | NM_001009273 | ||
| Forward | CAATCTGGCTGTCAATGCGCTCAA | 0.45 | |
| Reverse | AGTCAGTGGAAGAGAAGACGCACA | 0.45 | |
| SFTP-D | AJ133002.1 | ||
| Forward | GGCCACAGCCCAGAACAA | 0.3 | |
| Reverse | AAGTACCCTCCTTCCTGGTATCG | 0.3 | |
| YWAHZ | AY970970 | ||
| Forward | TGTAGGAGCCCGTAGGTCATCT | 0.45 | |
| Reverse | TTCTCTCTGTATTCTCGAGCCATCT | 0.45 |
Accession numbers refer to the published cDNA sequences from which the primer sequences were designed.
Quantification of type II AECs within the fetal lung
SFTP-B immunoreactivity to identify mature AECs
Immunohistochemistry was performed in a subset of animals (116-day saline-infused = 2; 116-day cortisol-infused = 4; 140-day saline-infused = 5) using a monoclonal antibody to SFTP-B (produced by Dr Y. Suzuki, Kyoto University, Japan and kindly donated by F. Possmayer, University of Western Ontario, Canada), staining of which is restricted to type II AECs in the alveolar epithelium and Clara cells in the bronchiolar epithelium (Weaver 1998; Lin et al. 1999). Paraformaldehyde-fixed, paraffin-processed lung tissue sections of 7-μm thickness were deparaffinized and rehydrated before endogenous peroxide solution activity was blocked, and followed by antigen retrieval. Slides were incubated overnight with the aforementioned SFTP-B antibody (1:1000) at 4°C. Negative control slides were performed in parallel with test slides. A Histostain-Plus broad spectrum kit (Zymed Laboratories Inc., South San Francisco, CA) was utilized with HRP and 3,3-diaminobenzidine (DAB) chromagen (Metal Enhanced DAB Substrate Kit; Pierce Biotechnology, Rockford, IL) for visualization of SFTP-B-positive cells. All sections were counterstained with Mayer's Hematoxylin.
Quantitative assessment of SFTP-B-positive cells in the fetal lung
Sections were examined using Visiopharm new Computer Assisted Stereological Toolbox (NewCAST) software (Visiopharm, Hoersholm, Denmark). Analysis was carried out by a single trained individual who was blinded to treatment groups. Sixty counting frames (400× magnification) of the alveolar epithelium were randomly selected per section. Point counting using an unbiased counting frame with an area of 20,000 μm2 was used to estimate the numerical density of SFTP-B-positive cells within the fetal lung. Using the four corners of the test frame, the reference space was estimated from the points falling on lung tissue. The numerical density of SFTP-B-positive cells expressed as SFTP-B-positive cells per mm2 of lung tissue was obtained using the following equation (Brüel et al. 2005):
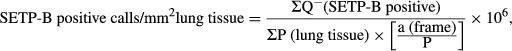 |
where 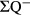 (SFTP-B Positive) represents the total number of SFTP-B-positive cells counted in all counting frames of one fetal lung tissue section;
(SFTP-B Positive) represents the total number of SFTP-B-positive cells counted in all counting frames of one fetal lung tissue section; 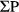 (lung tissue) represents the total number of points falling on lung tissue (i.e., the reference space); P is the number of points which were used to count the points hitting the reference space (i.e., four corners per counting frame); and a was the area of the counting frame. Tissue sections were photographed using a digital camera DP72 (Olympus Australia Pty. Ltd, Mt Waverley, Vic, Australia), which was connected to a BX53 Research Microscope (Olympus Australia Pty. Ltd).
(lung tissue) represents the total number of points falling on lung tissue (i.e., the reference space); P is the number of points which were used to count the points hitting the reference space (i.e., four corners per counting frame); and a was the area of the counting frame. Tissue sections were photographed using a digital camera DP72 (Olympus Australia Pty. Ltd, Mt Waverley, Vic, Australia), which was connected to a BX53 Research Microscope (Olympus Australia Pty. Ltd).
Statistical analyses
All statistical analyses were performed using Statistical Package for Social Sciences (SPSS) v20.0 (Chicago, IL). All fetal parameters and normalized mRNA expression data were analyzed using a one-way ANOVA for treatment followed by a Duncan post hoc test. The numerical density of SFTP-B-positive cells present in the alveolar epithelium was analyzed with an unpaired Student's t-test between 116-day cortisol and 140-day saline-infused fetuses. All data are presented as mean ± SEM. A probability level of 5% (P < 0.05) was considered statistically significant.
Results
No effect of cortisol infusion on fetal health and growth
There was no change in mean gestational Pao2 or Paco2 with age or cortisol infusion (Table 2). pH and hemoglobin content increased with age. There was a significant increase in fetal weight, crown-rump length, and lung weight as a result of age, but not cortisol infusion. Relative lung weight was not affected by age or cortisol infusion (Table 3).
Table 2.
Mean blood gas values for 116-day saline and cortisol-infused fetuses and 140-day saline-infused fetuses throughout the infusion period
| 116-day | 140-day | ||
|---|---|---|---|
| Saline-infused (n = 8) | Cortisol-infused (n = 9) | Saline-infused (n = 12) | |
| PaO2 (mmHg) | 21.5 ± 0.9 | 22.4 ± 0.9 | 22.6 ± 0.5 |
| PaCO2 (mmHg) | 49.6 ± 1.0 | 47.7 ± 0.5 | 49.6 ± 0.9 |
| pH | 7.335 ± 0.012a | 7.352 ± 0.008a | 7.379 ± 0.006b |
| Hb (g/dL) | 8.8 ± 0.1a | 9.0 ± 0.4a | 10.1 ± 0.3b |
Data are expressed as mean ± SEM. Data were analyzed by one-way ANOVA followed by Duncan post hoc test. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other. PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; Hb, hemoglobin.
