Abstract
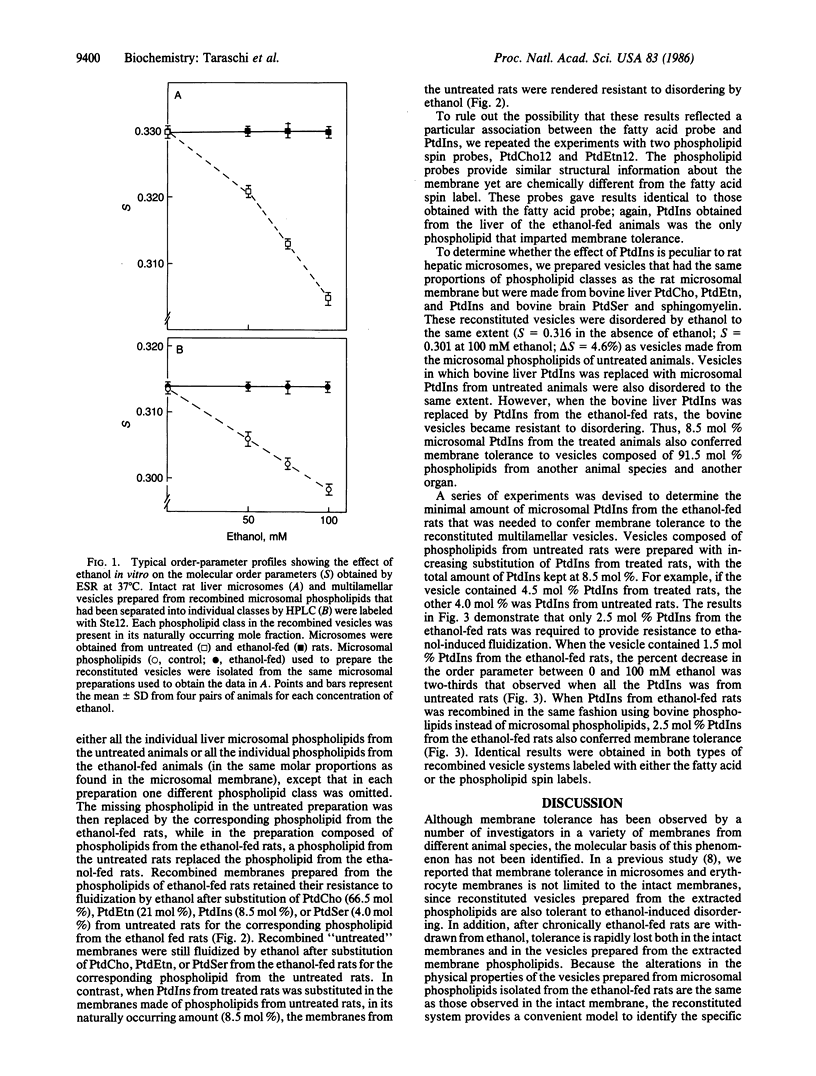
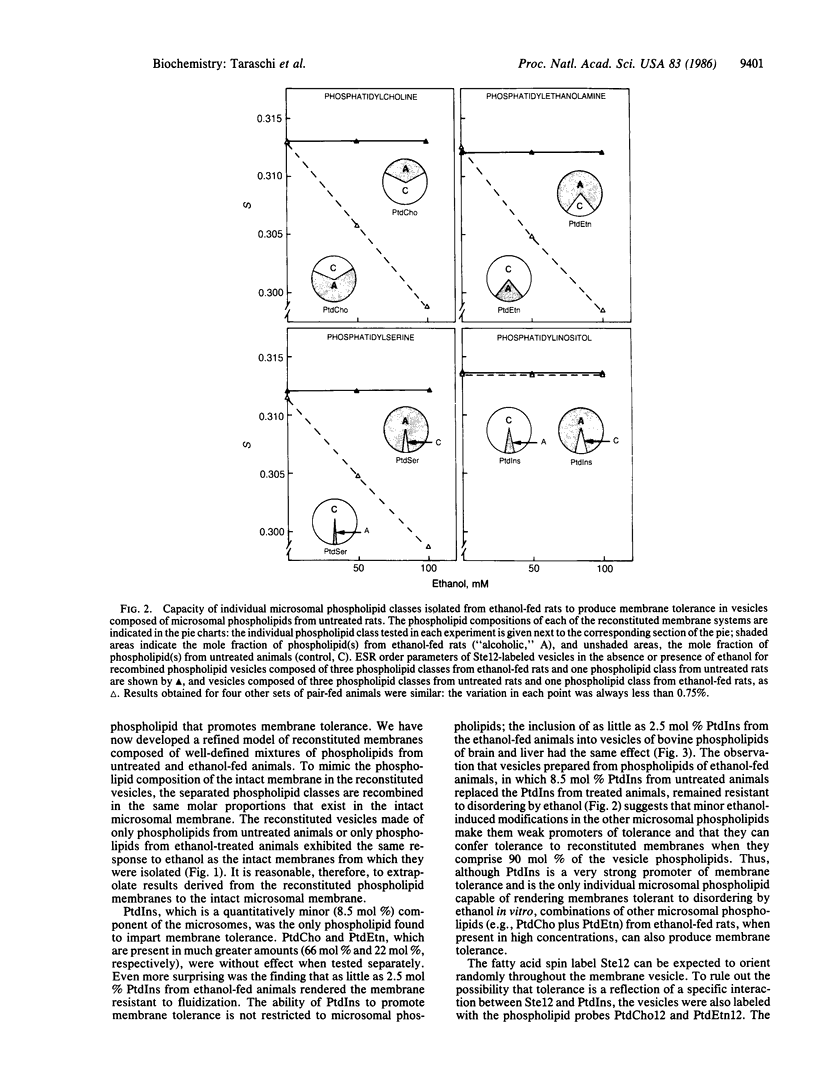
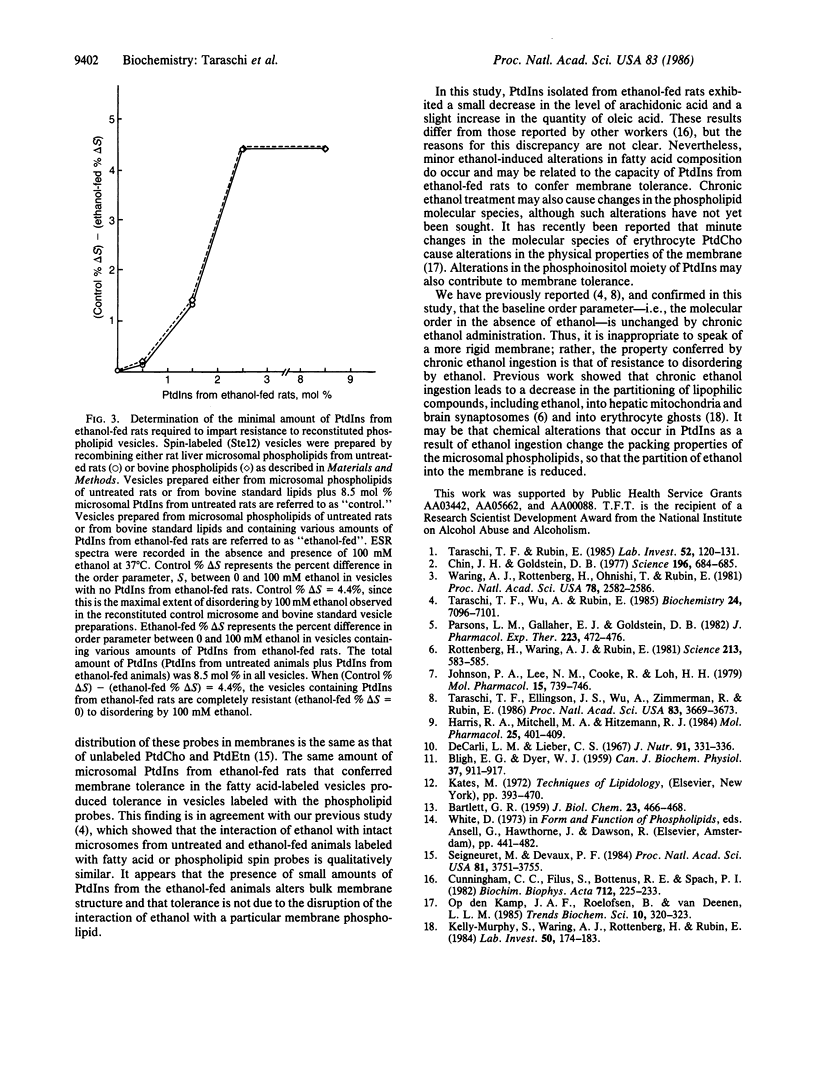
The presence of ethanol disorders (fluidizes) biological membranes, but its chronic administration confers resistance to this perturbation (membrane tolerance). The latter effect has been invoked as an explanation for behavioral tolerance in alcoholics, but the molecular basis for membrane tolerance is obscure. To study the molecular mechanisms of this acquired resistance to disordering, we fed rats ethanol (36% of total calories) for 35 days, after which we quantitatively separated the phospholipids of hepatic microsomal membranes by high-performance liquid chromatography. Multilamellar vesicles were prepared from the recombined phospholipid classes, and their physical properties were examined by electron spin resonance. Vesicles composed of phospholipids from untreated rats were disordered (fluidized) in the presence of ethanol, whereas those made from phospholipids of ethanol-fed rats were resistant to this effect. When phosphatidylcholine (66.5 mol %), phosphatidylethanolamine (21 mol %), or phosphatidylserine (4.0 mol %) from ethanol-fed rats replaced their corresponding phospholipids in vesicles prepared from microsomal phospholipids from untreated rats, the membranes were still disordered by ethanol. In contrast, when 2.5-8.5 mol % phosphatidylinositol from ethanol-fed rats replaced phosphatidylinositol from untreated rats, the reconstituted membranes were rendered resistant to ethanol-induced disordering. Liver microsomal phosphatidylinositol (2.5-8.5 