Table 3.
Effect of fetal intravenous saline or cortisol infusion on fetal and lung growth
| 116-day | 140-day | ||
|---|---|---|---|
| Saline-infused (n = 8) | Cortisol-infused (n = 9) | Saline-infused (n = 12) | |
| Gestational age at postmortem (days) | 116 ± 1a | 116 ± 1a | 140 ± 1b |
| Fetal weight (kg) | 2.17 ± 0.1a | 2.12 ± 0.1a | 4.81 ± 0.2b |
| Crown-rump length (cm) | 44.6 ± 0.8a | 43.1 ± 0.4a | 58.3 ± 1.4b |
| Lung weight (g) | 75.8 ± 3.5a | 66.2 ± 3.8a | 158.0 ± 6.7b |
| Relative lung weight (g/kg) | 37.4 ± 1.9 | 32.0 ± 1.2 | 33.8 ± 1.3 |
Data expressed as mean ± SEM. Data were analyzed by one-way ANOVA followed by Duncan post hoc test. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other.
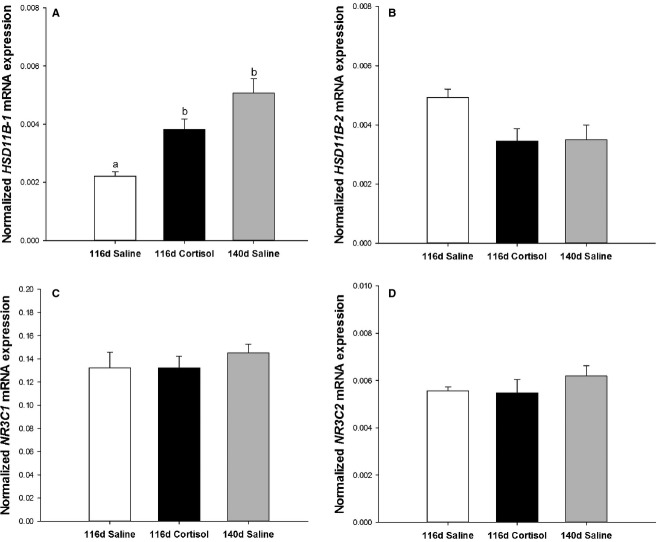
Effect of cortisol infusion on GC availability and signaling in the fetal lung
There was an increase in the mRNA expression of HSD11B-1 with both age and cortisol infusion (Fig. 1A). However, there was no effect of age or cortisol infusion on mRNA expression of HSD11B-2, NR3C1 or NR2C2 (Fig. 1B–D).
Figure 1.
Fetal intravenous cortisol infusion increased glucocorticoid activating enzyme mRNA expression in the fetal lung. Normalized mRNA expression of 11β hydroxysteroid dehydrogenase isoform 1 (HSD11B-1, A) was increased with age and cortisol infusion. There was no change in normalized mRNA expression of the glucocorticoid deactivating enzyme (HSD11B-2, B), glucocorticoid receptor (NR3C1, C) or the mineralocorticoid receptor (NR3C2, D) with age or cortisol infusion. Data expressed as mean ± SEM. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other. 116-day saline-infused, open bars; 116-day cortisol-infused, black bars; 140-day saline-infused, gray bars.
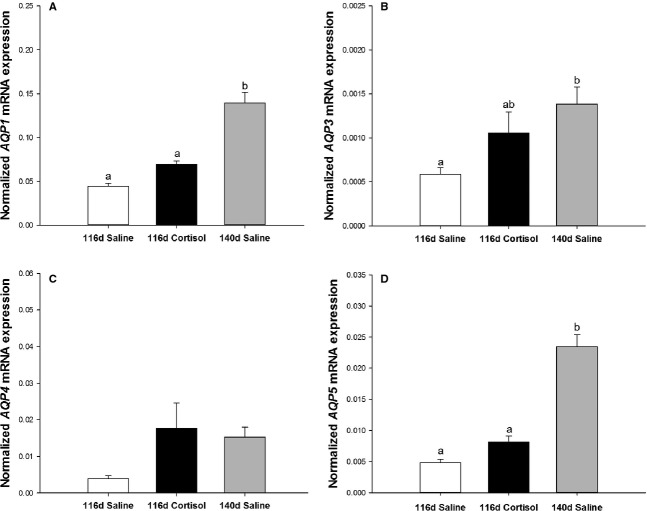
Effect of cortisol infusion on the expression of genes regulating lung liquid reabsorption
mRNA expression of SCNN1 -A, -B, and -G subunits increased with age, but not cortisol infusion (Fig. 2). mRNA expression of ATP1A1, but not B1, increased with age and cortisol infusion (Fig. 3). mRNA expression of AQP1, 3 and 5 were increased with age, but not cortisol infusion. AQP4 mRNA expression was not affected by age or cortisol infusion (Fig. 4).
Figure 2.
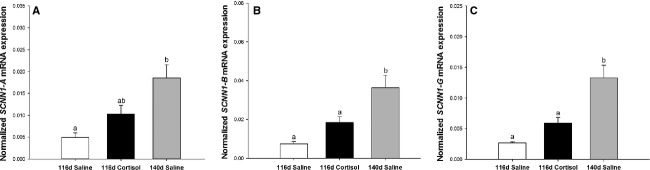
Fetal intravenous cortisol infusion from 109- to 116-days gestation had no impact on epithelial sodium channel (SCNN1) subunit mRNA expression compared to the late gestation fetal lung. There was an increase in normalized mRNA expression of SCNN1-A (A), SCNN1-B (B) and SCNN1-Y (C) subunits with age, but not cortisol infusion. Data expressed as mean ± SEM. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other. 116-day saline-infused, open bars; 116-day cortisol-infused, black bars; 140-day saline-infused, gray bars.
Figure 3.
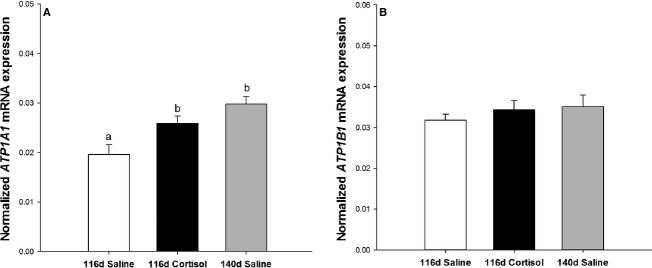
Fetal intravenous cortisol infusion increased sodium–potassium ATPase (ATP1) A1 pump subunit mRNA expression. There was an increase in normalized ATP1A1 subunit mRNA expression with age and cortisol infusion (A), but no impact on the ATP1B1 subunit (B). Data expressed as mean ± SEM. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other. 116-day saline-infused, open bars; 116-day cortisol-infused, black bars; 140-day saline-infused, gray bars.
Figure 4.
Fetal intravenous cortisol infusion did not increase expression of genes regulating water reabsorption in the fetal lung to the same extent as those observed in late gestation. There was an increase in aquaporin (AQP) 1, 3, and 5 mRNA expression with age, but not cortisol infusion (A, B, and D). AQP4 mRNA expression was not changed with age or cortisol infusion (C). Data expressed as mean ± SEM. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other. 116-day saline-infused, open bars; 116-day cortisol-infused, black bars; 140-day saline-infused, gray bars.
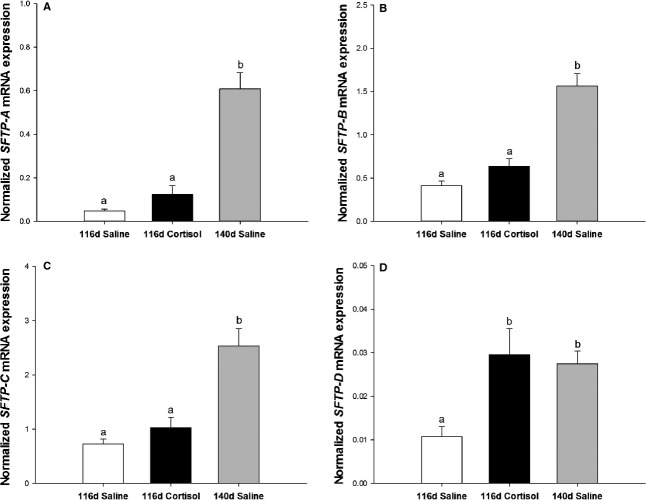
Cortisol infusion increased SFTP-D but not SFTP-A, -B or -C in the fetal lung
SFTP-A, -B, -C, and -D mRNA expression increased with age, but only SFTP-D mRNA expression increased with cortisol infusion (Fig. 5). The mRNA expression of NKX2-1, and its cofactor, GATA6, were not altered by age or cortisol infusion (Fig. 6).
Figure 5.
Fetal intravenous cortisol infusion does not increase surfactant protein (SFTP) -A, -B or -C mRNA expression in the lung compared to the late gestation fetus. There was only an increase in normalized mRNA expression of SFTP-A (A), SFTP-B (B) and SFTP-C (C) with age, but SFTP-D (D) expression was increased with both age and cortisol infusion. Data expressed as mean ± SEM. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other. 116-day saline-infused, open bars; 116-day cortisol-infused, black bars; 140-day saline-infused, gray bars.
Figure 6.
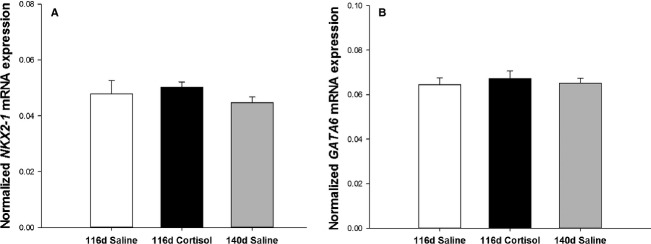
mRNA expression of transcription factor, NKX2-1, and its cofactor, GATA6, are not changed in the fetal lung with age or cortisol infusion. There was no change in normalized mRNA expression of either NKX2-1 (A) or GATA6 (B) with age or cortisol infusion. Data expressed as mean ± SEM. P < 0.05 was considered statistically significant; columns with different letters are significantly different from each other. 116-day saline-infused, open bars; 116-day cortisol-infused, black bars; 140-day saline-infused, gray bars.