mol %) from ethanol-fed rats also conferred membrane tolerance to vesicles composed of bovine liver and brain phospholipids, an effect which demonstrates that the ability of phosphatidylinositol to confer membrane tolerance is not restricted to the microsomal membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Goldstein D. B. Drug tolerance in biomembranes: a spin label study of the effects of ethanol. Science. 1977 May 6;196(4290):684–685. doi: 10.1126/science.193186. [DOI] [PubMed] [Google Scholar]
- Cunningham C. C., Filus S., Bottenus R. E., Spach P. I. Effect of ethanol consumption on the phospholipid composition of rat liver microsomes and mitochondria. Biochim Biophys Acta. 1982 Aug 18;712(2):225–233. doi: 10.1016/0005-2760(82)90338-1. [DOI] [PubMed] [Google Scholar]
- DeCarli L. M., Lieber C. S. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967 Mar;91(3):331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Baxter D. M., Mitchell M. A., Hitzemann R. J. Physical properties and lipid composition of brain membranes from ethanol tolerant-dependent mice. Mol Pharmacol. 1984 May;25(3):401–409. [PubMed] [Google Scholar]
- Johnson D. A., Lee N. M., Cooke R., Loh H. H. Ethanol-induced fluidization of brain lipid bilayers: required presence of cholesterol in membranes for the expression of tolerance. Mol Pharmacol. 1979 May;15(3):739–746. [PubMed] [Google Scholar]
- Kelly-Murphy S., Waring A. J., Rottenberg H., Rubin E. Effects of chronic ethanol consumption on the partition of lipophilic compounds into erythrocyte membranes. Lab Invest. 1984 Feb;50(2):174–183. [PubMed] [Google Scholar]