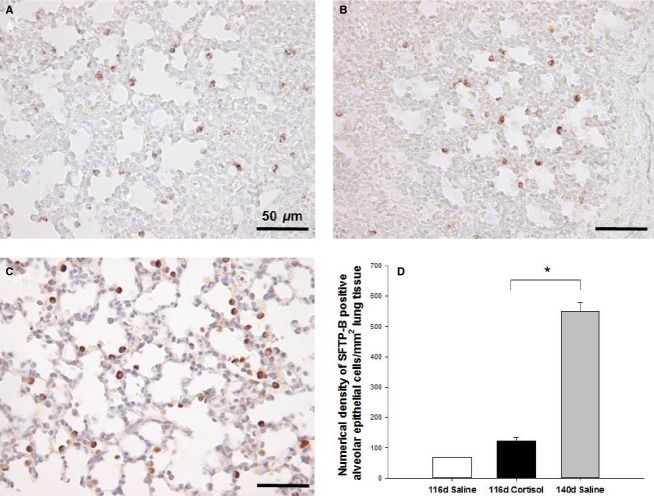
Effect of cortisol infusion on the numerical density of SFTP-B-positive cells in the fetal lung
The numerical density of SFTP-B-positive AECs in the alveolar epithelium increased with age, but not with cortisol infusion (Fig. 7).
Figure 7.
Evaluation of SFTP-B-positive alveolar epithelial cells in the fetal lung. Micrographs demonstrating SFTP-B immunoreactivity of alveolar epithelial cells in the 116-day saline-infused (A), 116-day cortisol infused (B) and 140-day saline-infused (C) fetal lung (200× magnification). Cortisol infusion did not increase the numerical density of SFTP-B-positive alveolar epithelial cells per mm2 of lung tissue when compared to the late gestation fetal lung (D). 116-day saline-infused (n = 2; open bars), 116-day cortisol-infused (n = 4; black bars) and 140-day saline-infused fetuses (n = 5; gray bars). Data expressed as mean ± SEM. Data were analyzed by an unpaired Student's t-test between 116-day cortisol and 140-day saline groups, *P < 0.05. Scale bar = 50 μm.
Discussion
It is widely documented that both endogenous and exogenous GCs regulate genes involved in lung liquid reabsorption and SFTP expression (Tan et al. 1999; Liu et al. 2003; Jesse et al. 2009), processes which are essential for a fetus to make a successful transition to air breathing after birth. However, we have shown that cortisol infusion during the late canalicular phase of lung development in the very preterm sheep fetus does not increase expression of genes involved in these processes to the same degree as occurs after exposure to the normal prepartum surge in cortisol. Fetal intravenous cortisol infusion did, however, increase mRNA expression of HSD11B-1 within the fetal lung, suggesting that despite a potential increase in the availability of bioactive GC, the very preterm fetal sheep lung is unable to respond to GCs during the late canalicular phase of lung development.
While previous studies have examined the developmental changes or the impact of cortisol infusion between 120- and 130-days gestation on the expression of some genes regulating lung liquid reabsorption in fetal sheep (Liu et al. 2003; Jesse et al. 2009), the relative efficacy of cortisol to mature the very preterm lung in comparison to late gestation has not previously been evaluated. The developmental changes observed in mRNA expression of the SCNN1-A, -B and -G subunits are consistent with those previously reported in sheep (Jesse et al. 2009; Keller-Wood et al. 2009), rats (Tchepichev et al. 1995) and mice (Nakamura et al. 2002). Although the optimal SCNN1 channel subunit combination has yet to be described (Firsov et al. 1998; Kosari et al. 1998; Eskandari et al. 1999), in this study the SCNN1-B subunit was the most abundantly expressed in each group, and SCNN1-A and -G subunits were the second and third most abundantly expressed, respectively. This observed distribution of the expression of subunits in all treatment groups was similar to those observed previously in the lung of the sheep fetus (Jesse et al. 2009).
Water reabsorption is controlled by the differential expression of AQP isoforms in lung tissue and we have observed an increase in mRNA expression of AQP1, 3, and 5, but not AQP4 with advancing gestation. The increased AQP5 and unchanged AQP4 mRNA expression profiles observed in this study were consistent with previous findings in the fetal sheep lung between 100 and 135-days gestation (Liu et al. 2003). However, AQP expression may be influenced by the composition of the lung tissue studied, for example, there is expression of AQP1 on the vascular endothelium, AQP3 in large airways and to some extent in type II AEC in adults (Kreda et al. 2001), AQP4 in large and small airways and AQP5 in type I AECs (Verkman et al. 2000; King et al. 2004). Unlike the previous study evaluating AQP expression in the fetal sheep (Liu et al. 2003), we have observed an increase in AQP1 and 3 mRNA expression with advancing gestation. These differences between the two studies may be due to the tissue sampling, therefore, the distribution of components expressing these markers as a result of the heterogeneity of lung tissue, such as expression patterns between smaller and larger airways and alveolar tissue.
In this study, we have demonstrated that high circulating concentrations of cortisol result in increased HSD11B-1 mRNA expression, suggesting a potential increase in GC availability in the fetal lung. However, despite these findings, there is no change in the expression of genes regulating lung liquid reabsorption, including SCNN1-A, -B, or -G subunits, ATP1B1, AQP1, AQP3, or AQP5 in the fetal sheep lung in the late canalicular phase of development relative to that which occurs during exposure to the normal prepartum increase in cortisol. It has previously been shown that cortisol infusion at ∼130-days gestation resulted in an increase in only the SCNN1-A subunit, but not SCNN1-B, SCNN1-G, ATP1A1, AQP1, or AQP5 mRNA expression in the sheep lung (Jesse et al. 2009). Similarly, maternal dexamethasone treatment from 17 to 19 days gestation (term = 20 days) in rats, during the less mature pseudoglandular phase and early canalicular phase of fetal lung development, enhanced protein expression of the SCNN1-A subunit but did not alter SCNN1-B or -G and ATP1A1 or ATP1B1 subunit expression (Tchepichev et al. 1995). In contrast, culture of human fetal lung explants (20–24 weeks gestation) and lung cell lines exposed to dexamethasone exhibited a dose-dependent increase in expression of the SCNN1 -A, -B, and -G subunits (Venkatesh and Katzberg 1997; Itani et al. 2002). The lack of response in the expression of these lung genes to an increase in fetal intravenous cortisol concentrations in this study may be a result of the responsiveness of the fetal sheep lung at 116-days gestation.
In this study, we have demonstrated an increase in gene expression of the catalytic A1 subunit, but not of the glycolytic B1 subunit of ATP1 in response to both cortisol infusion and advancing age. In the fetal rat lung, ATP1 subunit mRNA expression is altered in response to maternal dexamethasone exposure and gestational age at administration (14- to 19-days gestation) throughout the pseudoglandular and early canalicular stages (Ingbar et al. 1997), which is earlier in lung development than our model. Administration of GC for a shorter duration (1 or 3 days) and earlier ages (14- to 16-days gestation) increases ATP1B1, but not A1 subunit expression, whereas longer treatments (5 days) closer to term (14- to 18-days gestation) increase ATP1B1 and decrease A1 subunit expression. Interestingly, closer to term there was no impact of shorter exposure (1 or 3 days) on ATP1 subunit expression. However, in the rat, type II AEC cultures exposed to dexamethasone exhibit an increase in both ATP1A1 and B1 subunit mRNA expression (Barquin et al. 1997). The differing mRNA expression profiles from each study may be due to the developmental stage of the lung, drug type, dose or direct/indirect GC action regulating expression in each species. Despite the increase in ATP1A1 subunit with cortisol infusion, the findings from this study suggest that in comparison to the level of expression of genes involved in the regulation of lung liquid reabsorption near term, cortisol stimulation around 116 days gestation in the fetal sheep lung is not adequate to increase the expression of genes regulating ion pumps and water channels, which remove lung liquid and contribute to neonatal survival upon premature exposure to the extrauterine environment.
The second important component contributing to the successful transition of a fetus to air breathing at birth involves maturation of the pulmonary surfactant system. The regulation of surfactant proteins by GC is complex and varies as a function of dose and developmental stage (Weaver and Whitsett 1991; Boggaram 2003). The developmental pattern of mRNA expression for SFTP-A, -B and -C observed in this study is similar to previous studies in the sheep fetus (Tan et al. 1999; Flecknoe et al. 2003). Interestingly, lung SFTP-D was increased with both age and cortisol infusion. However, the period of cortisol infusion was not adequate to increase SFTP-A, -B or -C mRNA expression to the same extent as those increases in expression observed near term. These findings suggest that despite exposure to increased plasma cortisol concentrations during the late canalicular phase of development, the lung of the very preterm fetus is not responsive to GCs.
GCs stimulate the expression of surfactant proteins in an indirect manner. The promoter regions of the four SFTP genes contain regulatory elements, some of which are conserved between the four genes and others which are not, and hence there is specific regulation of each of the genes. NKX2-1 interacts with various cofactors, such as GATA6 (Bruno et al. 2000; Liu et al. 2002), and binds to NKX2-1 binding elements expressed on the promoter regions of SFTP-A, -B and -C gene constructs (Bruno et al. 1995; Kelly et al. 1996; Mendelson 2000). In the case of SFTP-D, NKX2-1 regulates gene transcription indirectly via interactions with nuclear factor of activated T cells (NFATs) (Davé et al. 2004). Here, we have evaluated gene expression of the transcription factor NKX2-1, and its co-factor GATA6, and have found that there was no difference in their expression with either age or cortisol infusion. Although there are many different mechanisms by which transcription factors can interact with DNA to influence expression, these data suggest that at the transcriptional level, these two factors may not play a role in surfactant protein expression or GC responsiveness in our cohort.
At ∼116-days gestation, there is differentiation of type I and type II AECs in the fetal sheep lung (Harding and Bocking 2001; Flecknoe et al. 2003). The fact that cortisol infusion from 109- to 116-days gestation did not stimulate greater production of surfactant proteins suggests that there may be a limited capacity of the surfactant system to respond because there are fewer or less mature type II AECs present at this stage of gestation. We found that there was an increase in the number of SFTP-B-positive cells in the alveolar epithelium with age, which is consistent with the increase in SFTP mRNA expression. However, there was no impact of cortisol infusion on the number of mature SFTP-B-positive cells in the alveolar epithelium when compared to the number present in the late gestation fetal lung. This result suggests that there is a limitation in the extent to which GCs can induce an increase in number and responsiveness of type II AECs, the cell responsible for an increase in SFTP expression. Taken together, the gene and immunohistochemistry results suggest that even during the last third of gestation, GCs may not be effective if the lung is at the late canalicular stage of development.