- Parsons L. M., Gallaher E. J., Goldstein D. B. Rapidly developing functional tolerance to ethanol is accompanied by increased erythrocyte cholesterol in mice. J Pharmacol Exp Ther. 1982 Nov;223(2):472–476. [PubMed] [Google Scholar]
- Rottenberg H., Waring A., Rubin E. Tolerance and cross-tolerance in chronic alcoholics: reduced membrane binding of ethanol and other drugs. Science. 1981 Jul 31;213(4507):583–585. doi: 10.1126/science.6264608. [DOI] [PubMed] [Google Scholar]
- Seigneuret M., Devaux P. F. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschi T. F., Ellingson J. S., Wu A., Zimmerman R., Rubin E. Membrane tolerance to ethanol is rapidly lost after withdrawal: a model for studies of membrane adaptation. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3669–3673. doi: 10.1073/pnas.83.11.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschi T. F., Rubin E. Effects of ethanol on the chemical and structural properties of biologic membranes. Lab Invest. 1985 Feb;52(2):120–131. [PubMed] [Google Scholar]
- Taraschi T. F., Wu A., Rubin E. Phospholipid spin probes measure the effects of ethanol on the molecular order of liver microsomes. Biochemistry. 1985 Dec 3;24(25):7096–7101. doi: 10.1021/bi00346a012. [DOI] [PubMed] [Google Scholar]
- Waring A. J., Rottenberg H., Ohnishi T., Rubin E. Membranes and phospholipids of liver mitochondria from chronic alcoholic rats are resistant to membrane disordering by alcohol. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2582–2586. doi: 10.1073/pnas.78.4.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]