In the clinical setting, antenatal GCs are administered to women at risk of preterm birth to promote fetal lung maturation, however, they also have benefits in reducing the risk of other morbidities in preterm infants. In sheep models, it has been demonstrated that both fetal and maternal injections have similar effects on lung development (Rebello et al. 1996). It is widely understood that antenatal GC administration to women at risk of preterm birth from 24 to 37 weeks gestation is the ‘gold standard’ treatment for reducing the risk of respiratory distress syndrome (Roberts and Dalziel 2006) in the 8–12% of babies born preterm in Australia, the UK, and the USA (Laws and Sullivan 2004; Tucker and McGuire 2004; Heron et al. 2010). However, with the primary outcome being to prepare the fetal lung for air breathing, it is important to consider a potential developmental limit to when the lung can respond to such treatments, particularly around the limit of fetal viability.
Acknowledgments
We acknowledge the assistance of Anne Jurisevic, Tim Butler and Jacob Ross in performing the surgical procedures, providing expert postsurgical care of the ewe and her fetus and performing the cortisol infusion. We thank Darran Tosh and Tamara Crittenden for assistance with optimizing housekeeping genes for real-time PCR and Stacey Dunn and Kimberley Botting for assistance with immunohistochemistry and cell counting, respectively.
Conflict of Interest
None declared.
References
- Barquin N, Ciccolella DE, Ridge KM, Sznajder JI. Dexamethasone upregulates the Na-K-ATPase in rat alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997;273:L825–L830. doi: 10.1152/ajplung.1997.273.4.L825. [DOI] [PubMed] [Google Scholar]
- Boggaram V. Regulation of lung surfactant protein gene expression. Front. Biosci. 2003;8:d751–d767. doi: 10.2741/1062. [DOI] [PubMed] [Google Scholar]
- Bolt RJ, Lafeber M, van Weissenbruch H, Delemarre van de Waal H. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr. Pulmonol. 2001;32:76–91. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- Bremner HR, Freywald T, O'brodovich HM, Otulakowski G. Promoter analysis of the gene encoding the β-subunit of the rat amiloride-sensitive epithelial sodium channel. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L124–L134. doi: 10.1152/ajplung.2002.282.1.L124. [DOI] [PubMed] [Google Scholar]
- Brüel A, Oxlund H, Nyengaard JR. The total length of myocytes and capillaries, and total number of myocyte nuclei in the rat heart are time-dependently increased by growth hormone. Growth Hormon. IGF Res. 2005;15:256–264. doi: 10.1016/j.ghir.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Bruno MD, Bohinski RJ, Huelsman KM, Whitsett JA, Korfhagen TR. Lung cell-specific expression of the murine surfactant protein A (SP-A) gene is mediated by interactions between the SP-A promoter and thyroid transcription factor-1. J. Biol. Chem. 1995;270:6531–6536. doi: 10.1074/jbc.270.12.6531. [DOI] [PubMed] [Google Scholar]
- Bruno MD, Korfhagen TR, Liu C, Morrisey EE, Whitsett JA. GATA-6 activates transcription of surfactant protein A. J. Biol. Chem. 2000;275:1043–1049. doi: 10.1074/jbc.275.2.1043. [DOI] [PubMed] [Google Scholar]
- Champigny G, Voilley N, Lingueglia E, Friend V, Barbry P, Lazdunski M. Regulation of expression of the lung amiloride-sensitive Na+ channel by steroid hormones. EMBO J. 1994;13:2177–2181. doi: 10.1002/j.1460-2075.1994.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuwels L, Haagsman L, van Golde H. The pulmonary surfactant system: biochemical and clinical aspects. Lung. 1997;175:1–39. doi: 10.1007/PL00007554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais A, Denis C, Vives MF, Girouard S, Massé C, Nguyen T, et al. Modulation of α-ENaC and α1-Na+-K + -ATPase by cAMP and dexamethasone in alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L217–L230. doi: 10.1152/ajplung.2001.281.1.L217. [DOI] [PubMed] [Google Scholar]
- Davé V, Childs T, Whitsett JA. Nuclear factor of activated T cells regulates transcription of the surfactant protein D gene (Sftpd) via direct interaction with thyroid transcription factor-1 in lung epithelial cells. J. Biol. Chem. 2004;279:34578–34588. doi: 10.1074/jbc.M404296200. [DOI] [PubMed] [Google Scholar]
- Eskandari S, Snyder PM, Kreman M, Zampighi GA, Welsh MJ, Wright EM. Number of subunits comprising the epithelial sodium channel. J. Biol. Chem. 1999;274:27281–27286. doi: 10.1074/jbc.274.38.27281. [DOI] [PubMed] [Google Scholar]
- Ewart HS, Klip A. Hormonal regulation of the Na (+)-K (+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am. J. Physiol. Cell Physiol. 1995;269:C295–C311. doi: 10.1152/ajpcell.1995.269.2.C295. [DOI] [PubMed] [Google Scholar]
- Firsov D, Gautschi I, Merillat AM, Rossier BC, Schild L. The heterotetrameric architecture of the epithelial sodium channel (ENaC) EMBO J. 1998;17:344–352. doi: 10.1093/emboj/17.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flecknoe S, Wallace M, Cock M, Harding R, Hooper S. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;285:L664–L670. doi: 10.1152/ajplung.00306.2002. [DOI] [PubMed] [Google Scholar]
- Gentili S, Morrison JL, McMillen IC. Intrauterine growth restriction and differential patterns of hepatic growth and expression of IGF1, PCK2, and HSDL1 mRNA in the sheep fetus in late gestation. Biol. Reprod. 2009;80:1121–1127. doi: 10.1095/biolreprod.108.073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke J, Clements J. Alveolar surface tension and lung surfactant. In: Macklem PT, Mead J, editors. Handbook of physiology, Section 3: the respiratory system. Vol III: mechanics of breathing. Part I. Washington D.C: American Physiological Society; 1985. [Google Scholar]
- Haagsman HP, Hogenkamp A, Veldhuizen M, van Eijk EJA. Surfactant collectins and innate immunity. Neonatology. 2008;93:288–294. doi: 10.1159/000121454. [DOI] [PubMed] [Google Scholar]
- Harding R, Bocking AD. Fetal growth and development. Cambridge, U.K: Cambridge Univ Press; 2001. [Google Scholar]
- Hawgood S, Derrick M, Poulain F. Structure and properties of surfactant protein B. Biochim. Biophys. Acta. 1998;1408:150–160. doi: 10.1016/s0925-4439(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, Speleman A, de Paepe F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19.1–R19.14. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- Hooper S, Harding R. Fetal lung liquid: a major determinant of the growth and functional development of the fetal lung. Clin. Exp. Pharmacol. Physiol. 1995;22:235–241. doi: 10.1111/j.1440-1681.1995.tb01988.x. [DOI] [PubMed] [Google Scholar]
- Ingbar D, Duvick S, Savick S, Schellhase D, Detterding R, Jamieson J, et al. Developmental changes of fetal rat lung Na-K-ATPase after maternal treatment with dexamethasone. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997;272:L665–L672. doi: 10.1152/ajplung.1997.272.4.L665. [DOI] [PubMed] [Google Scholar]
- Itani OA, Auerbach SD, Husted RF, Volk KA, Ageloff S, Knepper MA, et al. Glucocorticoid-stimulated lung epithelial Na+ transport is associated with regulated ENaC andsgk1 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L631–L641. doi: 10.1152/ajplung.00085.2001. [DOI] [PubMed] [Google Scholar]
- Jesse NM, McCartney J, Feng X, Richards EM, Wood CE, Keller-Wood M. Expression of ENaC subunits, chloride channels, and aquaporins in ovine fetal lung: ontogeny of expression and effects of altered fetal cortisol concentrations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R453–R461. doi: 10.1152/ajpregu.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J. Structure and properties of surfactant protein C. Biochim. Biophys. Acta. 1998;1408:161–172. doi: 10.1016/s0925-4439(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, Wood CE, Hua Y, Zhang D. Mineralocorticoid receptor expression in late-gestation ovine fetal lung. J. Soc. Gynecol. Investig. 2005;12:84–91. doi: 10.1016/j.jsgi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, McCartney MKWM, von Reitzenstein J. Is the fetal lung a mineralocorticoid receptor target organ? Induction of cortisol-regulated genes in the ovine fetal lung, kidney and small intestine. Neonatology. 2009;95:47–60. doi: 10.1159/000151755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SE, Bachurski CJ, Burhans MS, Glasser SW. Transcription of the lung-specific surfactant protein C gene is mediated by thyroid transcription factor 1. J. Biol. Chem. 1996;271:6881–6888. doi: 10.1074/jbc.271.12.6881. [DOI] [PubMed] [Google Scholar]
- King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- Kosari F, Sheng S, Li J, Mak DOD, Foskett JK, Kleyman TR. Subunit stoichiometry of the epithelial sodium channel. J. Biol. Chem. 1998;273:13469–13474. doi: 10.1074/jbc.273.22.13469. [DOI] [PubMed] [Google Scholar]
- Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am. J. Respir. Cell Mol. Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- Laws PJ, Sullivan EA. Australian mothers and babies 2002. Sydney, Australia: The Australian Institute of Health and Welfare; 2004. Perinatal Statistics Series No. 15. [Google Scholar]
- LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect. 2001;3:161–166. doi: 10.1016/s1286-4579(00)01363-0. [DOI] [PubMed] [Google Scholar]
- Lin S, Na CL, Akinbi HT, Apsley KS, Whitsett JA, Weaver TE. Surfactant protein B (SP-B)-/- mice are rescued by restoration of SP-B expression in alveolar type II cells but not Clara cells. J. Biol. Chem. 1999;274:19168–19174. doi: 10.1074/jbc.274.27.19168. [DOI] [PubMed] [Google Scholar]
- Liu H, Wintour EM. Aquaporins in development-a review. Reprod. Biol. Endocrinol. 2005;3:18–28. doi: 10.1186/1477-7827-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J. Biol. Chem. 2002;277:4519–4525. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- Liu H, Hooper S, Armugam A, Dawson N, Ferraro T, Jeyaseelan K, et al. Aquaporin gene expression and regulation in the ovine fetal lung. J. Physiol. 2003;551:503–514. doi: 10.1113/jphysiol.2003.044875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo LD, Reynolds LP. Some historical aspects of understanding placental development, structure and function. Int. J. Dev. Biol. 2010;54:237–255. doi: 10.1387/ijdb.082774ll. [DOI] [PubMed] [Google Scholar]
- Mendelson CR. Role of transcription factors in fetal lung development and surfactant protein gene expression. Annu. Rev. Physiol. 2000;62:875–915. doi: 10.1146/annurev.physiol.62.1.875. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R306–R313. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- Muhlhausler B, Adam C, Marrocco E, Findlay P, Roberts CT, McFarlane JR, et al. Impact of glucose infusion on the structural and functional characteristics of adipose tissue and on hypothalamic gene expression for appetite regulatory neuropeptides in the sheep fetus during late gestation. J. Physiol. 2005;565:185–195. doi: 10.1113/jphysiol.2004.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa SB, Digeronimo RJ, Petershack JA, Alcorn JL, Seidner SR. Postnatal glucocorticoids induce α-ENaC formation and regulate glucocorticoid receptors in the preterm rabbit lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L73–L80. doi: 10.1152/ajplung.00342.2002. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Stokes JB, McCray PB. Endogenous and exogenous glucocorticoid regulation of ENaC mRNA expression in developing kidney and lung. Am. J. Physiol. Cell Physiol. 2002;283:C762–C772. doi: 10.1152/ajpcell.00029.2002. [DOI] [PubMed] [Google Scholar]
- Orgeig S, Crittenden TA, Marchant C, McMillen IC, Morrison JL. Intrauterine growth restriction delays surfactant protein maturation in the sheep fetus. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;298:L575–L583. doi: 10.1152/ajplung.00226.2009. [DOI] [PubMed] [Google Scholar]
- Passmore M, Nataatmadja M, Fraser JF. Selection of reference genes for normalisation of real-time RT-PCR in brain-stem death injury in Ovis aries. BMC Mol. Biol. 2009;10:72–80. doi: 10.1186/1471-2199-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ID, Simonetta G, Owens JA, Robinson JS, Clarke IJ, McMillen IC. Placental restriction alters the functional development of the pituitary-adrenal axis in the sheep fetus during late gestation. Pediatr. Res. 1996;40:861–866. doi: 10.1203/00006450-199612000-00014. [DOI] [PubMed] [Google Scholar]
- Rebello CM, Ikegami M, Polk DH, Jobe AH. Postnatal lung responses and surfactant function after fetal or maternal corticosteroid treatment. J. Appl. Physiol. 1996;80:1674–1680. doi: 10.1152/jappl.1996.80.5.1674. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Roberts D, Dalziel SR. 2006. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 3:3.
- Ross JT, McMillen IC, Adams MB, Coulter CL. A premature increase in circulating cortisol suppresses expression of 11b hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid in the adrenal of the fetal sheep. Biol. Reprod. 2000;62:1297–1302. doi: 10.1095/biolreprod62.5.1297. [DOI] [PubMed] [Google Scholar]
- Silver M, Fowden A. Induction of labour in domestic animals: endocrine changes and neonatal viability. In: Künzel W, Jensen A, editors. The endocrine control of the fetus physiologic and pathophysiologic aspects. Berlin, Heidelberg: Springer; 1988. pp. 401–411. [Google Scholar]
- Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- Soo PS, Hiscock J, Botting KJ, Roberts CT, Davey AK, Morrison JL. Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod. Toxicol. 2012;33:374–381. doi: 10.1016/j.reprotox.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Stahlman MT, Gray ME, Whitsett JA. Expression of thyroid transcription factor-1 (TTF-1) in fetal and neonatal human lung. J. Histochem. Cytochem. 1996;44:673–678. doi: 10.1177/44.7.8675988. [DOI] [PubMed] [Google Scholar]
- Tan RC, Ikegami M, Jobe AH, Yao LY, Possmayer F, Ballard PL. Developmental and glucocorticoid regulation of surfactant protein mRNAs in preterm lambs. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999;277:L1142–L1148. doi: 10.1152/ajplung.1999.277.6.L1142. [DOI] [PubMed] [Google Scholar]
- Tchepichev S, Ueda J, Canessa C, Rossier B, O'brodovich H. Lung epithelial Na channel subunits are differentially regulated during development and by steroids. Am. J. Physiol. Cell Physiol. 1995;269:C805–C812. doi: 10.1152/ajpcell.1995.269.3.C805. [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Stewart PM. Cortisol metabolism and the role of 11 [beta]-hydroxysteroid dehydrogenase. Best Pract. Res. Clin. Endocrinol. Metab. 2001;15:61–78. doi: 10.1053/beem.2000.0119. [DOI] [PubMed] [Google Scholar]
- Tucker J, McGuire W. ABC of preterm birth: epidemiology of preterm birth. Br. Med. J. 2004;329:675–678. doi: 10.1136/bmj.329.7467.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, Pattyn K, De Preter F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh VC, Katzberg HD. Glucocorticoid regulation of epithelial sodium channel genes in human fetal lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 1997;273:L227–L233. doi: 10.1152/ajplung.1997.273.1.L227. [DOI] [PubMed] [Google Scholar]
- Verkman A, Matthay MA, Song Y. Aquaporin water channels and lung physiology. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L867–L879. doi: 10.1152/ajplung.2000.278.5.L867. [DOI] [PubMed] [Google Scholar]
- Warnes KE, Morris MJ, Symonds ME, Phillips ID, Clarke IJ, Owens JA, et al. Effects of increasing gestation, cortisol and maternal undernutrition on hypothalamic neuropeptide Y expression in the sheep fetus. J. Neuroendocrinol. 1998;10:51–57. doi: 10.1046/j.1365-2826.1998.00172.x. [DOI] [PubMed] [Google Scholar]
- Weaver TE. Synthesis, processsing and secretion of surfactant proteins B and C. Biochim. Biophys. Acta. 1998;1408:173–179. doi: 10.1016/s0925-4439(98)00066-0. [DOI] [PubMed] [Google Scholar]
- Weaver TE, Whitsett JA. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem. J. 1991;273:249–264. doi: 10.1042/bj2730249. [DOI] [PMC free article] [PubMed] [Google Scholar]